Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
64 results about "Cyclododecanone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyclododecanone is an organic compound with the formula (CH₂)₁₁CO. It is a cyclic ketone that exists as a white solid at room temperature. It is produced by the oxidation of cyclododecane via cyclododecanol.
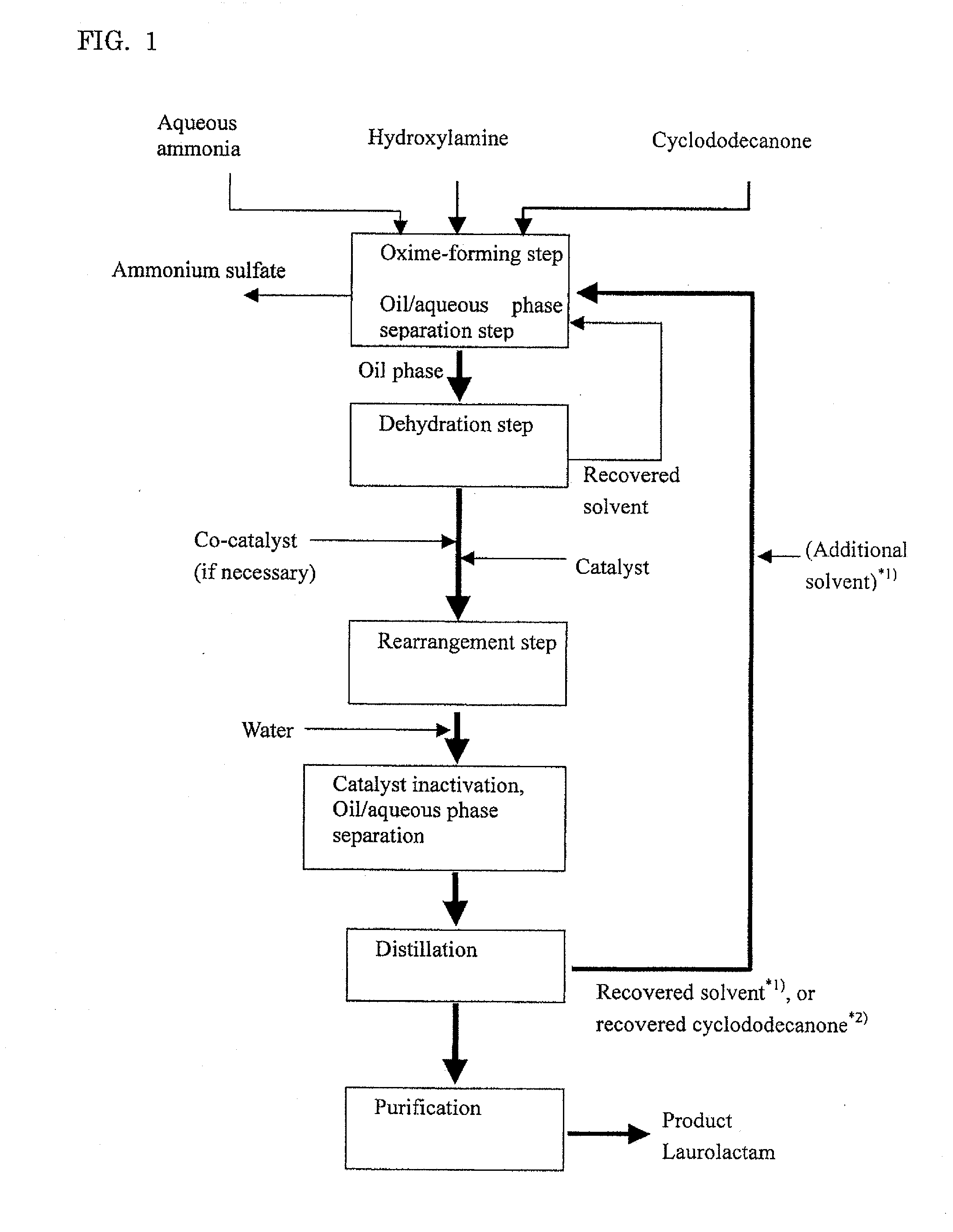
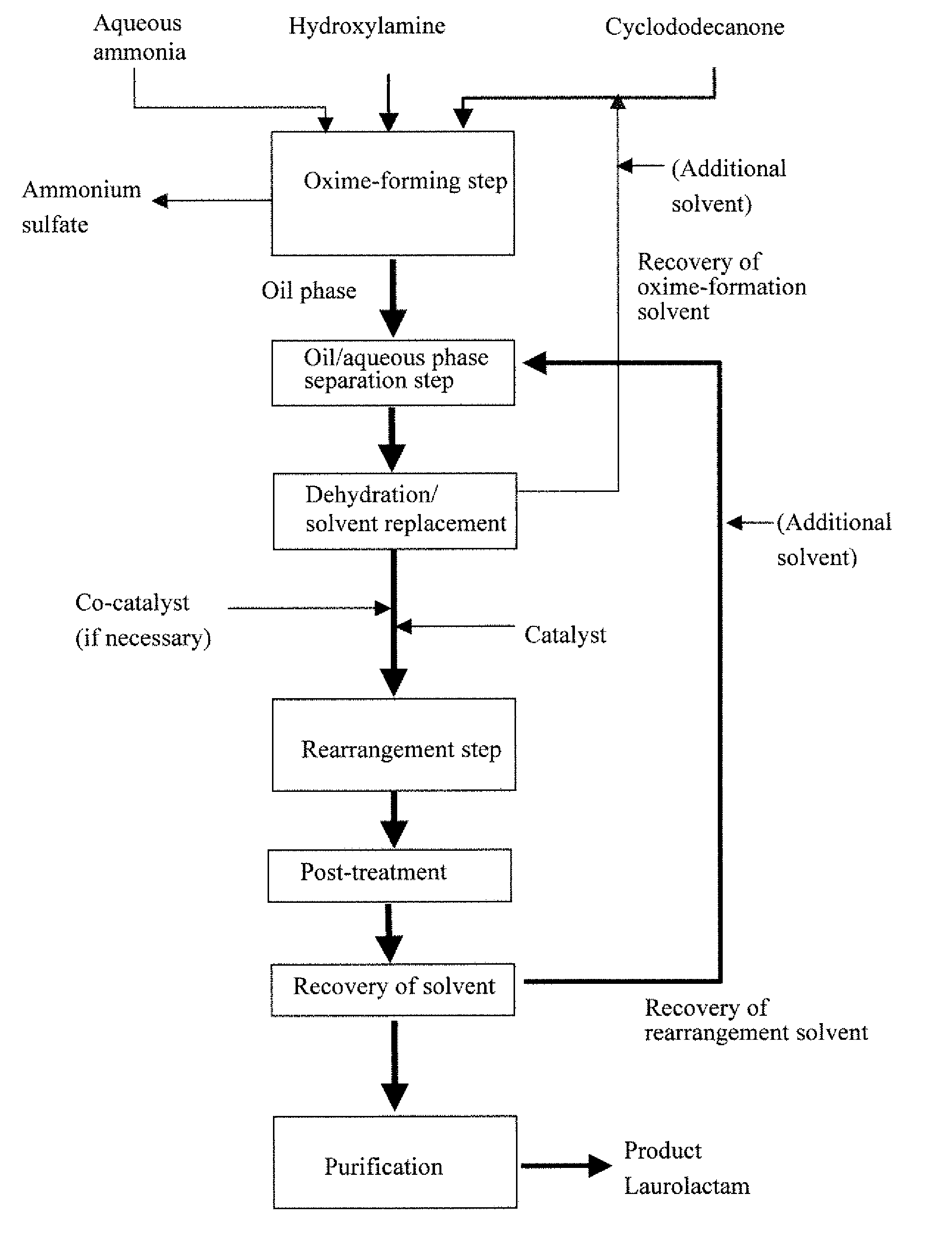
Process for producing laurolactam
ActiveUS20100324283A1Easy to processSimple processLactams preparationChemical recyclingLaurolactamHydroxylamine
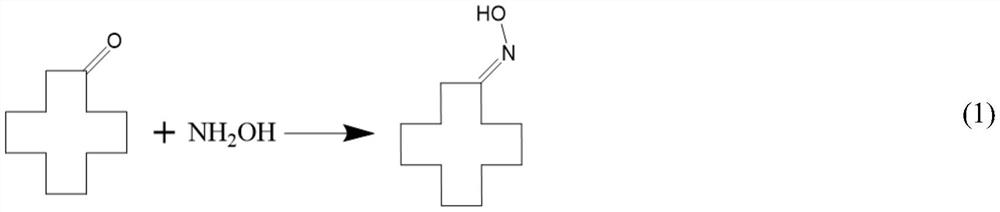
Disclosed is a method for producing laurolactam from cyclododecanone and hydroxylamine in a simple process and with high efficiency. The method comprises the following steps (a) to (e): (a) reacting cyclododecanone with hydroxylamine in an aqueous solution in the presence of an excess amount of cyclododecanone or a solvent to produce cyclododecanone oxime; (b) separating the reaction mixture obtained after the oxime-forming step into an oil and an aqueous phases and collecting a solution of cyclododecanone oxime of the oil phase as; (c) removing dissolved water from the solution of cyclododecanone oxime which is collected as an oily phase in the oil / aqueous phase separation step; (d) producing laurolactam from cyclododecanone oxime by rearrangement reaction using an aromatic-ring containing compound as a rearrangement catalyst; and (e) separating the produced laurolactam from the reaction mixture after the rearrangement step and purifying the laurolactam.
Owner:NAGOYA UNIVERSITY +1
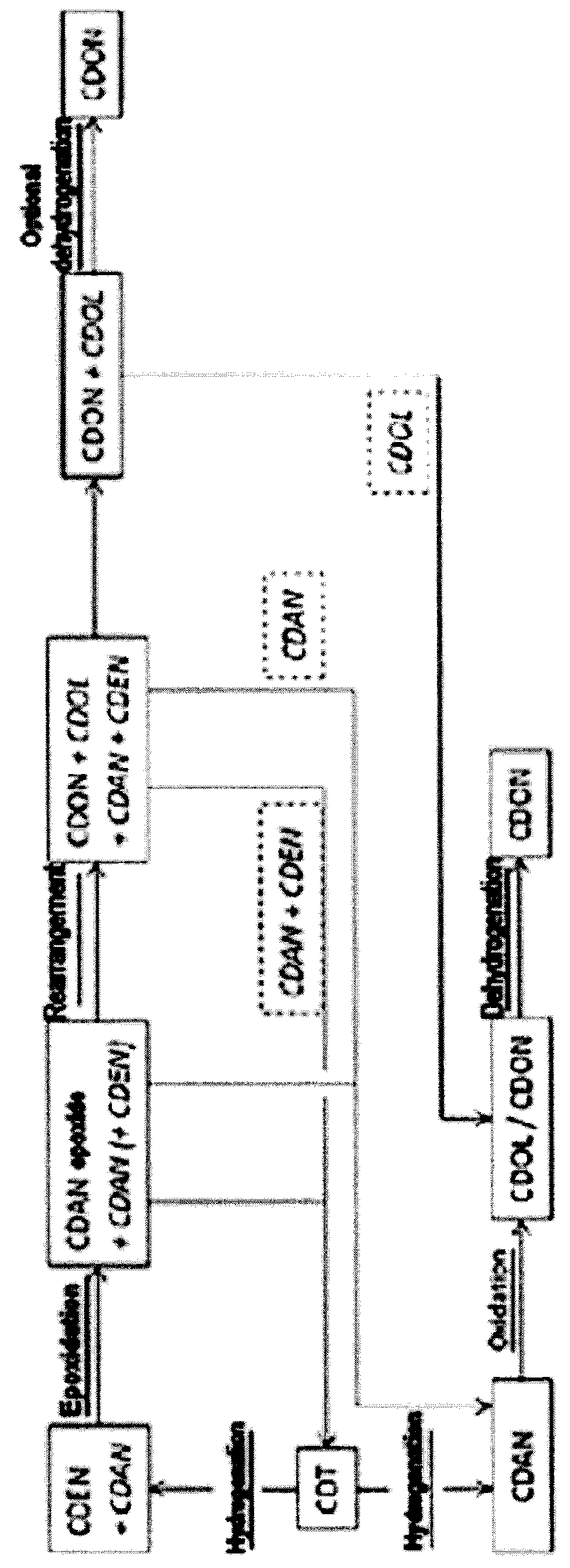
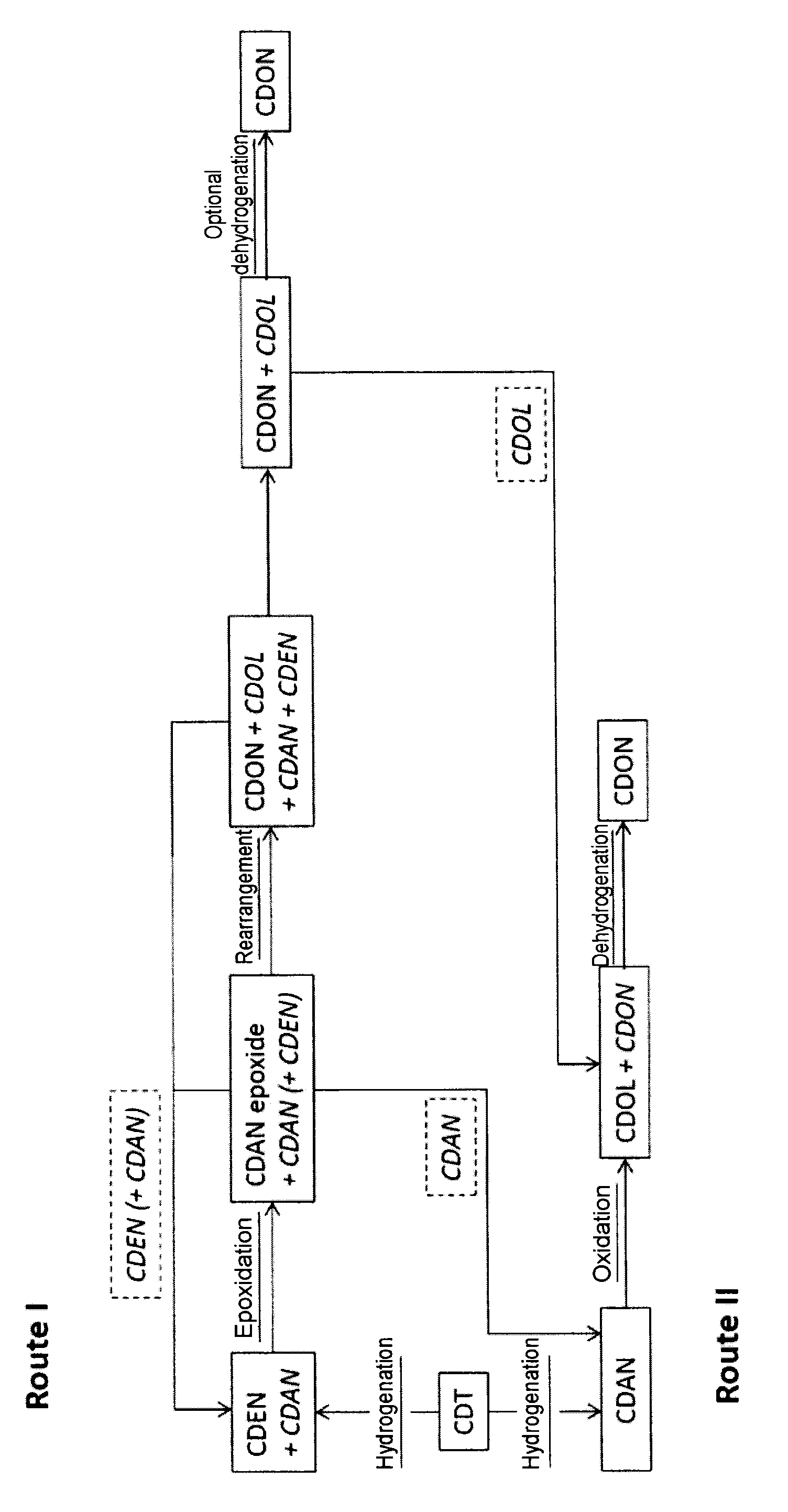
Process for preparing cyclododecanone
ActiveUS20160031784A1Increase productionReduce the ratioPreparation by oxidation reactionsLactams preparationCyclododecanoneEpoxide
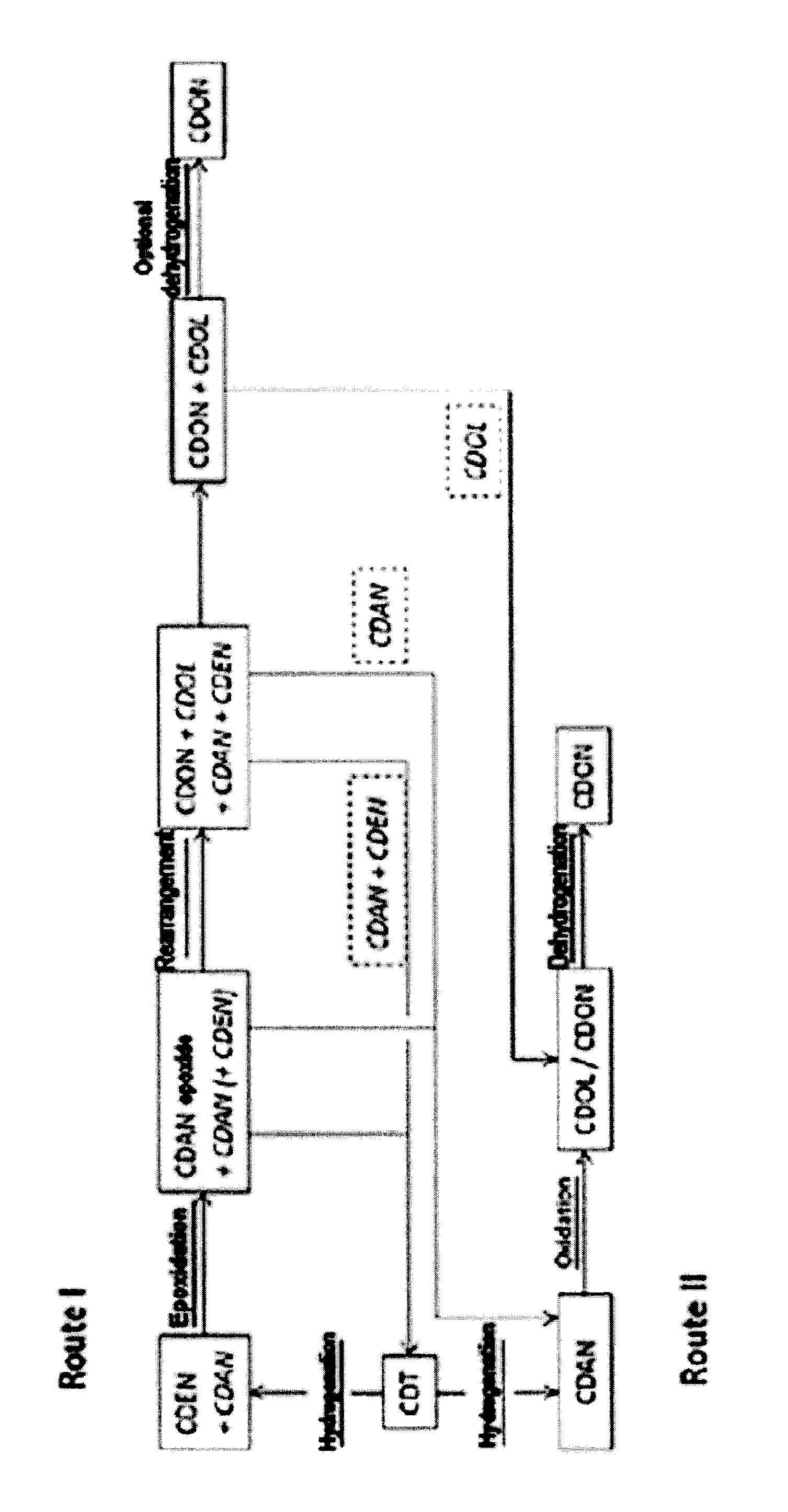
Cyclododecanone (CDON) is prepared by epoxidizing cyclododecene (CDEN) to epoxycyclododecane (CDAN epoxide), and rearranging the CDAN epoxide to CDON to obtain a mixture comprising said CDON and CDEN, wherein CDEN is separated from the CDON-containing mixture and sent to the epoxidation to CDAN epoxide in step a.
Owner:EVONIK OPERATIONS GMBH
Process for producing laurolactam
InactiveUS8309714B2Easy to processHigh yieldLactams preparationChemical recyclingLaurolactamHydroxylamine
Provided is a process for efficiently producing laurolactam by simple steps from cyclododecanone and hydroxylamine. This production process comprises the steps of: (a) reacting cyclododecanone with hydroxylamine in an aqueous solution in the presence of an oxime-formation solvent to produce cyclododecanone oxime; (b) separating the reaction mixture obtained after the oxime-forming step into an oil and an aqueous phases and collecting a solution of cyclododecanone oxime of the oil phase; (c) removing a part or all of the oxime-formation solvent and dissolved water from the solution of cyclododecanone oxime which is collected as an oil phase in the oil / aqueous phase separation step, whereby preparing a solution containing a rearrangement solvent to be used in a rearrangement reaction in a later step and the cyclododecanone oxime; (d) producing laurolactam from cyclododecanone oxime by rearrangement reaction using an aromatic-ring containing compound as a rearrangement catalyst; and (e) separating and removing the rearrangement solvent and the rearrangement catalyst from the reaction mixture after the rearrangement step, and purifying the laurolactam.
Owner:UBE IND LTD +1
Process for preparing cyclododecanone
ActiveUS20160031783A1Reduce the ratioEconomically viablePreparation by oxidation reactionsLactams preparationCyclododecanoneCyclododecane
Cyclododecanone (CDON) is prepared by epoxidizing cyclododecene (CDEN) to epoxycyclododecane (CDAN epoxide), and rearranging the CDAN epoxide to CDON to obtain a mixture comprising said CDON and cyclododecane (CDAN), wherein CDAN is separated from the CDON-containing mixture and oxidized to CDON.
Owner:EVONIK OPERATIONS GMBH
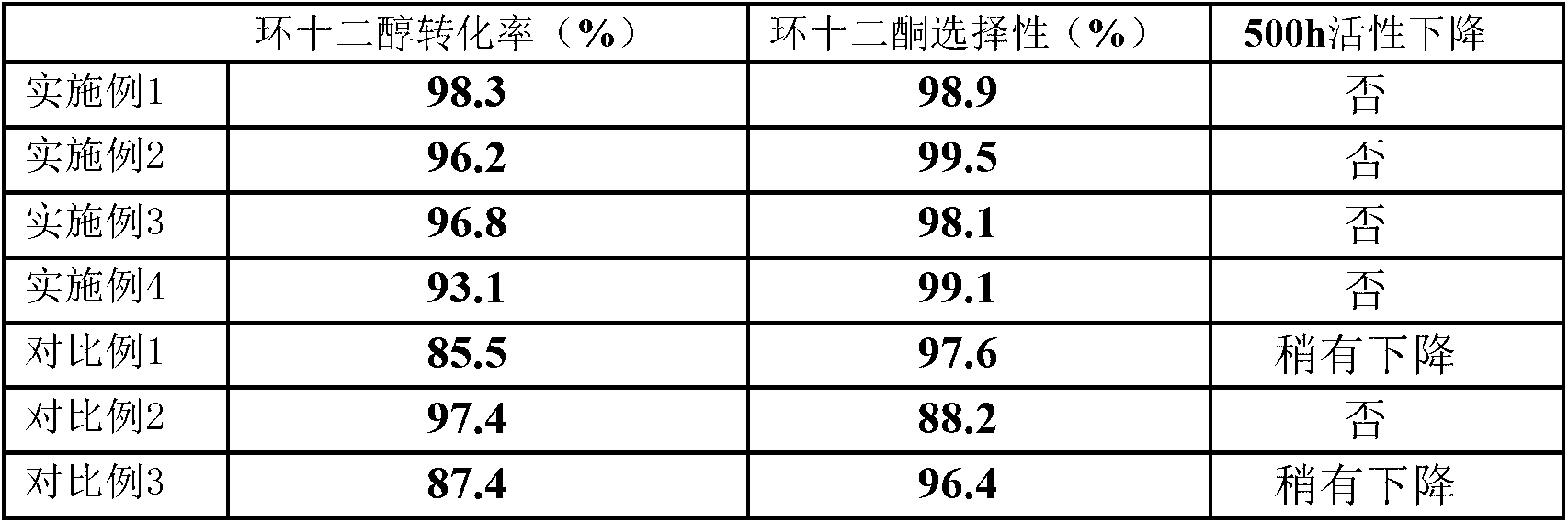
Catalyst for preparing ketone via cyclododecanol dehydrogenation and preparation method for same
ActiveCN103055880AImprove conversion rateOrganic compound preparationCarbonyl compound preparationDehydrogenationCerium
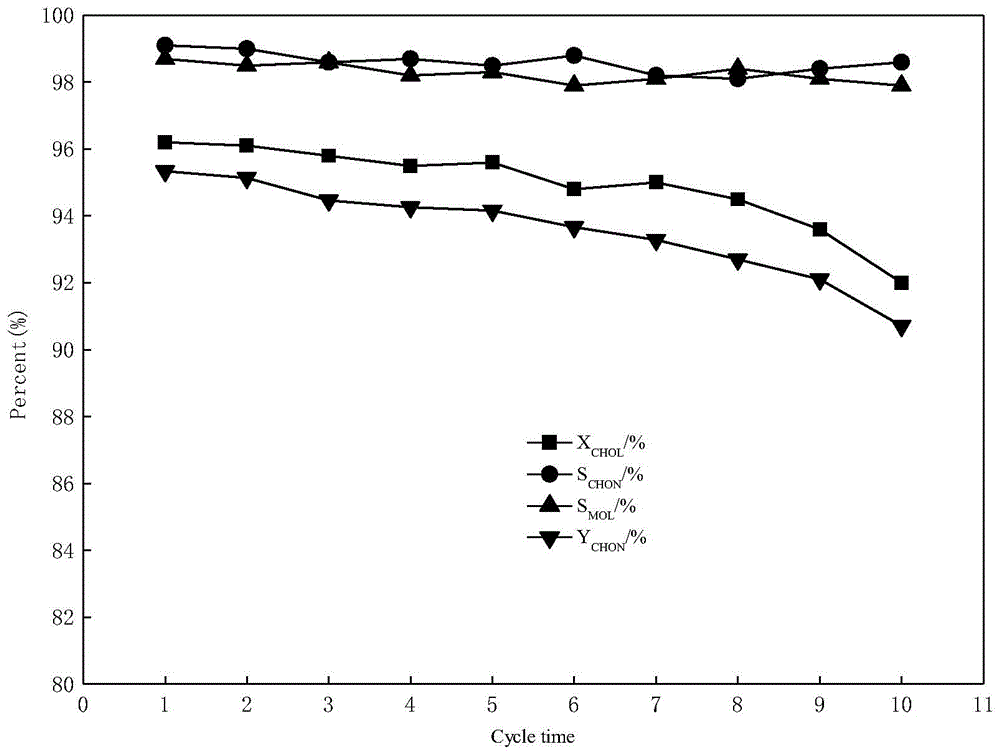
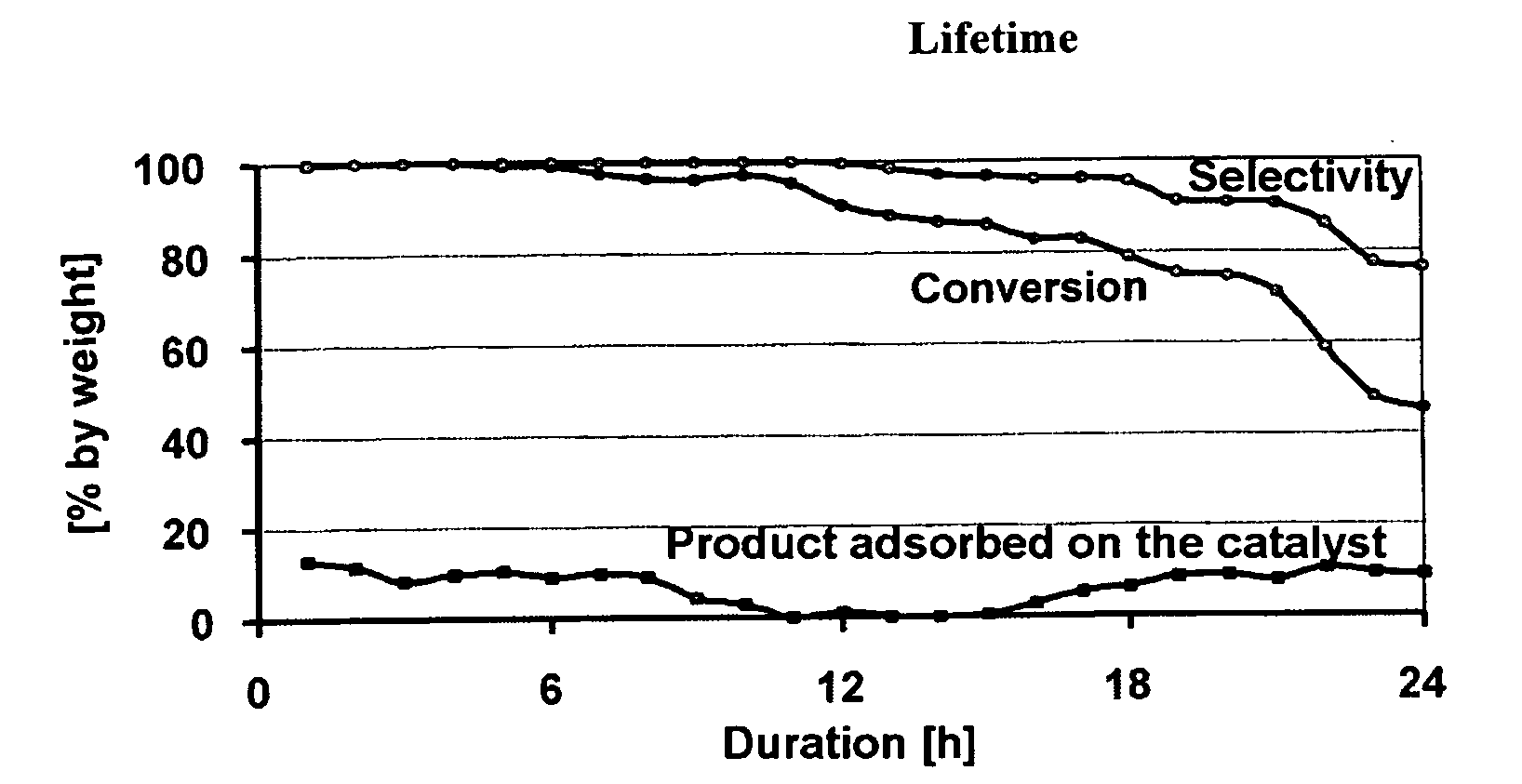
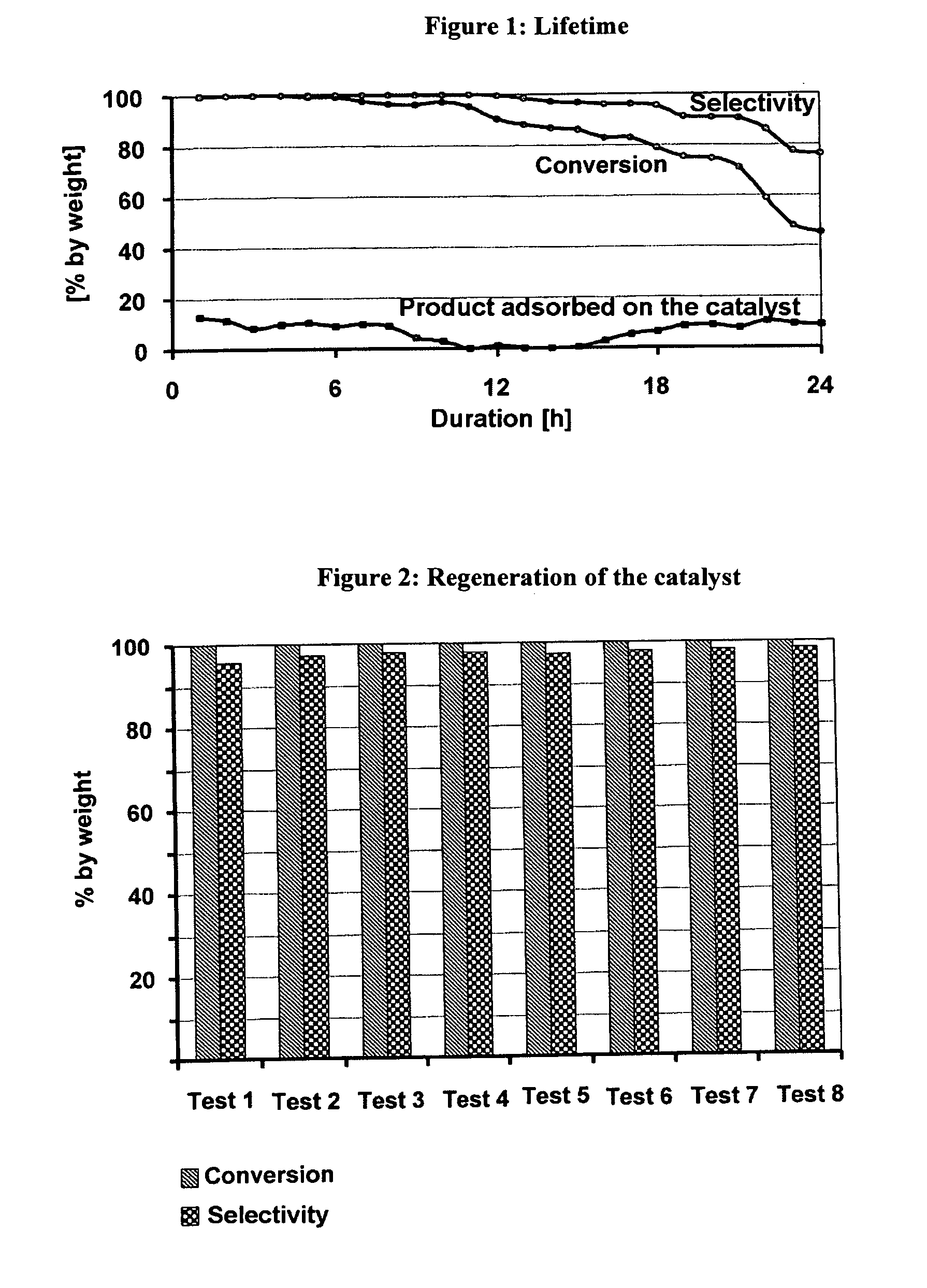
The invention discloses a catalyst for preparing ketone via cyclododecanol dehydrogenation and a preparation method for the same. The catalyst is characterized by comprising a main catalyst and an auxiliary, wherein the main catalyst is a copper-zinc mixed oxide taking copper element as a main active ingredient and taking zinc element as a carrier, and the molar ratio of the copper element to the zinc element is 1: 3 to 3: 1; and the auxiliary is a transition metal element with an inactive centre and selected from one of vanadium, vanadium, manganese, zirconium, lanthanum, cerium, praseodymium, neodymium, samarium, gadolinium or yttrium. Via an evaluation for the catalyst for cyclododecanol dehydrogenation prepared by the method disclosed by the invention on a micro reaction device in a laboratory, the conversion rate of cyclododecanol is greater than 95%, and the selectivity of cyclododecanone is greater than 98% in the conditions that a temperature is 230 DEG C, and a space velocity (WHSV (weight hourly space velocity)) is 0.4.
Owner:中瀚新材料科技有限公司
Process for the preparation of cyclododecanone
InactiveUS7838705B2Organic compound preparationCarbonyl compound preparation by oxidationCyclododecanoneCyclododecatriene
A process for preparing cyclododecanone by reacting cyclododecene with dinitrogen monoxide, comprising in particular steps (I) and (II):(I) preparing cyclododecene by partially hydrogenating cyclododecatriene;(II) reacting cyclododecene obtained in (I) with dinitrogen monoxide to obtain cyclododecanone.
Owner:BASF SE
Process for producing laurolactam
ActiveUS8163899B2Easy to processSimple processLactams preparationChemical recyclingLaurolactamHydroxylamine
Disclosed is a method for producing laurolactam from cyclododecanone and hydroxylamine in a simple process and with high efficiency. The method comprises the following steps (a) to (e): (a) reacting cyclododecanone with hydroxylamine in an aqueous solution in the presence of an excess amount of cyclododecanone or a solvent to produce cyclododecanone oxime; (b) separating the reaction mixture obtained after the oxime-forming step into an oil and an aqueous phases and collecting a solution of cyclododecanone oxime of the oil phase as; (c) removing dissolved water from the solution of cyclododecanone oxime which is collected as an oily phase in the oil / aqueous phase separation step; (d) producing laurolactam from cyclododecanone oxime by rearrangement reaction using an aromatic-ring containing compound as a rearrangement catalyst; and (e) separating the produced laurolactam from the reaction mixture after the rearrangement step and purifying the laurolactam.
Owner:NAGOYA UNIVERSITY +1
Process for producing laurolactam
InactiveUS20100267944A1Easy to processHigh yieldLactams preparationChemical recyclingLaurolactamHydroxylamine
Provided is a process for efficiently producing laurolactam by simple steps from cyclododecanone and hydroxylamine. This production process comprises the steps of: (a) reacting cyclododecanone with hydroxylamine in an aqueous solution in the presence of an oxime-formation solvent to produce cyclododecanone oxime; (b) separating the reaction mixture obtained after the oxime-forming step into an oil and an aqueous phases and collecting a solution of cyclododecanone oxime of the oil phase; (c) removing a part or all of the oxime-formation solvent and dissolved water from the solution of cyclododecanone oxime which is collected as an oil phase in the oil / aqueous phase separation step, whereby preparing a solution containing a rearrangement solvent to be used in a rearrangement reaction in a later step and the cyclododecanone oxime; (d) producing laurolactam from cyclododecanone oxime by rearrangement reaction using an aromatic-ring containing compound as a rearrangement catalyst; and (e) separating and removing the rearrangement solvent and the rearrangement catalyst from the reaction mixture after the rearrangement step, and purifying the laurolactam.
Owner:UBE IND LTD +1
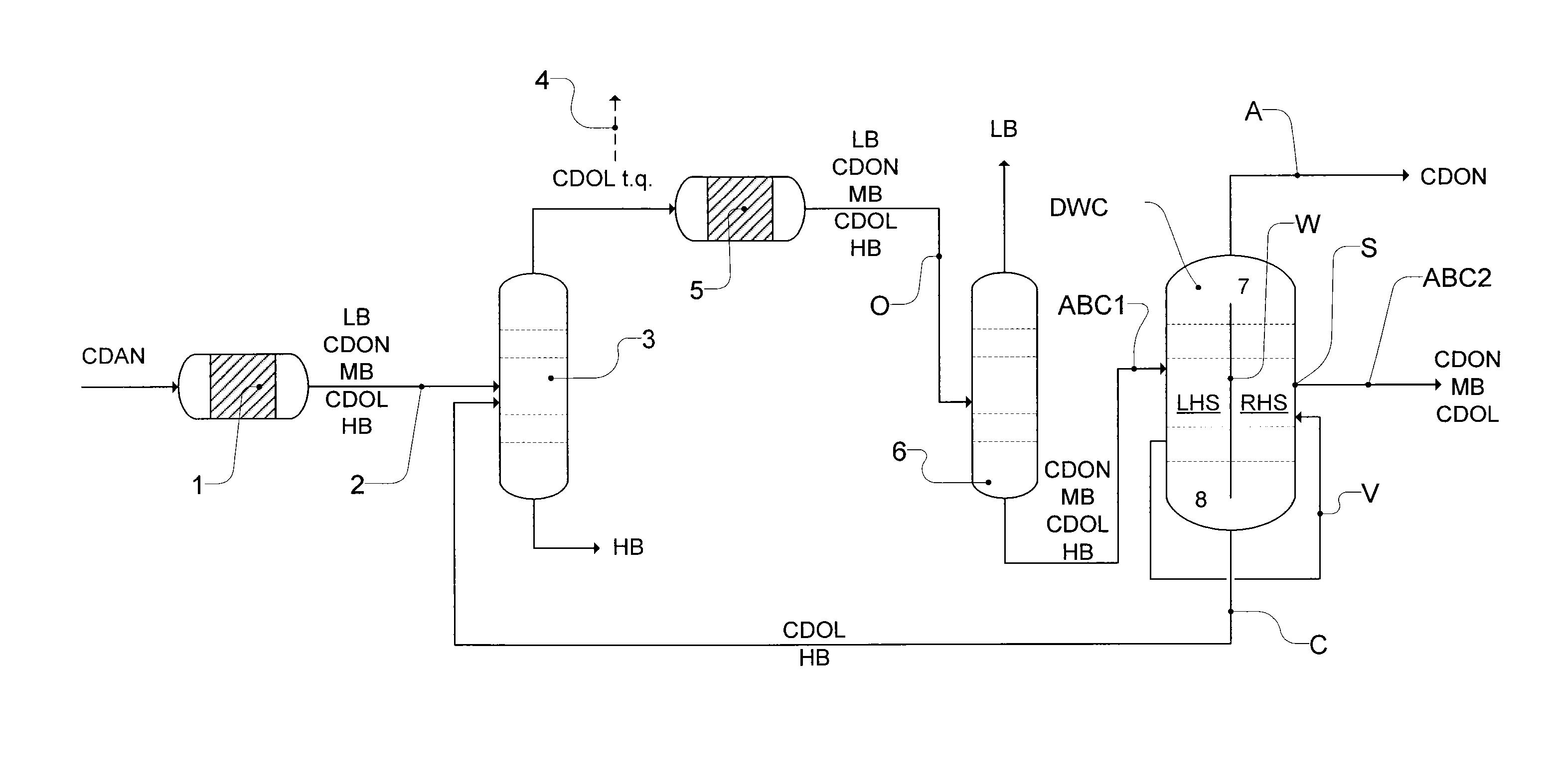
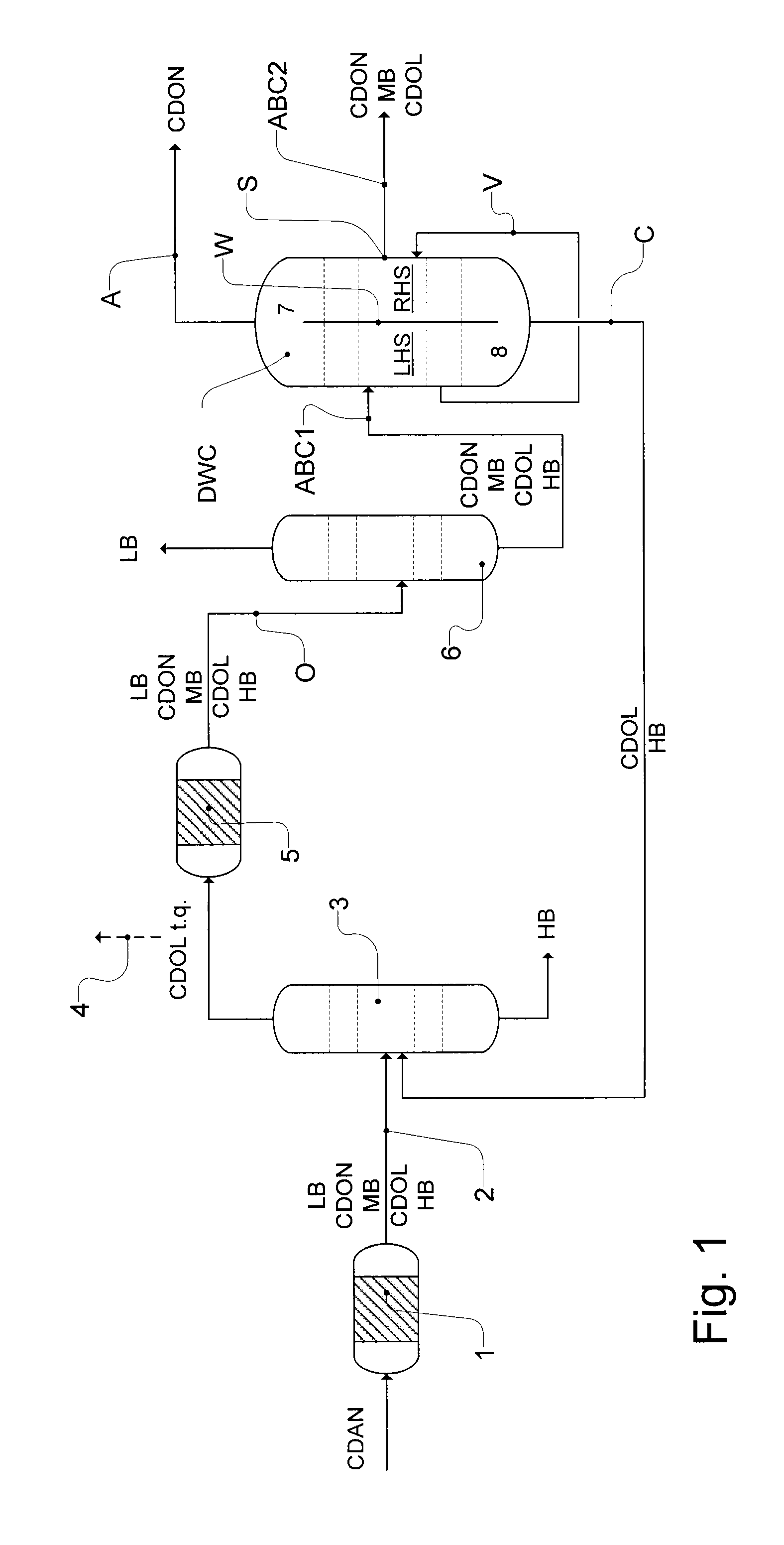
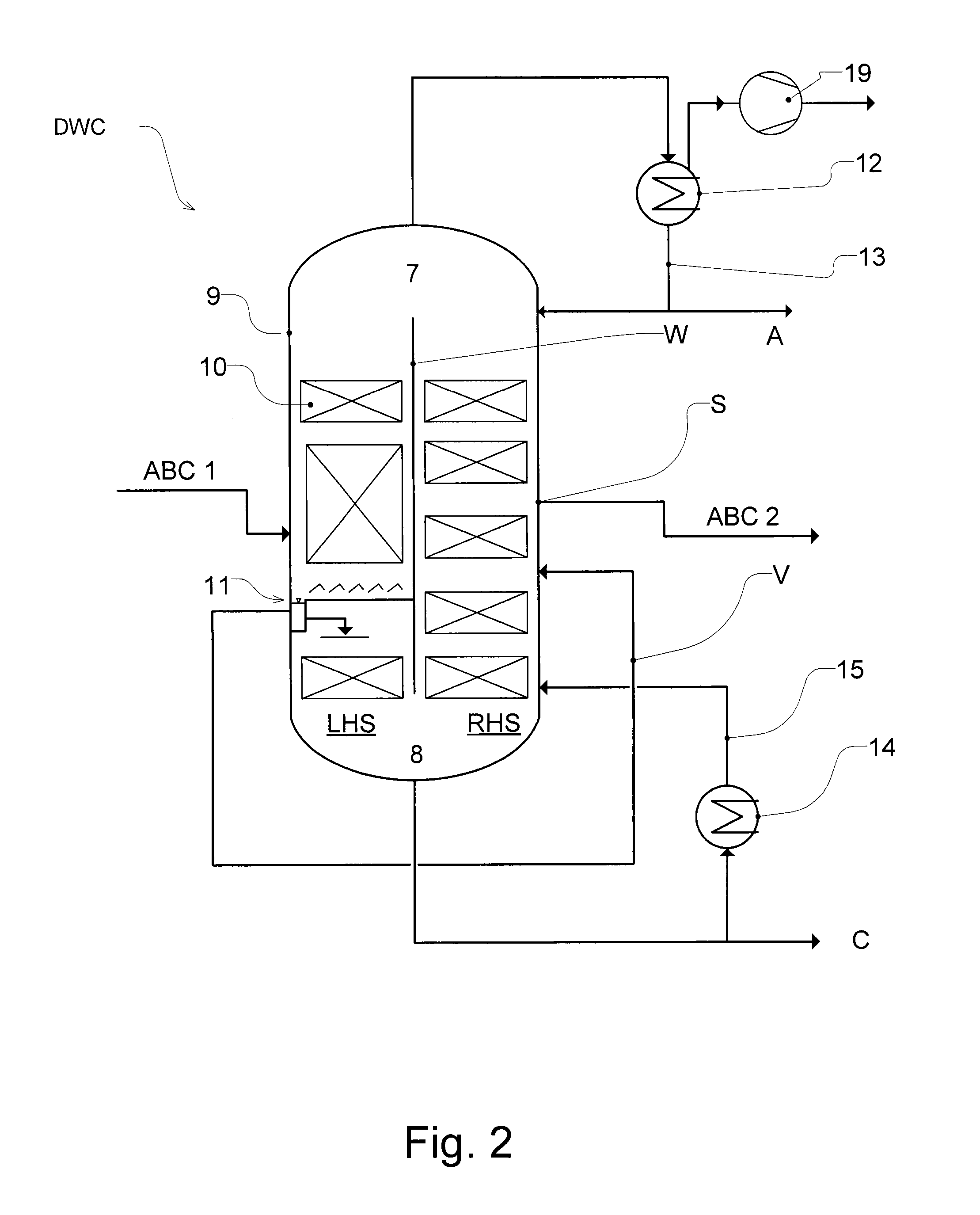
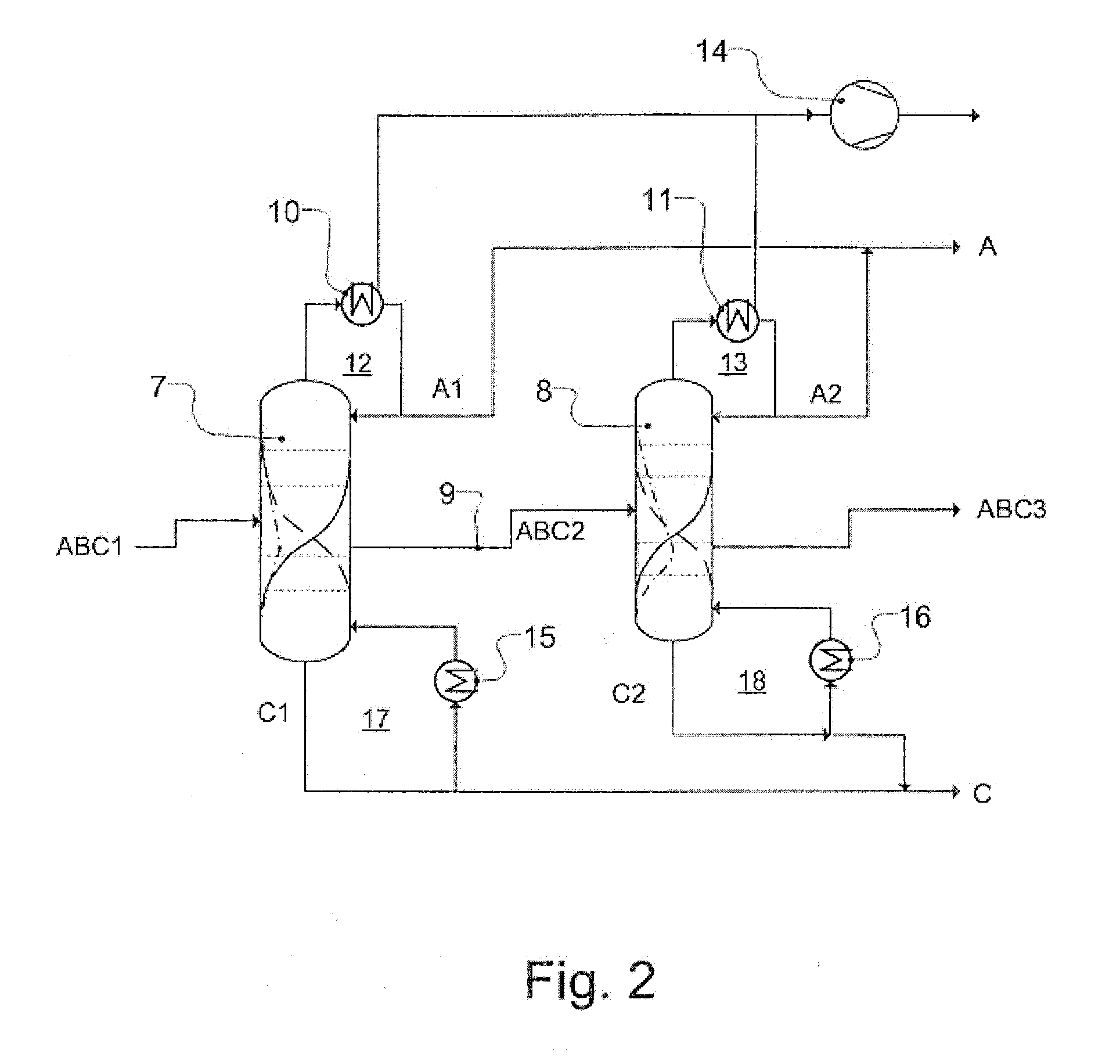
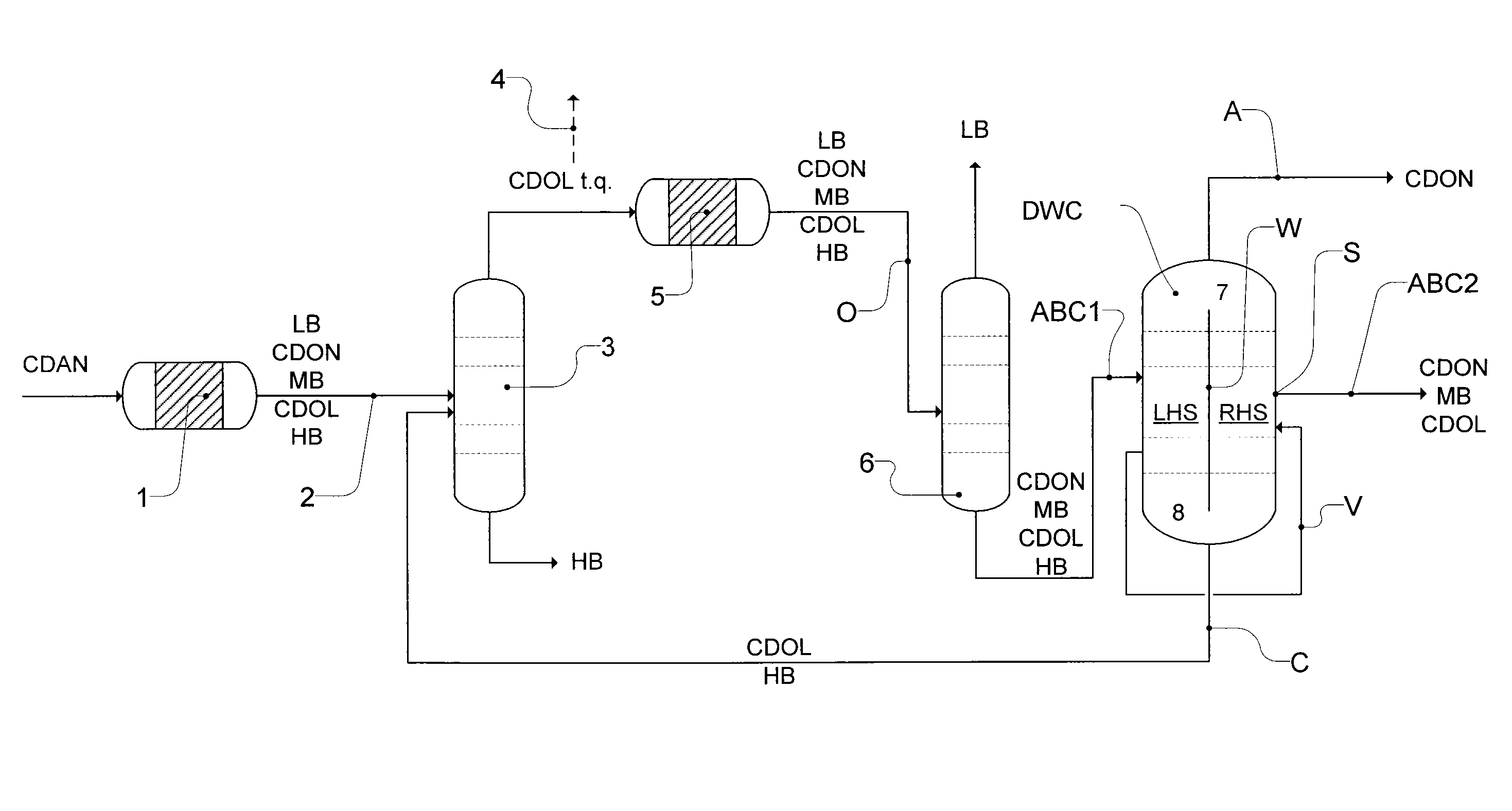
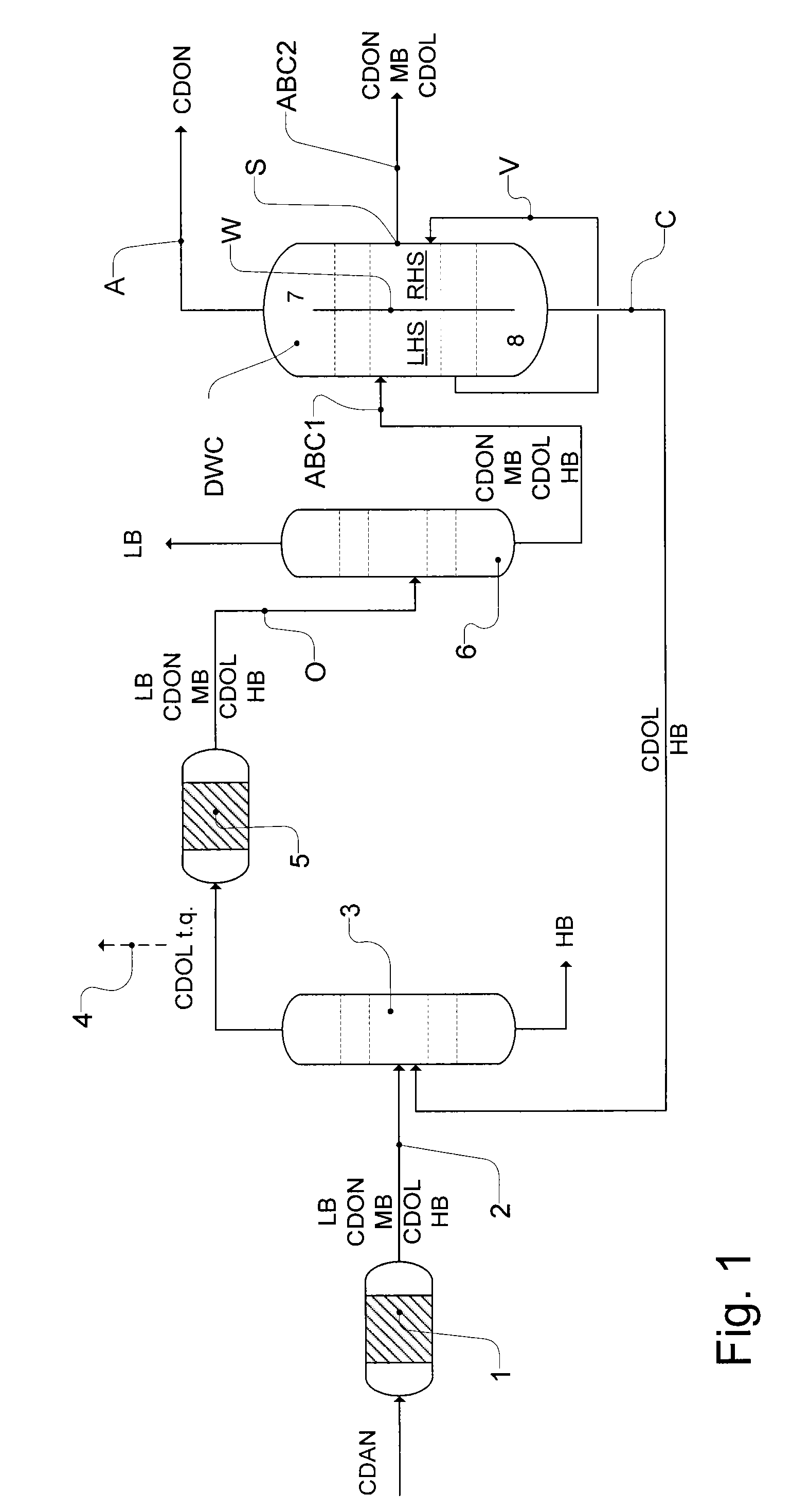
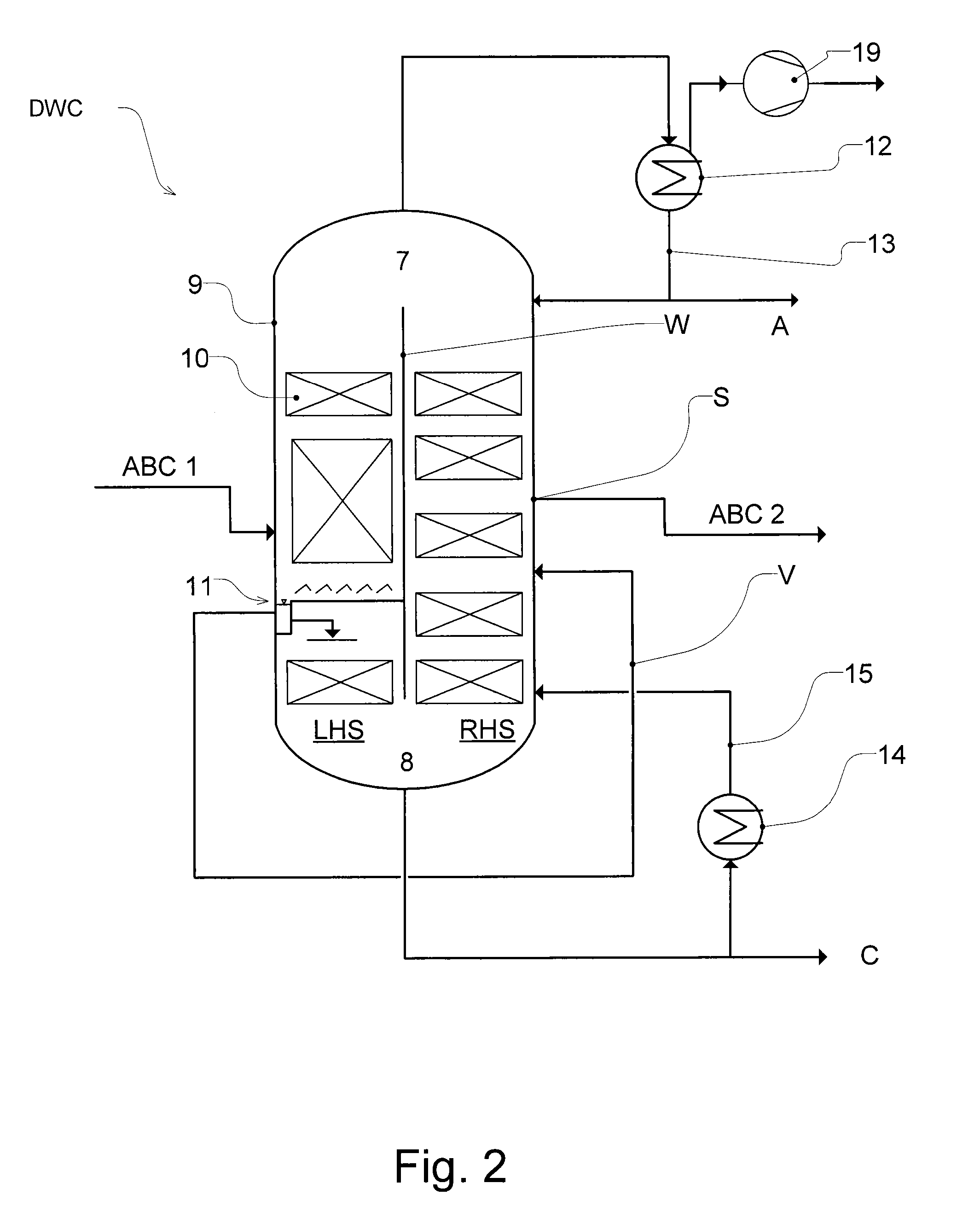
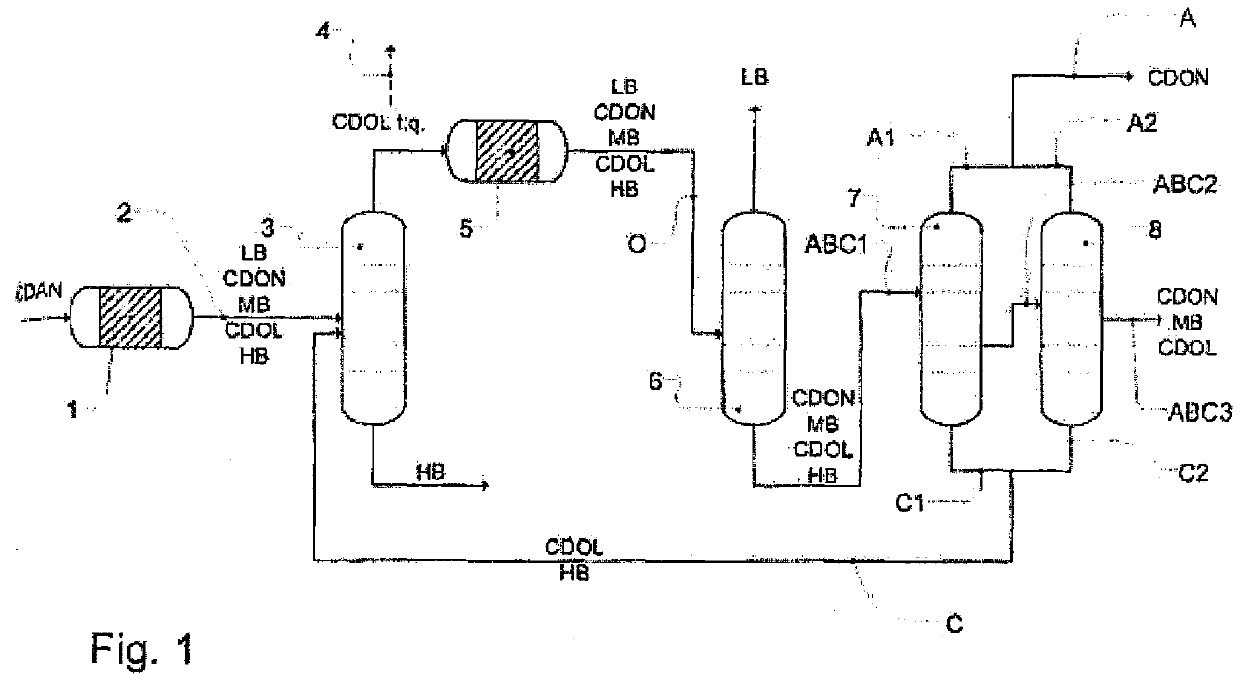
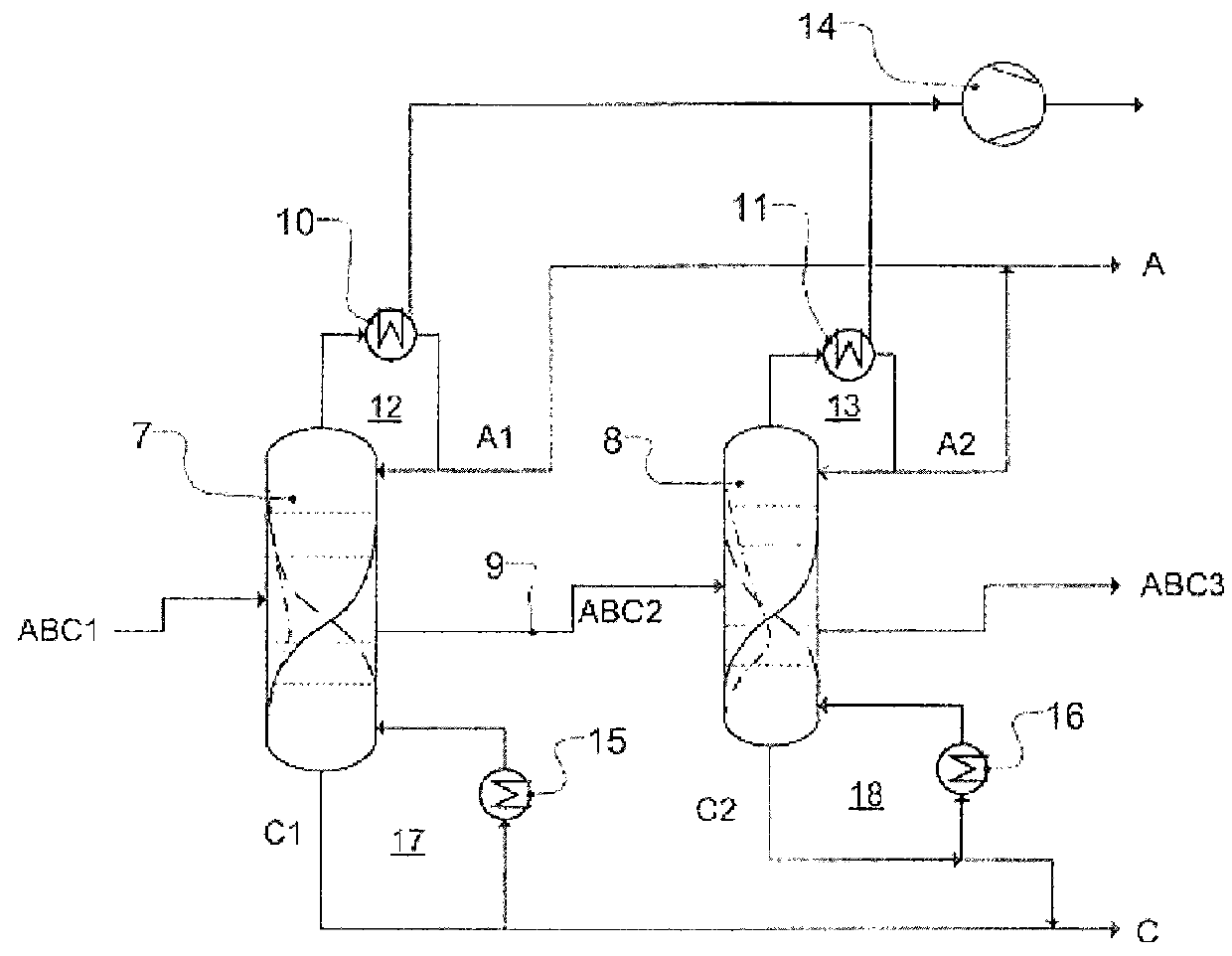
Workup of a cyclododecanone cyclododecanol mixture in a dividing wall column
ActiveUS20140166470A1Preparation by oxidation reactionsLactams preparationCyclododecanoneDehydrogenation
A process for removing a cyclododecanone-rich fraction from a dehydrogenation mixture comprising low boilers, cyclododecanone, medium boilers, cyclododecanol and high boilers is provided. According to the process, the cyclododecanone is separated from the cyclododecanol in a dividing wall column. The apparatus which is the dividing wall column is also provided within this invention.
Owner:EVONIK OPERATIONS GMBH
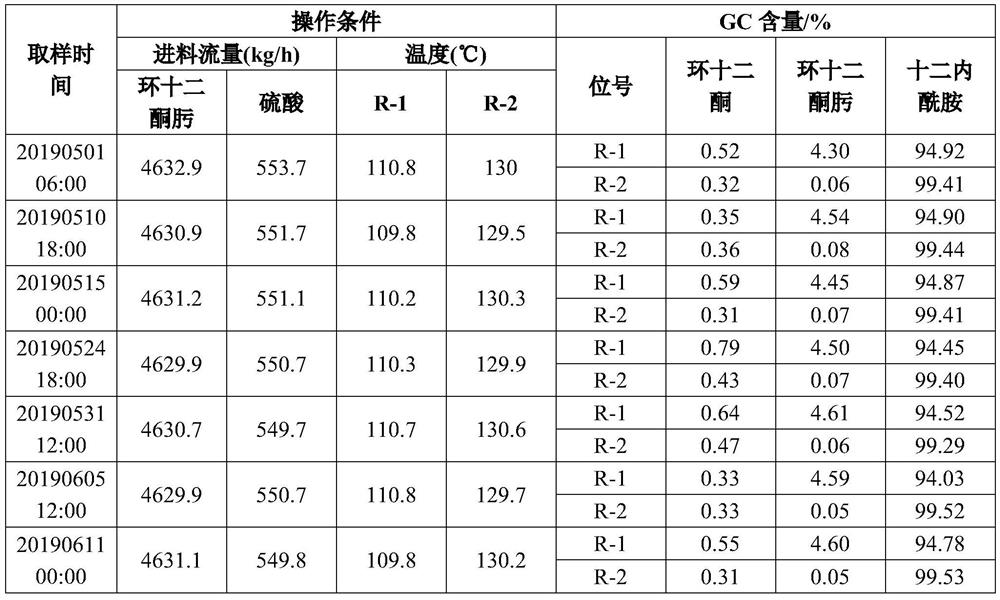
Production method of dodecalactam
ActiveCN109867616AThe oximation reaction is fastEasy to purifyLactams preparationBeckmann rearrangementHydroxylamine
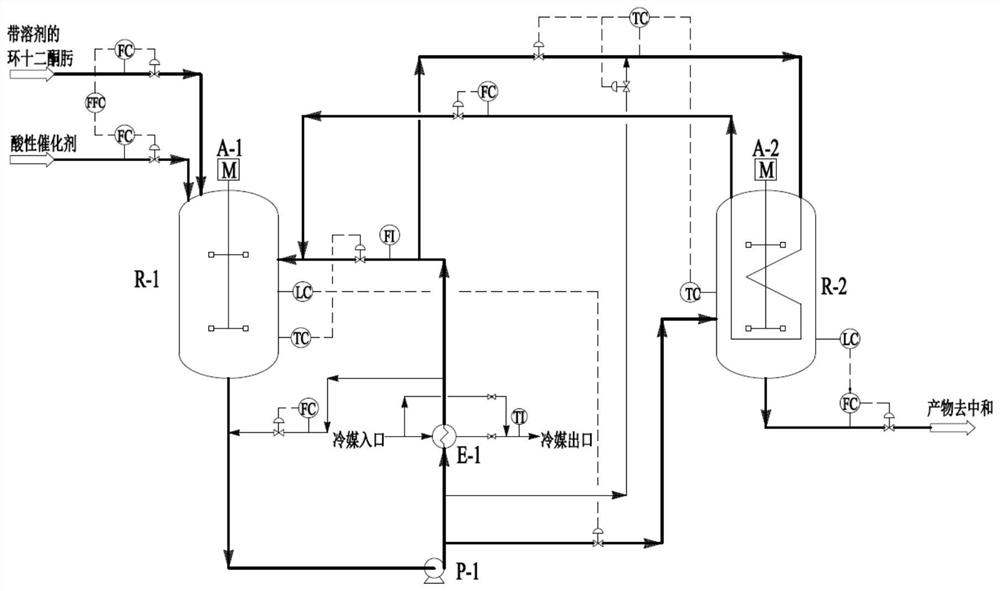
The invention relates to a production method of dodecalactam with high purity. The method comprises the following steps: carrying out a reaction of cyclododecanone and hydroxylamine, and adding a solvent a to assist the liquid separation; and recovering the remaining cyclododecanone from the oil phase obtained from the liquid separation by vacuum rectification, mixing the solution rich in cyclododecanoxime with concentrated sulfuric acid, carrying out a Beckmann rearrangement reaction, neutralizing the mixed solution with ammonia water after the reaction is finished, performing the liquid separation to obtain dodecalactam a solution, adsorbing impurities by alumina and then adding a solvent b, and cooling and crystallizing the solution in the mixed solvents of a and b to obtain the dodecalactam solid product. The dodecalactam product obtained by the method has high purity and light color, and is suitable for producing high-grade resin materials.
Owner:WANHUA CHEM GRP CO LTD
Workup of a cyclododecanone cyclododecanol mixture in a sequence of side draw columns
ActiveUS20140171636A1Preparation by oxidation reactionsLactams preparationCyclododecanoneDehydrogenation
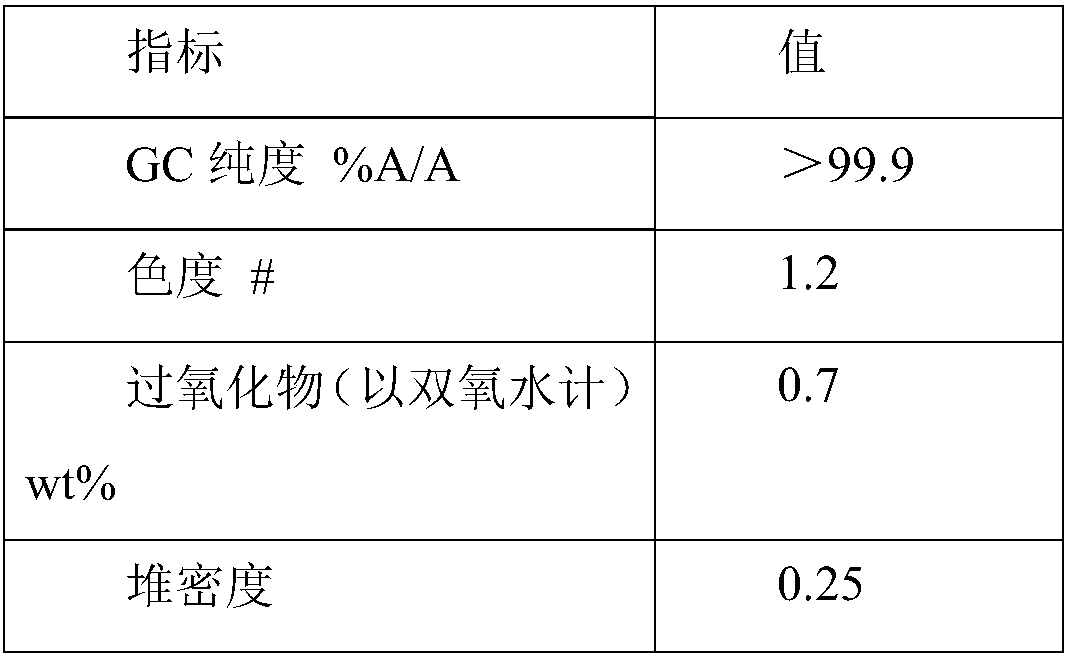
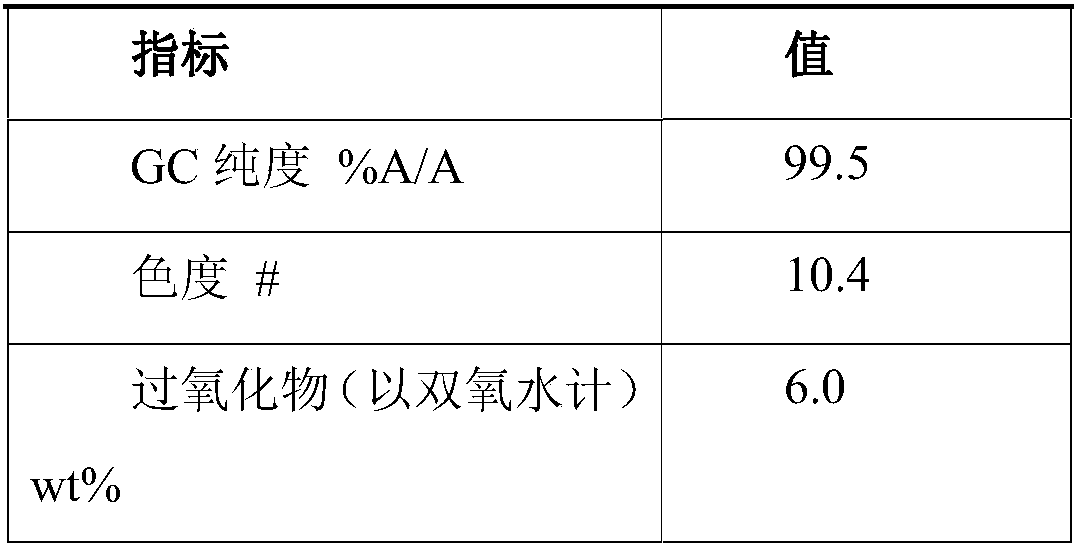
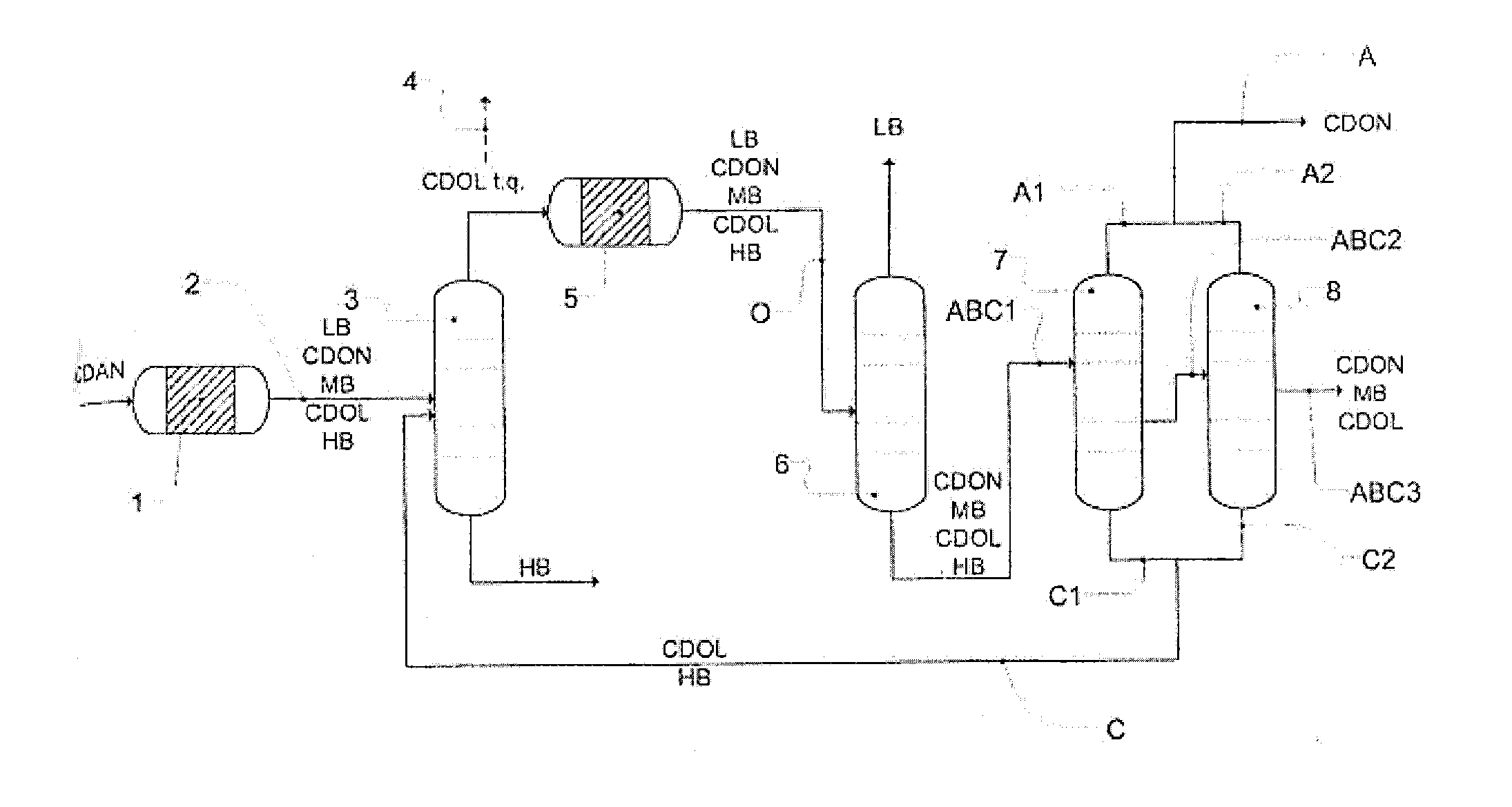
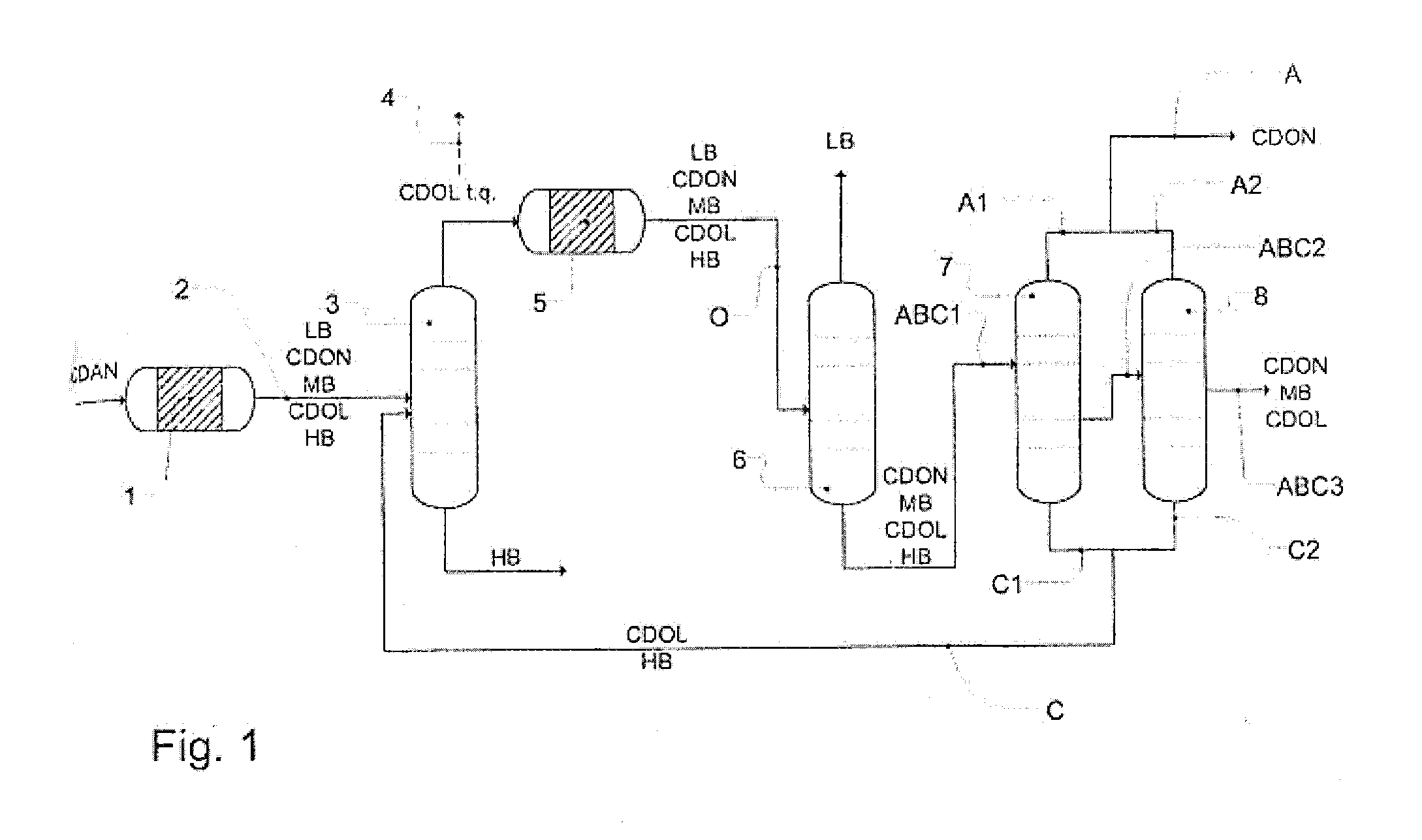
A process for removing a cyclododecanone-rich target fraction (A) from a dehydrogenation mixture (O) comprising low boilers (LB), cyclododecanone (CDON), medium boilers (MB), cyclododecanol (CDOL) and high boilers (HB) is provided. According to the process, substantially pure CDON is obtained via a distillative sequence of two side draw columns connected in series, wherein the sidestream of the primary side draw column is fed into the secondary side draw column. From the top of each of the two side draw columns, a CDON-rich fraction is drawn off, and these are combined to form a target fraction, which is essentially pure CDON.
Owner:EVONIK OPERATIONS GMBH
Process for the preparation of cyclododecanone
InactiveUS20090227815A1Organic compound preparationCarbonyl compound preparation by oxidationCyclododecanoneCyclododecatriene
A process for preparing cyclododecanone by reacting cyclododecene with dinitrogen monoxide, comprising in particular steps (I) and (II):(I) preparing cyclododecene by partially hydrogenating cyclododecatriene;(II) reacting cyclododecene obtained in (I) with dinitrogen monoxide to obtain cyclododecanone.
Owner:BASF AG
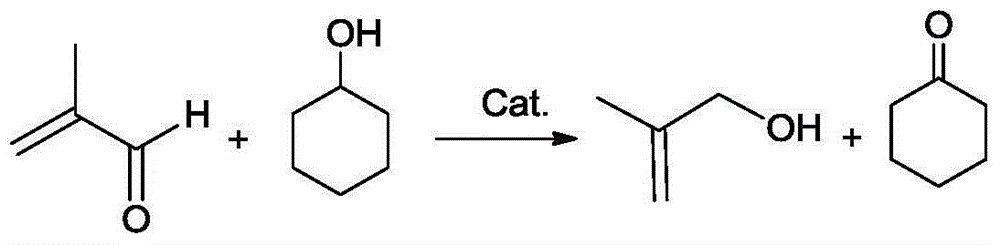
Method for simultaneously preparing methylallyl alcohol and cyclododecanone
ActiveCN106278814ALow costHigh yieldCarbonyl compound preparation by oxidationPreparation by oxygen reductionCyclohexanoneAlcohol
The invention provides a method for simultaneously preparing methylallyl alcohol and cyclododecanone. According to the method, cyclohexanol is adopted as a reducing agent to supply activity H, methylacrolein is reduced in the presence of a catalyst and a polymerization inhibitor to obtain methylallyl alcohol and cyclododecanone products. The process has the characteristics of high atom economy, high product yield, mild reaction condition and no pollution.
Owner:WANHUA CHEM GRP CO LTD
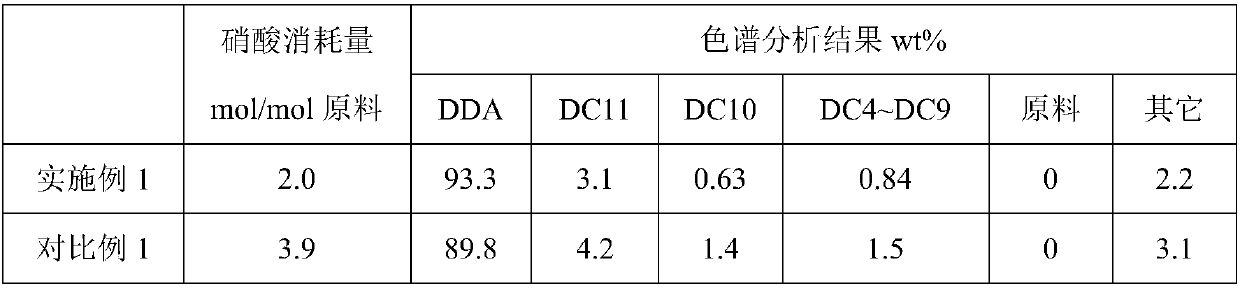
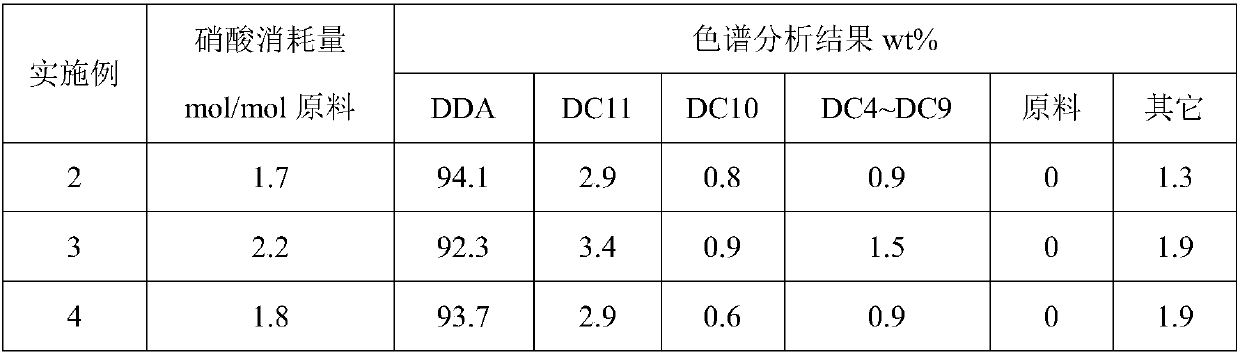
Method for preparation of dodecanedioic acid
ActiveCN108017533AIncrease unit consumptionOrganic compound preparationCarboxylic compound separation/purificationCyclododecanoneDistillation
The invention provides a method for preparation of dodecanedioic acid. According to the method, nitric acid, cyclododecanone and / or cyclododecanol are used as raw materials are production in the form of reaction crystallization in the presence of a catalyst, the concentration of the nitric acid in the reaction process is controlled at 65 to 90wt%, by controlling of the feeding speed and the grain size of the crystallized product, and in-time separation of the dodecanedioic acid solid, the side reaction is reduced, the selectivity of the reaction is increased, and the unit consumption of thenitric acid is reduced. A simple and easy method for recycling of mother liquor acid is provided, and a large-scale nitric acid distillation concentration process can be avoided.
Owner:WANHUA CHEM GRP CO LTD
Workup of a cyclododecanone cyclododecanol mixture in a dividing wall column
ActiveUS9278898B2Preparation by oxidation reactionsLactams preparationCyclododecanoneDehydrogenation
A process for removing a cyclododecanone-rich fraction from a dehydrogenation mixture containing low boilers, cyclododecanone, medium boilers, cyclododecanol and high boilers is provided. According to the process, the cyclododecanone is separated from the cyclododecanol in a dividing wall column. The apparatus which is the dividing wall column is also provided within this invention.
Owner:EVONIK OPERATIONS GMBH
Production method for heat-insulating flame-resistant material
The invention relates to a production method for a heat-insulating flame-resistant material. The production method has the advantages that specific monomers and ratios are adopted, and nano titanium dioxides are coated with polymers by a high-temperature polymerization process, so that agglomeration of nano components in the flame-resistant material is avoided effectively, and dispersity and stability of the nano titanium dioxides in the flame-resistant material are improved; in addition, a synergistic effect is achieved through compounding of modified nano titanium dioxide powder, composite resin, docusate sodium, cyclododecanone, four parts of propylene glycol n-butyl ether and N,N-diethyl aniline, and accordingly, flame resistance and heat insulation of the material are improved greatly; the flame-resistant material acquired according to the specific ratios has various excellent performance such as heat insulation, wear resistance, rigidity, strength and adhesiveness; the production method for the heat-insulating flame-resistant material is simple and easy to operate and the material is easy to produce industrially.
Owner:上海禾舟智能科技有限公司
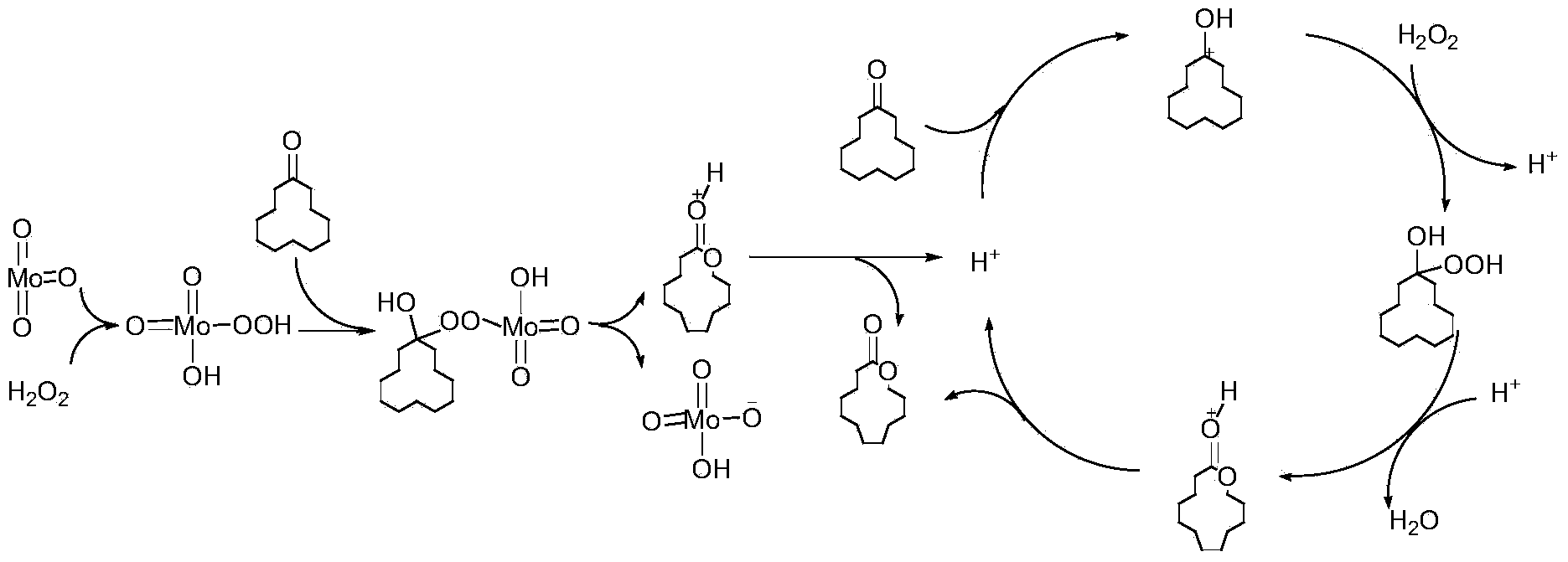
Method for synthesizing cyclododecalactone by catalytic oxidation of cyclododecanone
The invention discloses a method for synthesizing cyclododecalactone by catalytic oxidation of cyclododecanone. Cyclododecalactone is synthesized by using a molybdenum-containing compound as a catalyst, cyclododecanone as a raw material, a hydrogen dioxide solution as an oxidizing agent and acetonitrile as a solvent, wherein the molybdenum-containing compound is any one selected from ammonium molybdate, sodium molybdate, sodium phosphomolybdate and molybdenum oxide. With the molybdenum-containing compound used as the catalyst, there is no waste acid treatment or strong acid corrosion, energy conservation and emission reduction are realized and safety is high; with cyclododecanone used as the raw material, no high-temperature reflux reaction is required, and economic benefit and environmental benefit are raised; and by applying the hydrogen dioxide solution as an oxidizing agent, cleanability and safety of an industrial preparation reactions are raised, and environmental pollution is reduced.
Owner:NANJING UNIV OF SCI & TECH
Process for preparing cyclododecanone
ActiveUS9533933B2Increase productionReduce the ratioPreparation by oxidation reactionsLactams preparationCyclododecanoneEpoxide
Cyclododecanone (CDON) is prepared by epoxidizing cyclododecene (CDEN) to epoxycyclododecane (CDAN epoxide), and rearranging the CDAN epoxide to CDON to obtain a mixture comprising said CDON and CDEN, wherein CDEN is separated from the CDON-containing mixture and sent to the epoxidation to CDAN epoxide in step a.
Owner:EVONIK OPERATIONS GMBH
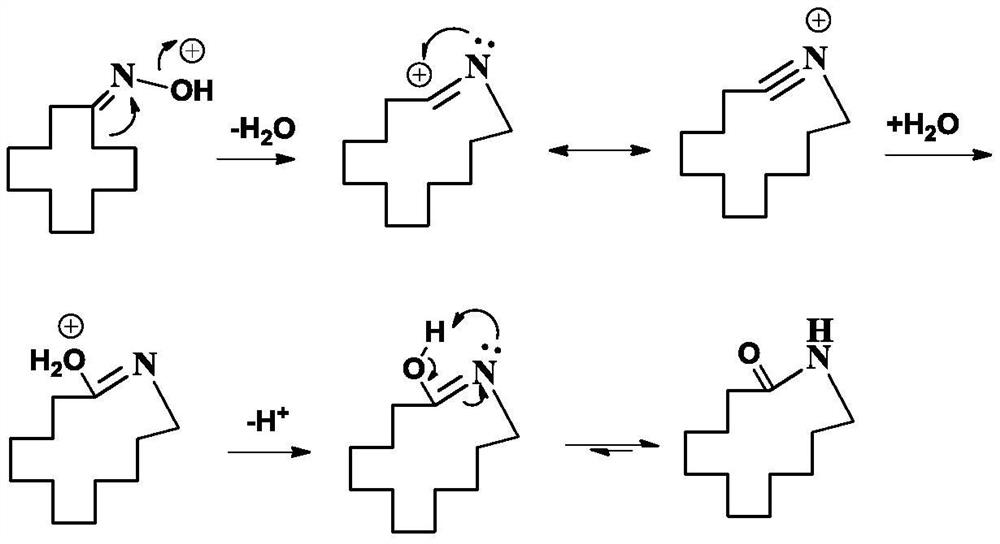
Applications of L-arginine and derivative of L-arginine in preparation of cyclododecanoneoxime, and method used for preparing cyclododecanoneoxime
ActiveCN110498748AReduce interfacial tensionReduce decomposition rateOximes preparationCyclododecanoneDecomposition
The invention discloses applications of L-arginine and a derivative of L-arginine in preparation of cyclododecanoneoxime, and a method used for preparing cyclododecanoneoxime. The derivative of L-arginine comprises one or a plurality of components selected from L-arginine-L-glutamic acid, an L-arginine hydrochloride, and an L-arginine succinate. According to the preparation method, one or more than one components selected from L-arginine and the derivative of L-arginine are taken as auxiliary agents, cyclododecanone is reacted with hydroxylamine sulphate to prepare cyclododecanoneoxime. The auxiliary agents are capable of controlling iron ion content in the reaction system, inhibiting hydroxylamine sulphate decomposition, inhibiting cyclododecylamine generation, and increasing reaction selectivity, and are convenient to separate and recycle.
Owner:WANHUA CHEM GRP CO LTD +1
Process for preparation of cyclododecanone
InactiveUS6861563B2Improve efficiencyIncrease response rateOrganic compound preparationPreparation from heterocyclic compoundsIsomerizationDodecane
In the process for producing a cyclododecanone by isomerizing an epoxycyclododecane-containing starting material in the presence of an isomerization reaction catalyst containing lithium bromide and / or lithium iodide, in order to perform the reaction with high efficiency (high reaction rate) and high selectivity and stably produce a high-purity cyclododecanone in industry while maintaining a high-level reaction rate, the epoxycyclododecane-containing starting material is produced by contacting an epoxycyclododecadiene with hydrogen in the presence of a hydrogen-reduction catalyst and has a content of the hydroxyl group-containing cyclododecane compounds contained in the epoxycyclododecane-containing starting material controlled to 5 mol % or less.
Owner:UBE IND LTD +1
Process for preparing cyclododecanone
ActiveUS9533932B2Reduce the ratioPreparation by oxidation reactionsLactams preparationCyclododecanoneEpoxide
Cyclododecanone (CDON) is prepared by epoxidizing cyclododecene (CDEN) to epoxycyclododecane (CDAN epoxide), and rearranging the CDAN epoxide to CDON to obtain a mixture comprising said CDON and cyclododecane (CDAN), wherein CDAN is separated from the CDON-containing mixture and oxidized to CDON.
Owner:EVONIK OPERATIONS GMBH
Method for the preparation of polymers from monomers comprising laurolactam
Polymers can be prepared from monomers comprising laurolactam, by a process including a. Beckmann rearrangement of cyclododecanone oxime to give laurolactam in the presence of a Beckmann rearrangementcatalyst, b. removal of impurities from the laurolactam to obtain purified laurolactam, and c. polymerization of monomers comprising purified laurolactam. For avoidance of discoloration or yellowingunder ageing conditions, prior to the polymerization, polycyclic substances containing 24 carbon atoms and at least one heteroatom selected from oxygen and nitrogen and having a molar mass between 300and 380 g / mol are limited to 500 ppm, based on laurolactam.
Owner:EVONIK OPERATIONS GMBH
Novel laurolactam preparation method and synthesis apparatus
PendingCN113227046AHigh yieldHigh purityLactams preparationHydrocarbon by hydrogenationLaurolactamBiochemical engineering
The present invention relates to a laurolactam preparation method and a synthesis apparatus, and epoxidation and a rearrangement reaction are carried out in the conversion of cyclododecene into cyclododecanone so that the preparation method can synthesize laurolactam having a higher purity with a higher selectivity and in a higher yield than a conventional preparation method.
Owner:HANWHA SOLUTIONS CORP
Process for the synthesis of lauryllactam (L12) by gas phase catalytic rearrangement of cyclododecanone oxime
InactiveUS20080214836A1Reduce accumulationReduce pressureLactams preparationMolecular sieve catalystsBeckmann rearrangementCyclododecanone
The present invention relates to a process for the preparation of lauryllactam in which a Beckmann rearrangement of cyclododecanone oxime is carried out. Said process is carried out in the gas phase at a temperature of between 180 and 450° C. in the presence of a microporous material having a three-dimensional inorganic main structure composed of tetrahedra connected via a common edge, called zeolite.
Owner:ARKEMA FRANCE SA
Process for producing laurolactam
The present invention relates to a process for producing laurolactam from cyclododecanone oxime by liquid-phase rearrangement reaction using trichlorotriazine as a rearrangement catalyst. The present invention can provide a process which can solve the problem of termination of the reaction at a certain conversion, can prevent an inactive precipitate generated from trichlorotriazine from precipitating in the course of the reaction process, and can remove an inactive precipitate, an active intermediate and a residual catalyst.
Owner:UBE IND LTD
Workup of a cyclododecanone cyclododecanol mixture in a sequence of side draw columns
ActiveUS9382181B2Preparation by oxidation reactionsLactams preparationCyclododecanoneDehydrogenation
A process for removing a cyclododecanone-rich target fraction (A) from a dehydrogenation mixture (O) comprising low boilers (LB), cyclododecanone (CDON), medium boilers (MB), cyclododecanol (CDOL) and high boilers (HB) is provided. According to the process, substantially pure CDON is obtained via a distillative sequence of two side draw columns connected in series, wherein the sidestream of the primary side draw column is fed into the secondary side draw column. From the top of each of the two side draw columns, a CDON-rich fraction is drawn off, and these are combined to form a target fraction, which is essentially pure CDON.
Owner:EVONIK OPERATIONS GMBH
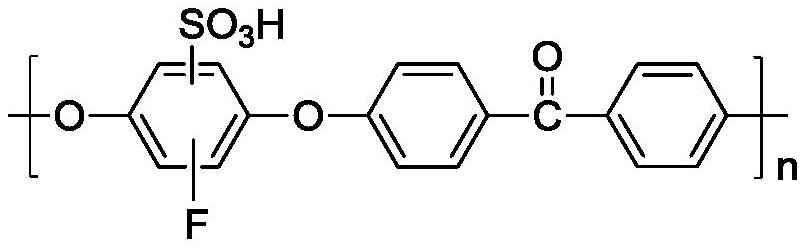
Catalyst and preparation method thereof, and method for preparing laurolactam through gas phase rearrangement reaction
ActiveCN112619673AHigh mechanical strengthImprove bindingLactams preparationPhysical/chemical process catalystsLaurolactamPtru catalyst
The invention discloses a catalyst and a preparation method thereof, and a method for preparing laurolactam through a gas phase rearrangement reaction. The catalyst comprises fluoride modified rare earth doped Al2O3-SiO2-ZrO2 and fluorine-containing sulfonated polyetheretherketone, and the loading capacity of the fluorine-containing sulfonated polyetheretherketone in the catalyst is 20-50wt%, preferably 30-45wt%. When the catalyst is used for catalyzing gas-phase rearrangement of cyclododecanone oxime, the conversion rate of cyclododecanone oxime reaches 99% or above, the selectivity of laurolactam reaches 99.5% or above, the service life of the catalyst reaches 3000h or above, no ammonium sulfate byproduct is produced in the reaction, the mechanical strength of the catalyst is high, and the problems of equipment corrosion and environmental pollution of a traditional liquid-phase rearrangement process are solved.
Owner:WANHUA CHEM GRP CO LTD
Synthesis method of macro cyclic ketone intermediate 14-methyl bicycle [10, 3, 0] pentadecyl-1-alkene
InactiveCN101624326AHigh reaction yieldEasy to operateHydrocarbon by hydrogenationSynthesis methodsSide chain
The invention relates to a synthesis method of macro cyclic ketone intermediate 14-methyl bicycle [10, 3, 0] pentadecyl-1-alkene. The method includes that cyclododecanone by product in petroleum industry is taken as raw material, methacrylic side chain is introduced to alpha site of carbonyl thereof, and dehydration cyclization by alumina and hydrogenation are carried out. The method of the invention is low in raw material cost, steady in reaction and safe and reliable and is applicable to industrialized production.
Owner:上海力智生化科技有限公司
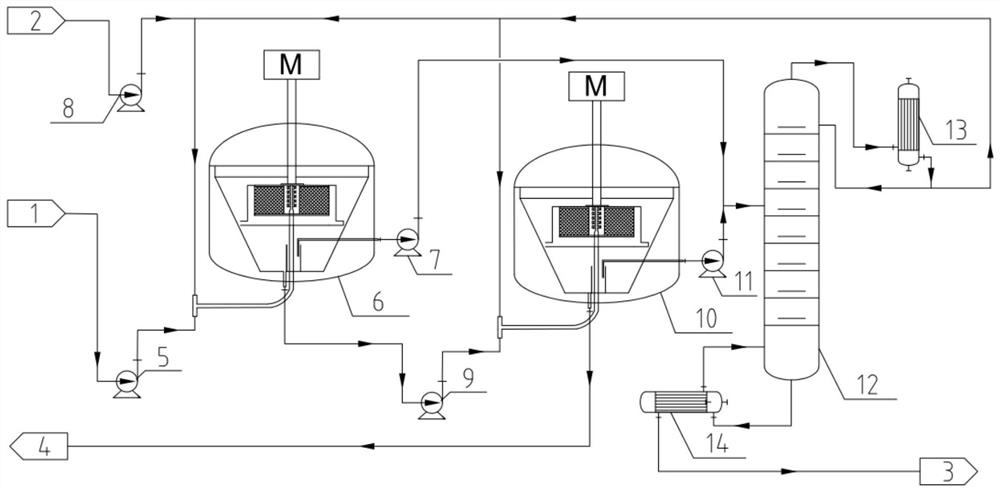
Continuous preparation system and method of cyclododecanone solution oxime
ActiveCN113877493AIncrease profitAvoid generatingProcess control/regulationChemical industryHydroxylamineCyclododecanone
Owner:浙江中巨海锐科技有限公司 +1
Method for preparing laurolactam through extraction rearrangement reaction of cyclododecanone oxime
ActiveCN112479964AGuaranteed conversion rateEliminate carbonizationLactams preparationLactams separation/purificationLaurolactamPtru catalyst
The invention discloses a method for preparing laurolactam through extraction rearrangement reaction of cyclododecanone oxime, which comprises the steps of (1) adding a cyclododecanone oxime solutionand an acidic catalyst into an extraction kettle in proportion, strongly stirring and mixing in the kettle to extract cyclododecanone oxime from a solvent phase to a catalyst phase, wherein the solution is liquid-liquid biphase in the reaction kettle, and the reaction is carried out in a catalyst phase; (2) removing extraction reaction heat and part of rearrangement reaction heat from the materialin the extraction kettle through an outer circulating pump and an outer circulating cooler, wherein one strand of material is led out from an outlet of the outer circulating pump to serve as feed ofa rearrangement kettle, and the other strand of material is led out from an outlet of the outer circulating cooler to serve as an inlet medium of a heat transfer inner coil of the rearrangement kettle; and (3) mixing the outlet material of the coil pipe in the rearrangement kettle with the outlet material of the outer circulating cooler of the extraction kettle, feeding the mixture into the extraction kettle, and feeding the rearrangement kettle material into a downstream neutralization process. The heat transfer mode is small in temperature difference in the rearrangement kettle, materials can be effectively prevented from being supercooled, and the conversion rate of reaction materials is guaranteed.
Owner:WANHUA CHEM GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




















![Synthesis method of macro cyclic ketone intermediate 14-methyl bicycle [10, 3, 0] pentadecyl-1-alkene Synthesis method of macro cyclic ketone intermediate 14-methyl bicycle [10, 3, 0] pentadecyl-1-alkene](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a623a486-693d-4d6d-a9f4-4531e637a165/A20091005670300051.PNG)