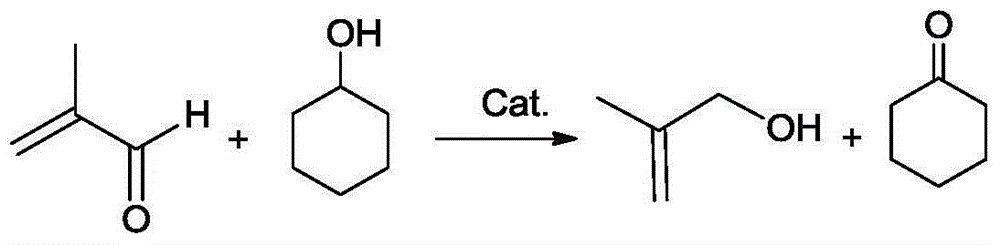
Method for simultaneously preparing methylallyl alcohol and cyclododecanone
A technology of methallyl alcohol and methacrolein, which is applied in the fields of oxidative preparation of carbonyl compounds, reduction preparation of oxygen-containing functional groups, organic chemistry, etc., to achieve the effects of high atom economy, high catalytic activity, and equipment cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of heteroatom-containing Y-type zeolite molecular sieve catalyst:
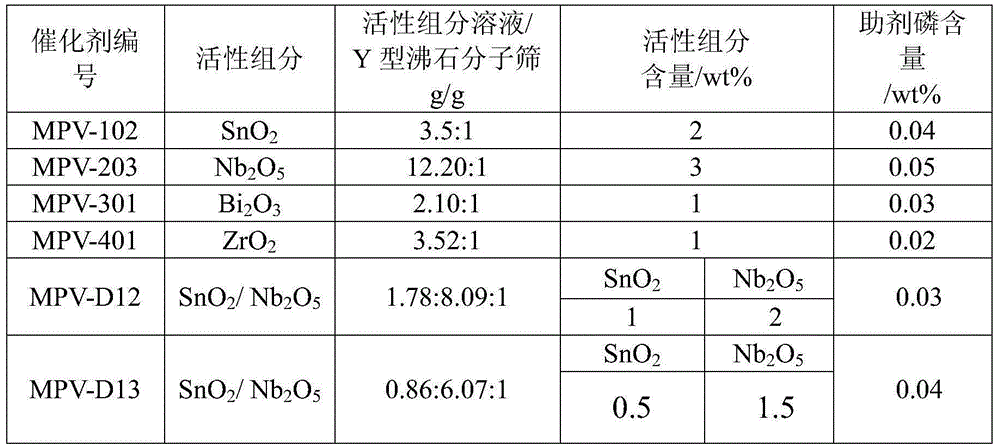
[0039] Add 3000g nitric acid solution (10wt%) and 600g Y-type zeolite molecular sieve into a 10L glass reactor, reflux at 90°C for 8h, filter after reflux, wash with deionized water until neutral, and dry at 120°C for 8h to obtain a Number of Y-type zeolite molecular sieves.
[0040] Dissolve tin tetrachloride and / or niobium oxalate, bismuth nitrate, and zirconium nitrate in 2% dilute nitric acid solution to obtain an active component solution, the mass concentration of the above solution is 1%, and one or more of the above solutions are used to prepare The obtained Y-type zeolite molecular sieve with a certain number of vacancies was impregnated, and then added a certain amount of auxiliary phosphoric acid, impregnated for 5 hours at 25°C, dried at 120°C for 8 hours after impregnation, and roasted at 550°C for 6 hours to obtain a finished catalyst. The specific composition is shown in Tabl...
Embodiment 2
[0044] Preparation of methallyl alcohol and cyclohexanone:
[0045] Using a 3L stainless steel reactor to synthesize methallyl alcohol and cyclohexanone, first add 1500g methacrolein, 1.5g inhibitor ZJ-701 (p-hydroxytetramethylpiperidine nitroxide free radical) and 45g catalyst, N 2 Replace the air in the system, and ensure a certain pressure, heat, stir, increase the temperature to 90 ° C, add N 2 When the pressure reached 3 bar, 214.3 g of cyclohexanol was gradually added using a parallel flow pump at a rate of 2 g / min. After the cyclohexanol was added, the reaction was continued for a total of 9 hours. After the reaction, use N 2 The materials in the system are pressed out, and the reaction kettle has its own filter head to filter to obtain a mixed solution containing cyclohexanone, methallyl alcohol and methacrolein.
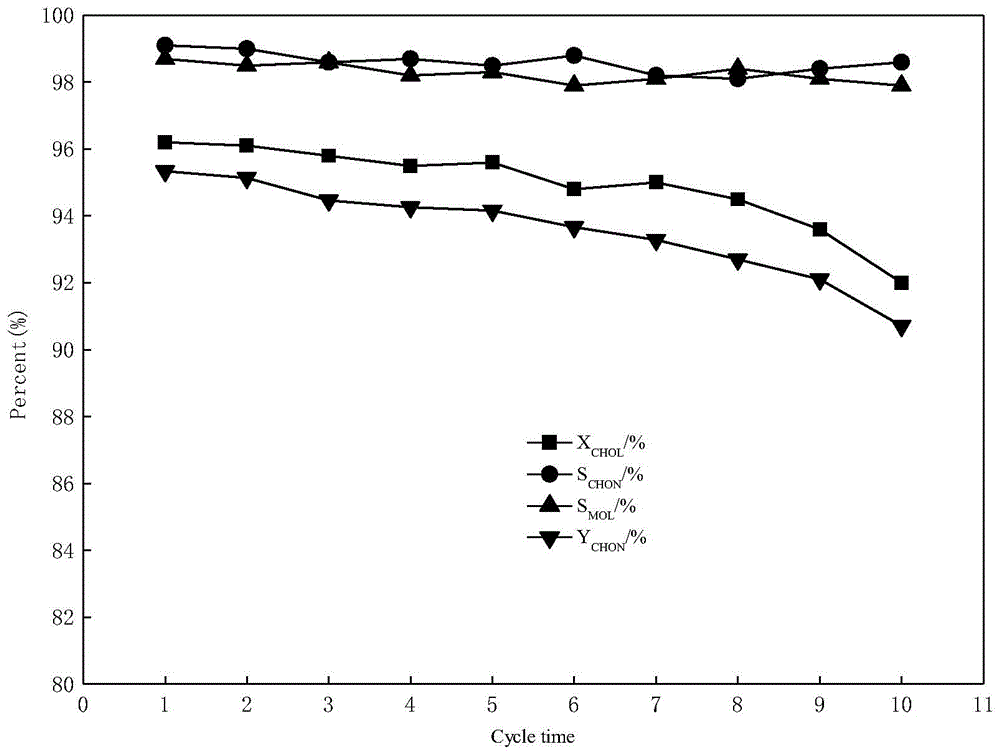
[0046] The product composition was analyzed by gas chromatography. The analysis results are shown in Table 2.
[0047] Table 2 Catalytic performance of...
Embodiment 3
[0054] Different from Example 2, the reaction performance of MPV-D12 catalyst for synthesizing methallyl alcohol and cyclohexanone under different process conditions was investigated. (catalyst consumption is based on the weight of methacrolein)
[0055] Table 3 Catalyst Reaction Performance under Different Process Conditions
[0056]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com