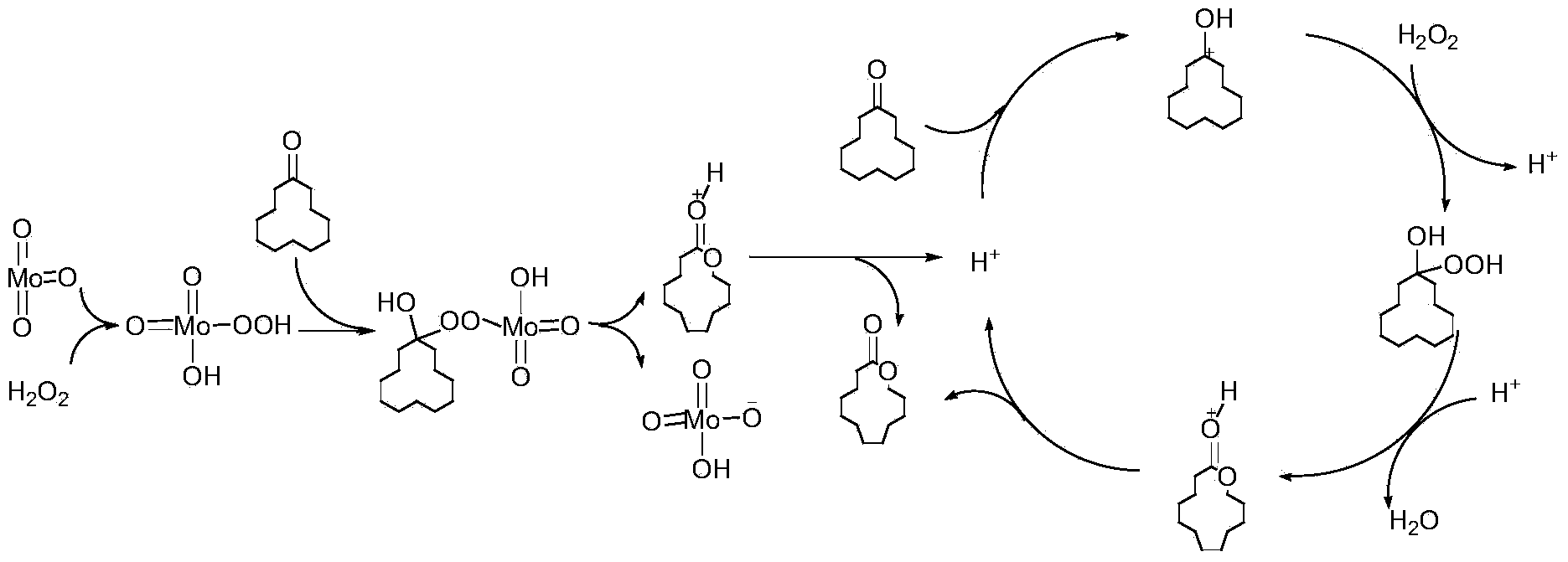
Method for synthesizing cyclododecalactone by catalytic oxidation of cyclododecanone
A technology of cyclododecone and cyclododecanone, which is applied in the field of organic chemical synthesis, can solve the problems of separation and unfriendly environment, difficult handling of corrosion equipment, and low selectivity, so as to improve economic and environmental benefits, improve Cleanliness and safety, the effect of reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 25mL three-necked flask, successively add cyclododecanone (0.45g), acetonitrile (1mL), ammonium molybdate (0.05g), dropwise add a 50% hydrogen peroxide solution, hydrogen peroxide and 2-heptyl The molar ratio of cyclopentanone was 2.5 / 1, stirred by magnetic force, and reacted at 55° C. for 24 hours. After the reaction, the reaction product was extracted with ethyl acetate. Take 1mL of 5% sodium bicarbonate aqueous solution to wash the organic phase three times, then wash with distilled water until the organic phase is neutral, adjust the aqueous phase to weak alkalinity with 5% sodium bicarbonate aqueous solution and extract with ethyl acetate. The ethyl ester extract was combined with the organic phase, the solvent was evaporated in vacuo, weighed and analyzed by gas chromatography. The conversion of cyclododecanone was 9%, and the yield of cyclododecanone was 6%.
Embodiment 2
[0021] In a 25mL three-necked flask, successively add cyclododecanone (0.45g), acetonitrile (1mL), sodium molybdate (0.05g), dropwise add a 50% hydrogen peroxide solution, hydrogen peroxide and 2-heptyl The molar ratio of cyclopentanone was 2.5 / 1, stirred by magnetic force, and reacted at 55° C. for 24 hours. After the reaction, the reaction product was extracted with ethyl acetate. Take 1mL of 5% sodium bicarbonate aqueous solution to wash the organic phase three times, then wash with distilled water until the organic phase is neutral, adjust the aqueous phase to weak alkalinity with 5% sodium bicarbonate aqueous solution and extract with ethyl acetate. The ethyl ester extract was combined with the organic phase, the solvent was evaporated in vacuo, weighed and analyzed by gas chromatography. The conversion of cyclododecanone was 15%, and the yield of cyclododecanone was 10%.
Embodiment 3
[0023] In a 25mL three-necked flask, successively add cyclododecanone (0.45g), acetonitrile (1mL), sodium phosphomolybdate (0.10g), and dropwise add a mass fraction of 50% hydrogen peroxide solution, hydrogen peroxide and 2- The molar ratio of heptylcyclopentanone is 2.5 / 1, and the reaction is carried out at 55° C. for 24 hours with magnetic stirring. After the reaction, the reaction product was extracted with ethyl acetate. Take 1mL of 5% sodium bicarbonate aqueous solution to wash the organic phase three times, then wash with distilled water until the organic phase is neutral, adjust the aqueous phase to weak alkalinity with 5% sodium bicarbonate aqueous solution and extract with ethyl acetate. The ethyl ester extract was combined with the organic phase, the solvent was evaporated in vacuo, weighed and analyzed by gas chromatography. The conversion of cyclododecanone was 18%, and the yield of cyclododecanone was 13%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com