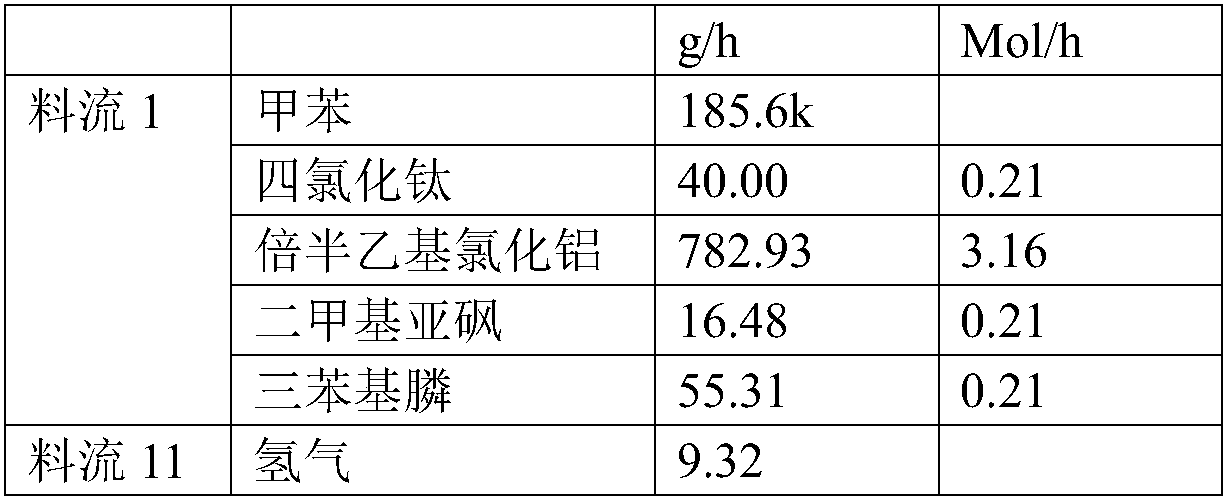
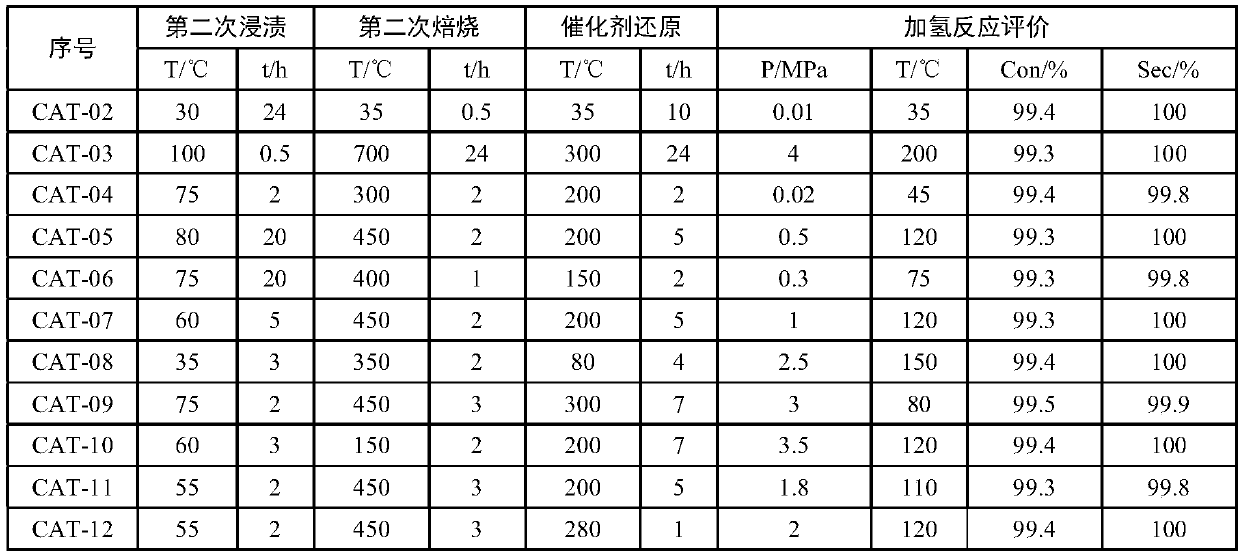
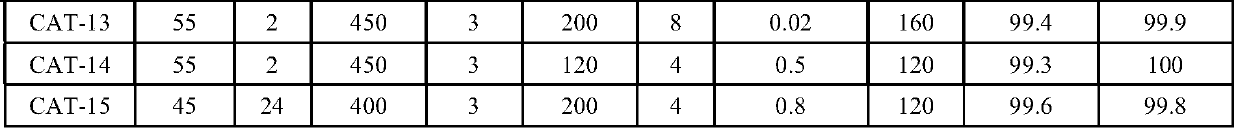
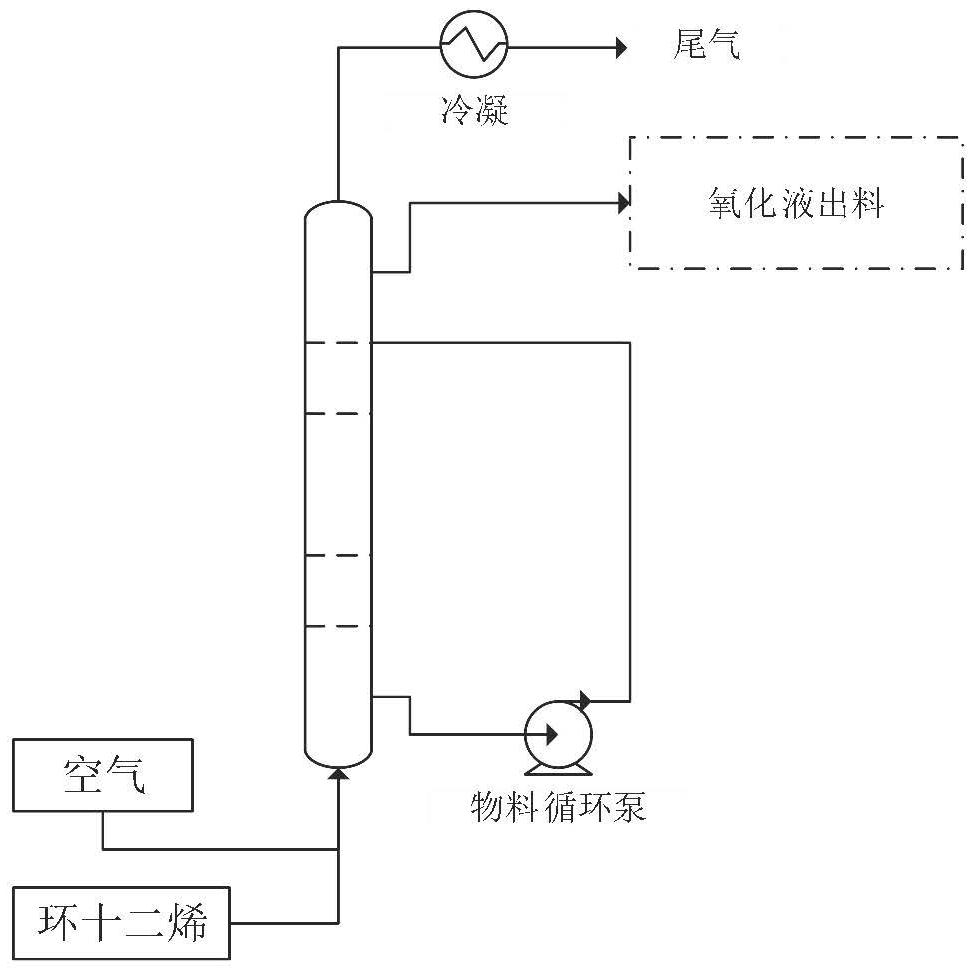
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
68 results about "Cyclododecatriene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyclododecatrienes are cyclic trienes with the formula C₁₂H₁₈. Four isomers are known for 1,5,9-cyclododecatriene. The trans,trans,cis-isomer is a precursor in the production of nylon-12.
Process for preparing cyclododecatriene
ActiveUS8168841B2High selectivityHigh yieldCosmetic preparationsHydrocarbon by isomerisationCyclododecatrieneDistillation
Preparation of cyclododecatriene in a continuous or discontinuous process by trimerization of butadiene in the presence of a catalyst system and a solvent, the crude cyclododecatriene obtained being able to be isolated by means of distillation. The cyclooctadiene formed as by-product can likewise be isolated from the crude product.
Owner:EVONIK OPERATIONS GMBH
Method for the production of alpha-alane
A method of forming α-alane. The method includes reacting aluminum trichloride and an alkali metal hydride to form an alane-ether complex solution. An aqueous diethyl ether solution is optionally added to the alane-ether complex solution to form a partially hydrolyzed ether / alane-ether complex solution. A solution of a first crystallization additive is added to the alane-ether complex solution or to the aqueous ether / alane-ether complex solution to form a crystallization solution. The first crystallization additive is selected from the group consisting of polystyrene, polybutadiene, polystyrene-co-polybutadiene, polyisoprene, poly-alpha-methylstyrene, polystyrene-co-polyindene, poly-alpha-pinene, and mixtures thereof. Optionally, a second crystallization additive is added to the crystallization solution. The second crystallization additive is selected from the group consisting of squalene, cyclododecatriene, norbornylene, norbornadiene, a phenyl terminated polybutadiene, 2,4-dimethyl anisole, 3,5-dimethyl anisole, 2,6-dimethyl anisole, polydimethyl siloxane, and mixtures thereof. Solvents are removed from the crystallization solution to crystallize the α-alane.
Owner:NORTHROP GRUMMAN SYST CORP
Process for preparing cyclododecatriene
ActiveUS20070265184A1High selectivityHigh yieldCosmetic preparationsHydrocarbon by isomerisationDistillationCyclododecatriene
Preparation of cyclododecatriene in a continuous or discontinuous process by trimerization of butadiene in the presence of a catalyst system and a solvent, the crude cyclododecatriene obtained being able to be isolated by means of distillation. The cyclooctadiene formed as by-product can likewise be isolated from the crude product.
Owner:EVONIK OPERATIONS GMBH
Method for the production of alpha-alane
ActiveUS20050222445A1Reduce impurityHydrogenThiosulfates/dithionites/polythionitesCyclododecatrienePolydimethyl siloxane
A method of forming alpha-alane. The method includes reacting aluminum trichloride and an alkali metal hydride to form an alane-ether complex solution. An aqueous ether solution is optionally added to the alane-ether complex solution to form a partially hydrolyzed ether / alane-ether complex solution. A solution of a crystallization additive is added to the alane-ether complex solution or to the aqueous ether / alane-ether complex solution to form a crystallization solution. The crystallization additive is selected from the group consisting of squalene, cyclododecatriene, norbomylene, norbomadiene, a phenyl terminated polybutadiene, 2,4-dimethyl anisole, 3,5-dimethyl anisole, 2,6-dimethyl anisole, polydimethyl siloxane, and mixtures thereof. Ether is removed from the crystallization solution to crystallize the alpha-alane.
Owner:NORTHROP GRUMMAN SYST CORP
Method for the production of alpha-alane
ActiveUS20070066839A1Lower the volumeMultiple metal hydridesGroup 3/13 element organic compoundsAlkaneCyclododecatriene
A method of forming α-alane. The method includes reacting aluminum trichloride and an alkali metal hydride to form an alane-ether complex solution. An aqueous diethyl ether solution is optionally added to the alane-ether complex solution to form a partially hydrolyzed ether / alane-ether complex solution. A solution of a first crystallization additive is added to the alane-ether complex solution or to the aqueous ether / alane-ether complex solution to form a crystallization solution. The first crystallization additive is selected from the group consisting of polystyrene, polybutadiene, polystyrene-co-polybutadiene, polyisoprene, poly-alpha-methylstyrene, polystyrene-co-polyindene, poly-alpha-pinene, and mixtures thereof. Optionally, a second crystallization additive is added to the crystallization solution. The second crystallization additive is selected from the group consisting of squalene, cyclododecatriene, norbornylene, norbornadiene, a phenyl terminated polybutadiene, 2,4-dimethyl anisole, 3,5-dimethyl anisole, 2,6-dimethyl anisole, polydimethyl siloxane, and mixtures thereof Solvents are removed from the crystallization solution to crystallize the α-alane.
Owner:NORTHROP GRUMMAN SYST CORP
Method for continuously producing cyclododecatriene
ActiveCN109867578AActive influenceEffective quenchingDistillation purification/separationHydrocarbonsCyclododecatrienePre treatment
The invention relates to a method for continuously producing cyclododecatriene. The method comprises the following steps: (1) mixing butadiene with an organic metal compound to obtain a material flowcontaining butadiene, carrying out a butadiene cyclization tripolymerization reaction on the material flow containing butadiene in a polymerization reactor under the action of a catalyst solution to obtain a reaction liquid containing cyclododecatriene; (2) feeding the reaction liquid containing cyclododecatriene and the other part of the catalyst solution into an aging reactor for carrying out areaction; and (3) feeding the reaction liquid obtained by the aging reactor into a quenching reactor, and quenching the reaction by adopting a polyamine substance as a quenching agent to obtain a quenched reaction liquid containing cyclododecatriene. According to the method disclosed by the invention, the butadiene is pretreated first, so that the polymerization reaction process is not influencedby impurities which are carried in by raw materials, and efficiency of the catalyst is increased; and meanwhile, the reaction is quenched by adopting a catalyst quenching agent, so that a post-treatment process is simplified, and production efficiency is improved.
Owner:WANHUA CHEM GRP CO LTD
Method for continuously synthesizing 1, 5, 9-cyclododecatriene
InactiveCN103420777AHigh yieldHigh purityOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbonsCyclododecatrieneProcess efficiency
The invention provides a method for continuously synthesizing 1, 5, 9-cyclododecatriene. The method is completed through the processes of preparation of a homogeneous phase complex catalyst system, cascade reaction, catalyst deactivation processing, refining processing of a reaction product and follow-up processing of the reaction product. According to the method, 1, 3-butadiene can continuously react in a cascade reaction system and can be smoothly and efficiently converted into the 1, 5, 9-cyclododecatriene in an energy-saving manner, especially an effect of converting the 1, 3-butadiene into trans, trans, cis-1, 5, 9-cyclododecatriene isomers in a high-selectivity manner is relatively obvious. According to the method, the reaction time is shortened, the reaction cost is reduced, and the process efficiency is improved.
Owner:李翔
Method for the production of alpha-alane
A method of forming α-alane. The method includes reacting aluminum trichloride and an alkali metal hydride to form an alane-ether complex solution. An aqueous ether solution is optionally added to the alane-ether complex solution to form a partially hydrolyzed ether / alane-ether complex solution. A solution of a crystallization additive is added to the alane-ether complex solution or to the aqueous ether / alane-ether complex solution to form a crystallization solution. The crystallization additive is selected from the group consisting of squalene, cyclododecatriene, norbornylene, norbornadiene, a phenyl terminated polybutadiene, 2,4-dimethyl anisole, 3,5-dimethyl anisole, 2,6-dimethyl anisole, polydimethyl siloxane, and mixtures thereof. Ether is removed from the crystallization solution to crystallize the α-alane.
Owner:NORTHROP GRUMMAN SYST CORP
Process for the preparation of cyclododecanone
InactiveUS7838705B2Organic compound preparationCarbonyl compound preparation by oxidationCyclododecanoneCyclododecatriene
A process for preparing cyclododecanone by reacting cyclododecene with dinitrogen monoxide, comprising in particular steps (I) and (II):(I) preparing cyclododecene by partially hydrogenating cyclododecatriene;(II) reacting cyclododecene obtained in (I) with dinitrogen monoxide to obtain cyclododecanone.
Owner:BASF SE
Antrodia camphorata cyclonene compound for restraining growth of bone cancer cells
The invention relates to a novel application of a compound. In the invention, 4-hydroxy-2,3-dimethoxy-6-methyl-5(3,7,11-trimethyl-2,6,10- cyclododecatriene)-2- cyclonene(4-hydroxy-2,3-dimethoxy-6-mehty-5(3,7,11-trimethyl-dodeca-2,6,10-trienyl)-cyclohex-2-enone) is separated and purified from an antrodia camphorate extract. The cyclonene compound can be applied to restricting the growth of a bone cancer cell and the medical composition for restricting the growth of bone cancer cells.
Owner:GOLDEN BIOTECH
Novel process for producing hexabromocyclododecane
InactiveCN101139245AGuaranteed low unit consumptionImprove solubilityPreparation by halogen additionOrganic solventBromine
The present invention provides a new method to produce hexabromocyclododecane, which belongs to the synthesizing and manufacturing technology field for halogenating alkyl and hydrocarbon. The new manufacturing process for the hexabromocyclododecane is that: the hexabromocyclododecane solution material is prepared by the synthesizing reaction between 1,5,9-cyclododecatriene and bromide under the catalyzing function of bromination ammonium ammonium salt in an organic solution. The hexabromocyclododecane solution material after being washed, decolored and debromined is to be made with a separating process or an emulsification process in the mixing solution between surface active agent and the water. The separated solution or the emulsification solution for being prepared is to be heated to get rid of the organic solution. So the powder-shaped or the grain-shaped hexabromocyclododecane will be directly obtained.
Owner:HUAIHAI INST OF TECH +1
Process for the preparation of cyclododecanone
InactiveUS20090227815A1Organic compound preparationCarbonyl compound preparation by oxidationCyclododecanoneCyclododecatriene
A process for preparing cyclododecanone by reacting cyclododecene with dinitrogen monoxide, comprising in particular steps (I) and (II):(I) preparing cyclododecene by partially hydrogenating cyclododecatriene;(II) reacting cyclododecene obtained in (I) with dinitrogen monoxide to obtain cyclododecanone.
Owner:BASF AG
Process for producing hexabromocyclododecane
InactiveUS6284935B1Improve overall utilizationHigh yieldPreparation by halogen additionWater basedCyclododecatriene
This invention relates to the production of an hexabromocyclododecane product, which process comprises brominating cyclododecatriene in the presence of a 1,4-dioxane and water based solvent and from about 0.5 to about 30 wt % bromide ion in the liquid phase of the reaction mass. Optional post-reaction heat treatment in a finishing step increases process yields if needed. The hexabromocyclododecane product is unrecrystallized and contains no more than about 1.5 wt % tetrabromocyclododecene impurities.
Owner:ALBEMARLE CORP
Catalyst for preparing cyclododecene through selective hydrogenation, preparation method and applications thereof
PendingCN110743540AHigh selectivityImprove conversion rateMolecular sieve catalystsHydrocarbon by hydrogenationRhodium MetallicumIridium
The invention provides a catalyst for preparing cyclododecene through selective hydrogenation, a preparation method and applications thereof, wherein the catalyst comprises a carrier, a main metal rhodium attached to the carrier and an auxiliary metal attached to the carrier, a weight ratio of the main metal rhodium to the carrier is 0.03-5.0%, a weight ratio of the main metal to the auxiliary metal is (0.001-1):1, preferably (0.05-0.1):1, the carrier is one selected from a gamma-aluminum oxide, active carbon, silicon dioxide, calcium carbonate, an all-silicon molecular sieve, a titanium-silicon molecular sieve TS-1, a ZSM-5 molecular sieve, a beta-molecular sieve, hydrotalcite and kaolin, and the auxiliary metal is one or more than two selected from ruthenium, gold, silver, platinum, iridium, iron, bismuth, lead, tin, cerium, nickel, copper, zinc and cadmium. According to the present invention, with the catalyst, the cyclododecene preparation process route is simple, and can be completed through the one-step selective hydrogenation reaction by using 1,5,9-cyclododecyltriene as the raw material, wherein the selectivity can achieve 99.3-99.6%, and the conversion rate can achieve 99.8-100%.
Owner:CHINA TIANCHEN ENG
Preparation method of organic fluorine hydrophobic chain extender containing ionic liquid
A preparation method of an organic fluorine hydrophobic chain extender containing ionic liquid is disclosed, wherein styrene, a 1-vinyl-3-ethylimidazole hexafluorophosphate solution, a 1-vinyl-3-butylimidazole tetrafluoroborate solution, 6,6,7,7,8,8,8-heptafluoro-5,5-bis(trifluoromethyl)-1,3-octadiene, and 1,5,9-cyclododecatriene are participate in polymerization, thus preparing the organic fluorine hydrophobic chain extender which can improve mechanical performance and hydrophobic capability of a polyurethane product.
Owner:上海鲁聚聚合物技术有限公司
Prepn process of hexabromo-cyclododecane
The present invention belongs to the field of chemical synthesis of cyclo alkane halide. The preparation of hexabromo-cyclododecane includes the following steps: reaction of 1,5,9-cyclododecatriene and liquid bromine in mixed solvent; separation of the reacted mixture to obtain coarse hexabromo-cyclododecane product; washing with hot water; and vacuum drying to obtain hexabromo-cyclododecane product. The present invention has the features of use of mixed solvent containing C2-C4 saturated alcohol, use of no catalyst, no neutralizing step of coarse product, vacuum drying, high product yield and high product smelting point.
Owner:邵林高
Catalyst for catalyzing butadiene cyclization trimerization and application method thereof
InactiveCN102688775AEasily hydrolyzedAvoid hydrolysisOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbonsPolymer sciencePtru catalyst
The invention relates to a catalyst for catalyzing butadiene cyclization trimerization and an application method thereof. The components of the catalyst include titanium naphthenate and AlRyZ3-y, wherein Z is a halogeno group, R is an alkyl group, and Y is more than 0 and less than or equal to 3; and the catalyst also includes a small amount of zirconium naphthenate. When the catalyst is used for catalyzing the butadiene cyclization trimerization, methylbenzene, the zirconium naphthenate, the AlRyZ3-y, the butadiene and the titanium naphthenate are added into a reaction kettle in sequence and then mixed; the mixture is reacted for 20-40 hours at the temperature of 30-90 DEG C to obtain a reaction product; and sedimentation, distillation and purification treatments are carried out on the reaction product to obtain a butadiene trimer. Through measuring, the conversion of the butadiene is 90-95%, and the content of trans, trans, cis-1,5,9-cyclododecatriene in the trimer is 99.99-100%.
Owner:WUDI SINORANCE CHEM
Antrodia camphorata cyclonene compound for restraining growth of bone cancer cells
The invention relates to a novel application of a compound. In the invention, 4-hydroxy-2,3-dimethoxy-6-methyl-5(3,7,11-trimethyl-2,6,10- cyclododecatriene)-2- cyclonene(4-hydroxy-2,3-dimethoxy-6-mehty-5(3,7,11-trimethyl-dodeca-2,6,10-trienyl)-cyclohex-2-enone) is separated and purified from an antrodia camphorate extract. The cyclonene compound can be applied to restricting the growth of a bone cancer cell and the medical composition for restricting the growth of bone cancer cells.
Owner:GOLDEN BIOTECH
Method for preparing 1,5,9-cyclododecatriene
InactiveCN103232316AHigh yieldHigh purityOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbonsOrganic solventButadiene Dioxide
The invention provides a method for preparing 1,5,9-cyclododecatriene. The method comprises the steps that: in an organic solvent, a catalytic system composed of sesqui-alkyl aluminum chloride, TiX4 and at least one compound with a structure represented below is adopted, such that 1,3-butadiene is polymerized into 1,5,9-cyclododecatriene under catalysis. The X in the TiX4 is independently F, Cl, Br or I; R and R' are independently C1-C5 straight-chain alkyl or alkyl with free branched-chain. With the method provided by the invention, 1,3-butadiene is highly selectively converted into 1,5,9-cyclododecatriene, and is especially highly selectively converted into trans,trans,cis-1,5,9-cyclododecatriene. Through refining, fine 1,5,9-cyclododecatriene with purity higher than 99% can be obtained. Therefore, the method has important industrial application prospect.
Owner:李翔 +1
Preparation method of hexabromocyclododecane
ActiveCN104892349AHalogenated hydrocarbon separation/purificationPreparation by halogen additionIsobutanolCyclododecatriene
The invention discloses a preparation method of hexabromocyclododecane. The preparation method comprises three steps of bromination, washing separation and drying, wherein the bromination step comprises the steps of respectively dropwise adding raw materials including cyclododecatriene and bromine into a mixed solvent of isobutanol and dichloroethane, and heating to 50+ / -2 DEG C in three stages of the dropwise adding process, wherein the molar ratio of bromine to cyclododecatriene is (3.05-3.08) to 1, and the total reaction time is 3-4 hours; the washing separation step comprises the steps of adding a solid phase separated from a finished solution of the bromination reaction into the prepared mixed solvent of dichloroethane and isobutanol, after uniformly stirring and mixing, separating the solid phase out, adding ethanol, and washing twice, so as to obtain the filtered solid phase; the drying step comprises the step of drying the solid phase obtained in the washing separation step at 80+ / -2 DEG C, so as to obtain a white solid powder product. According to the preparation method of hexabromocyclododecane, completely-bromized impurities which are entrained by the product and can influence the melting point are removed by virtue of a solvent washing method, so that the product impurity is improved, and the melting point of products is increased.
Owner:TIANJIN SEA WATER DESALINATION & COMPLEX UTILIZATION INST STATE OCEANOGRAPHI
Process for cyclic dodecatriene selecting hydrogenated cyclic dode cene
A process for preparing cyclododecene by selective gas-phase hydrogenation of at least one starting material selected from cyclododecatriene, cyclododecadiene and mixtures thereof, wherein the starting material present in the gas phase is hydrogenated in the presence of a catalyst in a fixed-bed reactor and the Bodenstein number for the process is greater than 100.
Owner:EVONIK OPERATIONS GMBH
Preparation method for co-production of cyclododecene ether and cyclododecanol
PendingCN114105911AReduce processingEmission reductionOrganic compound preparationPreparation by hydrogenationPtru catalystCyclododecatriene
The invention provides a preparation method for co-producing cyclododecene ether and cyclododecanol, which comprises three continuous processes of oxidation, epoxidation and hydrogenation which are connected in series: in the oxidation process, any one or a mixture of cyclododecene, cyclododecadiene and cyclododecatriene is taken as a raw material, an initiator is added, and mixed gas of nitrogen and oxidizing gas is introduced; an epoxidation process: directly adding an epoxy catalyst into a product obtained by the oxidation process to prepare an epoxy structure product; a hydrogenation process: rectifying and separating out unreacted raw materials and an epoxy catalyst in the epoxidation process, and adding a hydrogenation catalyst into the residual solution to prepare cyclododecene ether and cyclododecanol; the preparation method disclosed by the invention is low in cost and green and environment-friendly, byproducts in the whole reaction process are few, and the product yield is increased.
Owner:CHINA TIANCHEN ENG +1
Preparation method of carbon tetrachloride purification adsorbent
InactiveCN106040206ALarge specific surface areaDense pore size distributionOther chemical processesHalogenated hydrocarbon preparationPentamethylcyclopentadieneSorbent
The invention provides a preparation method of carbon tetrachloride purification adsorbent. The preparation method comprises the steps of adding water and polyvinyl alcohol into a reaction kettle and evenly mixing to form a water phase; adding styrene, 1,5,9-cyclododecatriene, 3-allyloxy-2-hydroxy propane sulfonic acid, methacrylamide and pentamethyl cyclopentadienyl isopropyl titanate into a reaction kettle and evenly mixing to form an oil phase; adding an oil-phase solution into the reaction kettle where the water phase is prepared; reacting in an appropriate temperature; acetone extracting reaction mixture after reaction is finished; drying in a vacuum mode to obtain the carbon tetrachloride purification adsorbent. The carbon tetrachloride purification adsorbent is large in specific surface area, stable in property and high in product purity after adsorption.
Owner:王金明
Production of hexabromocyclododecane of enhanced gamma isomer content
Owner:ALBEMARLE CORP
Catalyst and application of catalyst in catalyzing butadiene cyclization trimerization
InactiveCN102688781ABreak interactionHigh selectivityOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbonsDistillationCyclododecatriene
The invention relates to a catalyst and application of the catalyst in catalyzing butadiene cyclization trimerization. The components of the catalyst include TiX4 and AlRyZ3-y, wherein X and Z are halogeno groups; R is alkyl; Y is more than 0 and less than or equal to 3; the catalyst is characterized by also includin CP2ZrC12. When the catalyst is used for catalyzing the butadiene cyclization trimerization, toluene, CP2ZrC12, AlRyZ3-y, butadiene and TiX4 are added to a reaction kettle in sequence and then mixed; the mixture reacts for 20-40 hours at 30-90 DEG C to obtain a reaction product; and sedimentatin, distillation and purification treatments are carried out on the reaction product to obtain a butadiene trimer. Through measuring, the conversion rate of the butadiene is 90-95%; and the content of trans, trans, cis-1, 5, 9-cyclododecatriene in the trimer is 99.99-100%.
Owner:WUDI SINORANCE CHEM
Separation method for oligomerization products of 1,3-butadiene ring
InactiveCN103265393AAvoid pollutionSave resourcesDistillation purification/separationButadiene DioxideCyclododecatriene
The invention brings forward a separation method for oligomerization products of 1,3-butadiene ring. The method comprises steps of adding an extractant into residues, distilling, fractionating distillate, cooling and settling residual oily substances, and filtering and separating crystal substances, so that an oil liquid of C16-C32 isomer substances, petroleum ether and a 2-butanone mixed solvent extractant are separated from residue distillation ingredients generated in a synthesis of cyclododecatriene, thereby obtaining 1,3-butadiene, cyclododecatriene, cyclooctadiene and vinyl cyclohexene substances. According to the invention, the method can separate the oligomerization products of the 1,3-butadiene ring, and obtain useful substances used for reusing or receiving valuable compounds, thereby reducing discharge, eliminating pollution, protecting the environment, and conserving resources, and being suitable as a method for processing the oligomerization products of the 1,3-butadiene ring.
Owner:李明泉
Method for preparing hexabromocyclododecane
The present invention discloses a preparation method of hexabromocyclododecane. In the method, bromine and cyclododecatriene are simultaneously dropped in the mixed solvent of substances, which are used for providing halogen ions, and protic solvent; the molar ratio of the bromine and the cyclododecatriene is equal to 3 to from 3.5 to 1; thus the hexabromocyclododecane can be prepared through the reaction. Compared with the hexabromocyclododecane prepared in the prior art, the hexabromocyclododecane prepared in the method has an increased melting point, which reaches above 186 DEG C. The preparation method has the advantages of low cost, simple operation, safety, environmental protection, and suitability for the industrial application.
Owner:ZHENJIANG SANWA FLAME RETARDANT ENG TECH CO LTD
Process for producing hexabromocyclododecane
InactiveUS6420617B1Improve overall utilizationImprove solubilityPreparation by halogen additionFluid phaseCyclododecatriene
This invention relates to the production of an hexabromocyclododecane product, which process comprises brominating cyclododecatriene in the presence of a 1,4-dioxane and water based solvent and from about 0.5 to about 30 wt % bromide ion in the liquid phase of the reaction mass. Optional post-reaction heat treatment in a finishing step increases process yields if needed. The hexabromocyclododecane product is unrecrystallized and contains no more than about 1.5 wt % tetrabromocyclododecene impurities.
Owner:ALBEMARLE CORP
Method for preparing adsorbent for diethyl sebacate purification
InactiveCN106111092AHigh purityImprove adsorption capacityOther chemical processesOrganic compound preparationSorbentCyclododecatriene
A preparation method of an adsorbent for purification of diethyl sebacate: add water and polyvinyl alcohol in a reactor, and stir to obtain an aqueous phase; in the reactor, methyl methacrylate, bicyclo[2.2. 1] Hepta-2,5-diene, monomer containing rare earth ruthenium, 1,5,9-cyclododecatriene, vinyl laurate, stir evenly to obtain an oil phase; add the oil phase solution to the Put it in a reaction kettle with prepared water phase, and then react at a suitable temperature. After the reaction, the reaction mixture is extracted with acetone and dried in vacuum to obtain an adsorbent for purifying diethyl sebacate.
Owner:王金明
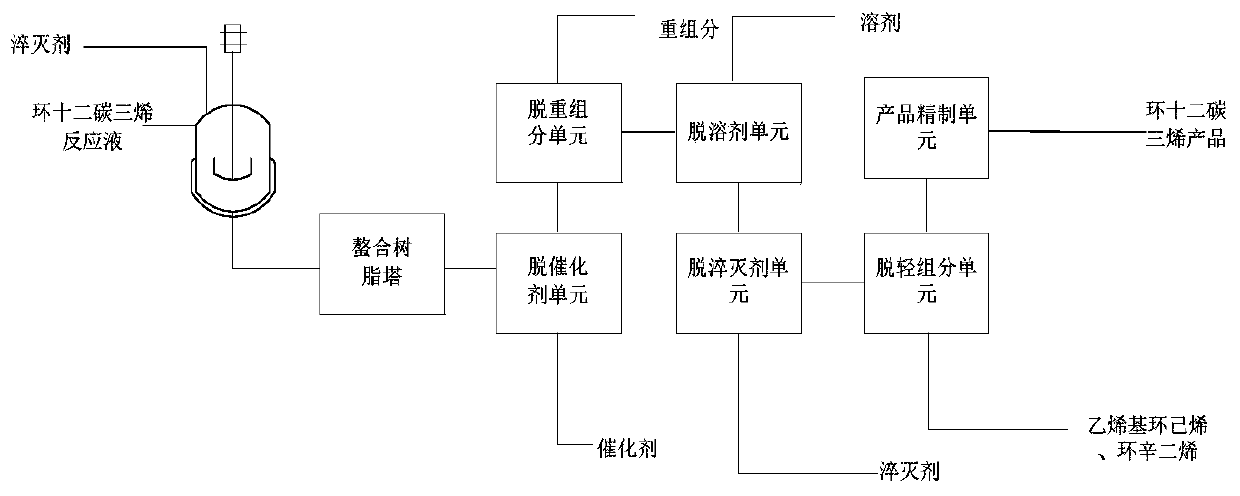
Method for separating and purifying cyclododecatriene
ActiveCN111153761AReduce corrosionReduce the risk factorDistillation purification/separationHydrocarbonsPtru catalystCyclododecatriene
The invention provides a method for separating and purifying cyclododecatriene. The method comprises the following steps: dissolving a quenching agent in a solvent to prepare a quenching agent solution; mixing the quenching agent solution with a cyclododecatriene reaction solution, quenching in a quenching reactor to obtain a cyclododecatriene crude product; introducing the cyclododecatriene crudeproduct into a chelating resin tower at first, and then making the crude product sequentially pass through a catalyst removal unit, a heavy component removal unit, a solvent removal unit, a quenchingagent removal unit, a light component removal unit and a product refining unit in a distillation tower to obtain a cyclododecatriene product. According to the method, corrosion to equipment is reduced, the danger coefficient is low; the side reaction is reduced, the yield of cyclododecatriene is increased; the stability of CDT and other heat-sensitive substances in the separation process is improved, the purity of cyclododecatriene is improved; insoluble substances in a crude product can be avoided, so that the separation process is continuous, and industrial amplification is easy to realize.
Owner:WANHUA CHEM GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com