Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
99 results about "ARGININE HYDROCHLORIDE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
L-arginine hydrochloride (HCL) is a formulation for the amino acid L-arginine that is sometimes used in dietary supplement. L-arginine HCL is used for exercise performance, erectile dysfunction, immune system performance and cardiovascular conditions, such as hypertension (high blood pressure).
Compound amino acid injecta, and preparation method and detection method thereof
ActiveCN102440989AAccurate measurementExact reproductionOrganic active ingredientsMetabolism disorderIon chromatographySulfate radicals
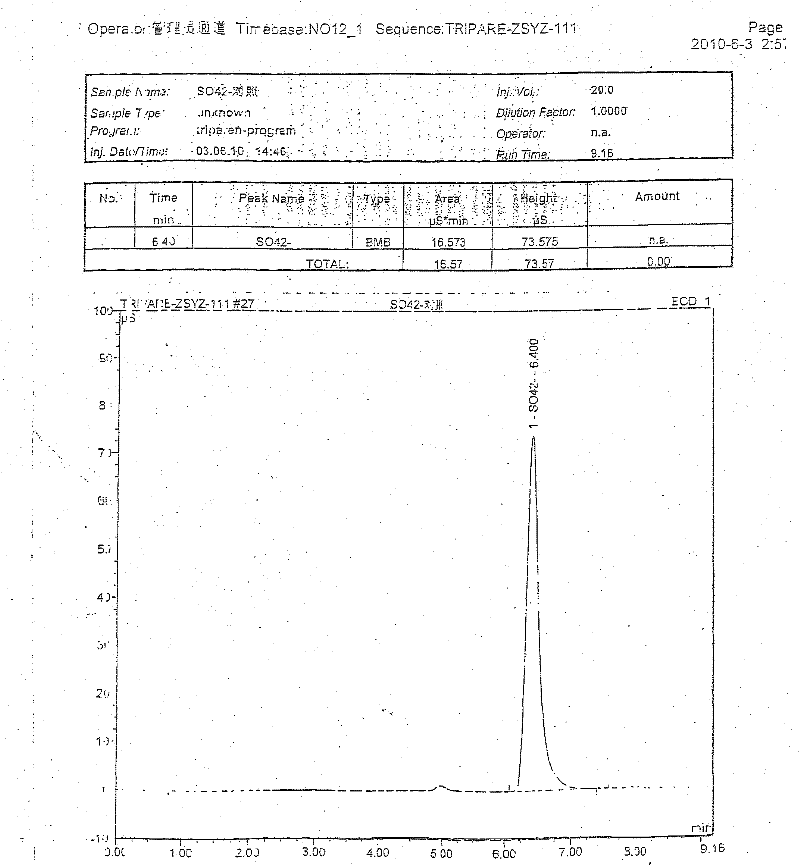
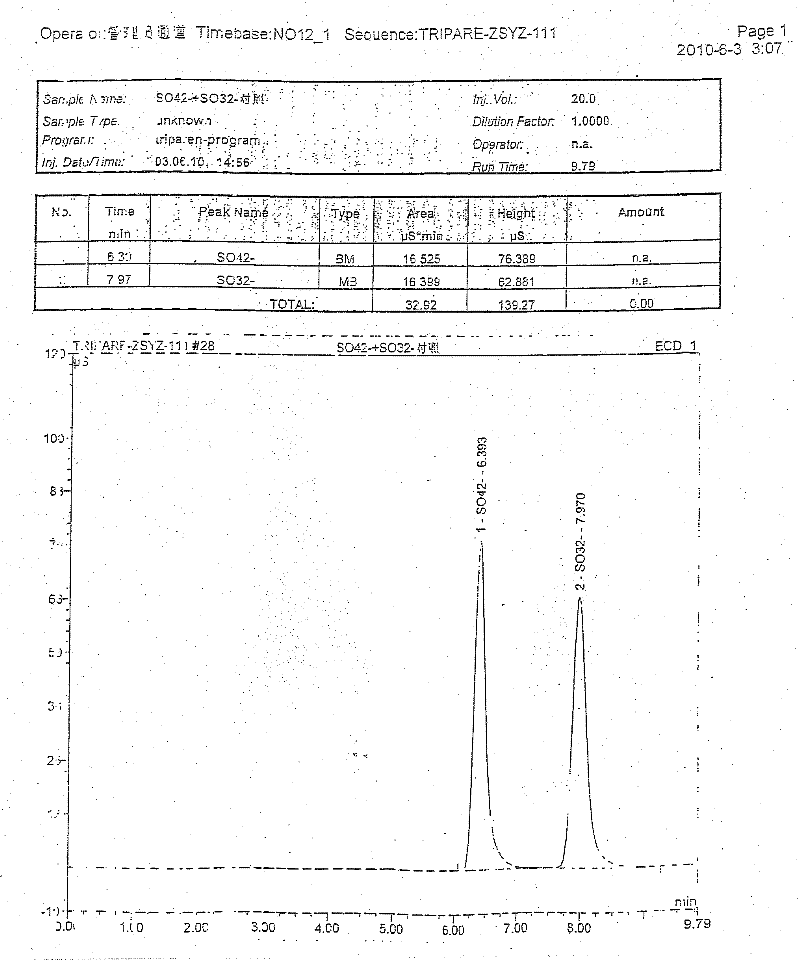
The invention discloses compound amino acid injecta, which contains arginine hydrochloride, histidine monohydrochloride, leucine, isoleucine, lysine hydrochloride and the like; meanwhile, the invention further discloses a preparation method of the compound amino acid injecta (18AA); in the event of ensuring the product quality, the compound amino acid injecta has the characteristics of saving energy and enhancing efficacy; by means of repetitive verification on precision, detection limit, quantitation limit, linear range and the like, a simple, convenient, rapid and reliable method with cleaning verification is ensured; furthermore, the residual quantity of antioxygen, namely sodium hydrogensulfite, in the compound amino acid injecta and sulphate ion generated by degrading sodium hydrogensulfite are detected according to an ion chromatography principle, thus, the amount of the antioxygen charged in the raw material proportioning can be accurately measured, and the stability of the raw material proportioning process is judged according to the measurement result; and a detection method of the compound amino acid injecta provided by the invention is an online derivative amino acid content measuring method, the speed is rapid, and the method is applied to large-scale detection.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Pharmaceutical composition containing 18 kinds of amino acid
ActiveCN101439036AInhibition of oxidative decomposition reactionsQuality assuranceOrganic active ingredientsMetabolism disorderAntioxidantTryptophan
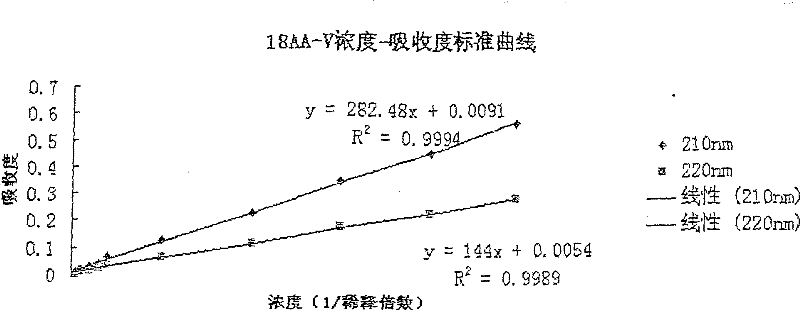
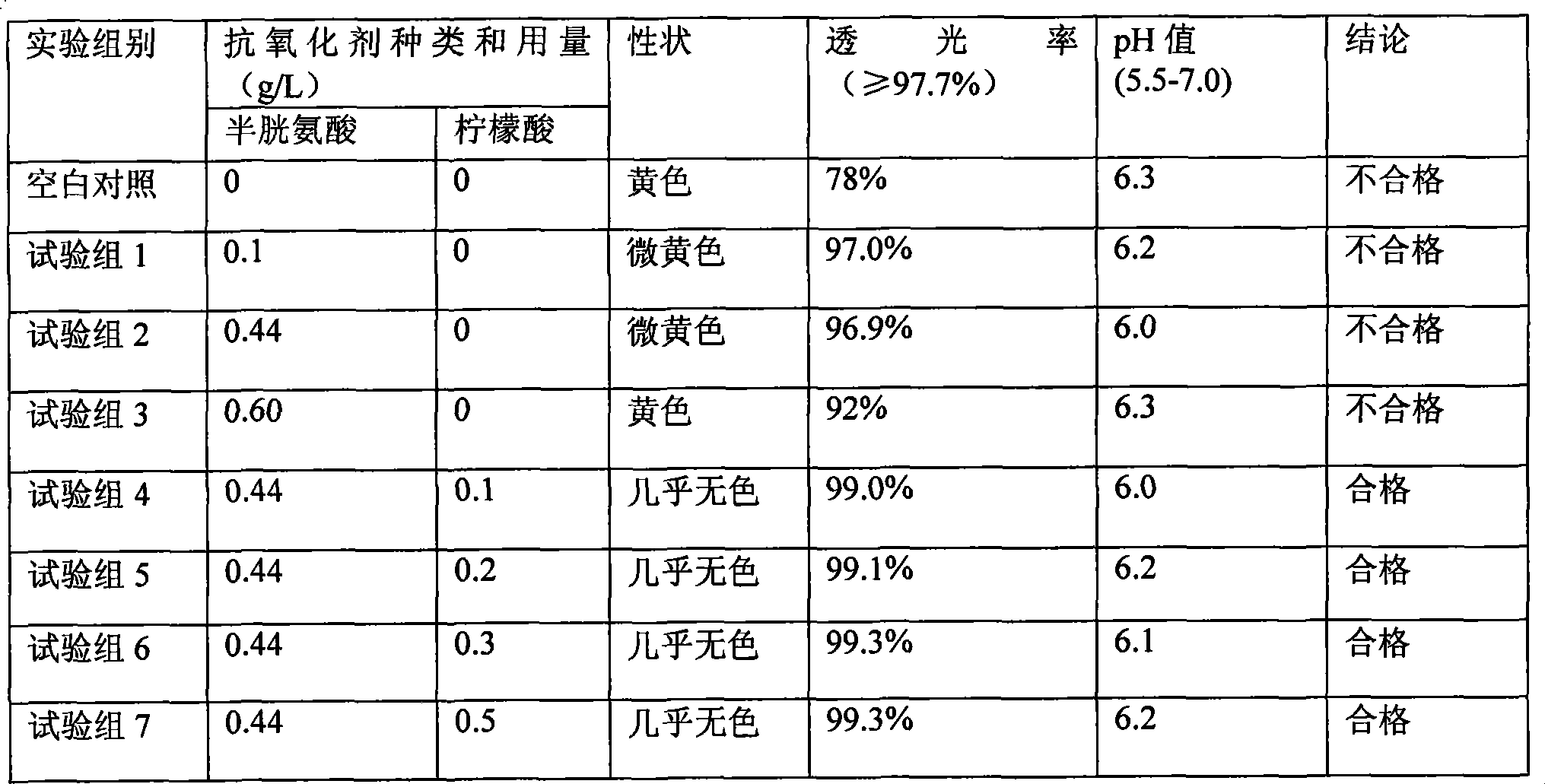
The invention discloses a pharmaceutical composition containing 18 amino acids. The pharmaceutical composition is characterized in that a compound amino acid injection (18AA-V) with varied concentration is prepared by using the following components according to the following ratios of parts by weight: 2.89 of arginine hydrochloride, 2.46 of histidine hydrochloride, 3.79 of leucine, 1.70 of isoleucine, 3.33 of lysine hydrochloride, 2.83 of phenylalanine, 1.97 of threonine, 1.36 of valine, 1.06 of methionine, 0.39 of tryptophan, 3.24 of glycine, 1.88 of alanine, 1.00 of proline, 0.11 of tyrosine, 0.67 of serine, 0.44 of cysteine hydrochloride, 1.15 of aspartic acid, 1.97 of glutamic acid, 50 of xylitol, 0.10 to 0.30 of citric acid and injection water with proper amount. The pharmaceutical composition does not contain a sulfite antioxidant so that the pharmaceutical composition is clinically used in a safer manner. After an accelerated test and a quality test, results show that the pharmaceutical composition is as stable as or more stable than like products (18AA-V) which are sold in the markets and contain sulfites.
Owner:福州凯瑞医药咨询有限公司
Composite amino acid and vitamine capsule preparation and its preparing method
The composite amino acid and vitamin capsule preparation includes coated amino acid granules and coated vitamin granules in the weight ratio of 1 to 0.68. The coated amino acid granules include isoleucine, leucine, lysine hydrochloride, phenylalanine, methionine, threonine, tryptophan, valine and arginine hydrochloride; and the coated vitamin granules include vitamin B1 nitrate, vitamin B2, vitamin B6, vitamin E acetate, and nicotinamide. The present invention also provides the preparation process of the composite amino acid and vitamin capsule preparation.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Dry heat treatment stabilizer for human blood coagulation factor VIII and application thereof
ActiveCN102924562AEffectively maintain activityHigh activityFactor VIIPeptide/protein ingredientsDry heatChloride
The invention discloses a dry heat treatment stabilizer for human blood coagulation factor VIII, which comprises the following proportions of components: 0.005M-0.015M of sodium citrate, 0.001M-0.002M of calcium chloride, 0. 15M-0.25M of arginine hydrochloride, and 6-10g / L of human serum albumin. The invention also discloses a human blood coagulation factor VIII preparation and a preparation method of the human blood coagulation factor VIII preparation. The dry heat treatment stabilizer for human blood coagulation factor VIII provided by the invention can maintain the activity of the human blood coagulation factor VIII, and is low in cost, thus having good prospect in clinical application.
Owner:CHENGDU RONGSHENG PHARMA
Bovine oocyte in-vitro fertilization and embryo culture method and transport culture solution
The invention relates to a bovine oocyte in-vitro fertilization and embryo culture method, and a transport culture solution. On one hand, the transport culture solution comprises glycine, alanine, arginine hydrochloride, aspartic acid, cystine dihydrochloride, glutamic acid, L-glutamine, histidine, threonine, tryptophan, tyrosine, valine, ascorbic acid, biotin, choline chloride, calcium pantothenate, folic acid, menadione and the like. The bovine oocyte in-vitro fertilization and embryo culture method comprises the following steps: oocyte collection and in-vitro maturation, in-vitro fertilization, and embryo in-vitro culture and preservation. The method and the related transport culture solution have excellent technical effects as described in the specification.
Owner:天津力牧生物科技有限公司
Amino acid injection and preparation method thereof
ActiveCN101401785APain reliefReduce clinical adverse reactionsOrganic active ingredientsMetabolism disorderAntioxidantThreonine
The invention discloses an amino acid injection, commonly known as propranolol, which relates to a pharmaceutical preparation containing 18 kinds of amino acids. The amino acid injection contains the following components per 1,000 milliliters: isoleucine, leucine, methionine, phenylalanine, threonine, tryptophan, valine, glycine, alanine, glutamic acid, aspartic acid, proline, serine, cystine, lysine hydrochloride or lysine acetate, arginine hydrochloride or arginine, histidine hydrochloride or histidine, tyrosine or acetyl tyrosine, and sodium bisulfite or sodium meta-bisulphite. The osmotic pressure ratio of the amino acid injection is lower than 1.8, which effectively reduces the pain for a patient in infusion; the added quantity of antioxidant is small, which can reduce the clinical adverse reactions; and an infusion bottle body is large, which is convenient to mix liquid in clinical application.
Owner:GUANGDONG LITAI PHARM CO LTD
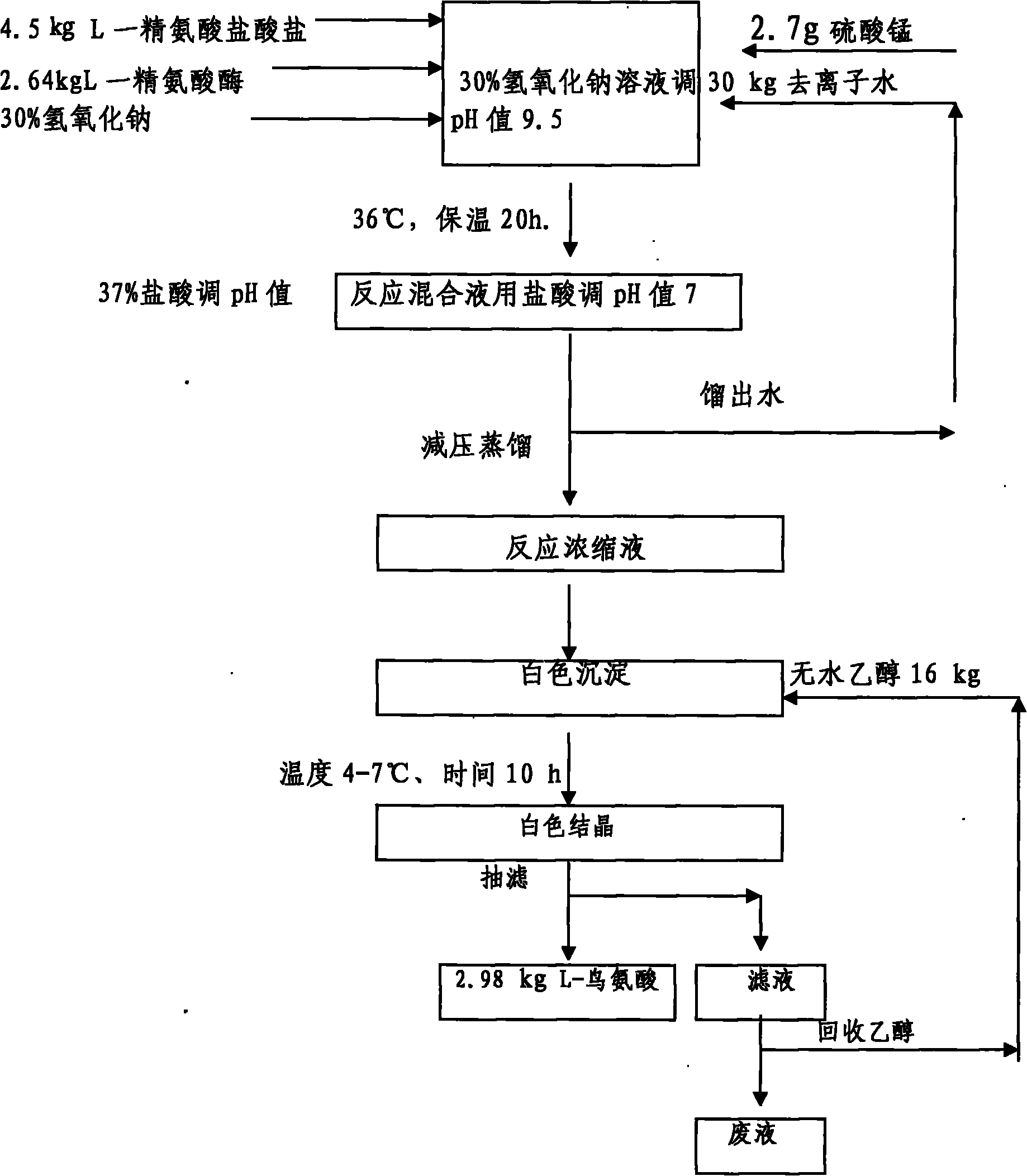
Method for preparing d-arginine hydrochloride and l-ornithine hydrochloride by splitting dl-arginine by microbial enzymatic method
InactiveCN102286602AIncrease enzyme activityIncrease vitalityMicroorganism based processesFermentationEscherichia coliD-Arginine
A method for preparing D-arginine hydrochloride and L-ornithine hydrochloride by splitting DL-arginine by microbial enzymatic method. The present invention utilizes the fermented bacterium of recombinant E. coli or its spray-dried preparation that highly expresses L-arginase as an enzyme source, uses DL-arginine as a substrate, and splits DL-arginine through an enzymatic reaction to produce D-arginine and L-ornithine. The resulting liquid is converted into L-ornithine monohydrochloride by adding hydrochloric acid, then adding a certain volume of ethanol to precipitate it, and collecting the precipitate to obtain L-ornithine hydrochloride; in the supernatant, D- The purification method of arginine is: adsorption and elution of ion exchange resin, concentration and drying under reduced pressure, to obtain D-arginine hydrochloride; this patent provides a new D-arginine hydrochloride and L- Green production process route of ornithine hydrochloride.
Owner:天津启仁医药科技有限公司
Healthcare food composition for relieving physical fatigue and preparation method of healthcare food composition
The invention discloses a healthcare food composition for relieving physical fatigue and a preparation method of the healthcare food composition. The healthcare food composition is made from the following medical components: maca powder, cordyceps militaris, cistanche and L-arginine hydrochloride. The invention also discloses the preparation method of the healthcare food composition. Animal tests indicate that the healthcare food composition disclosed by the invention has the function of relieving physical fatigue and has no side and toxic effects.
Owner:中科乐仁(北京)科技发展有限公司
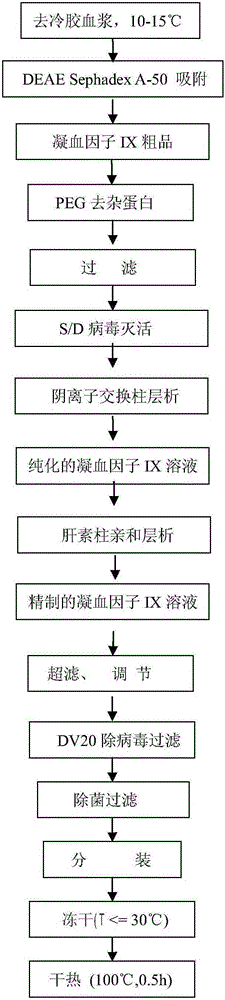
Preparation method of high-purity human coagulation factor IX
InactiveCN105175486AReduce lossesPrevent denaturation and inactivationPeptide preparation methodsUltrafiltrationPolyethylene glycol
The invention relates to a preparation method of a high-purity human coagulation factor IX, which comprises the following steps: melting refrigerated plasma, and carrying out low-temperature centrifugation; adsorbing with a DEAE (diethylaminoethanol) Sephadex A-50 gel to remove the coagulation factor IX in the cold-glue plasma; removing impure proteins in the solution by using polyethyleneglycol; carrying out S / D virus inactivation; carrying out anion exchange column chromatography to obtain a purified coagulation factor IX solution; passing through a heparin affinity column for further chromatography to obtain a high-purity coagulation factor IX solution; carrying out ultrafiltration, dialysis and concentration, and adding arginine hydrochloride and glycinate as protective agents; filtering through a 20nm filter element to remove viruses; carrying out freeze-drying; and carrying out dry heat virus inactivation. The protein protective agents are added during the gel adsorption, column chromatography and ultrafiltration dialysis, thereby lowering the activation probability of the FIX product thrombin and enhancing the qualification rate of the product. The technique has high product yield; the FIX specific activity can reach 150 IU / mg or so which is much higher than that of the traditional product; and by performing the three-step virus inactivation, the product is safe and reliable to use.
Owner:上海洲跃生物科技有限公司
Compound amino acid injection and preparation method thereof
InactiveCN110507604AReduce degradationStable in natureOrganic active ingredientsMetabolism disorderTryptophanBottle
The invention provides a preparation method of a compound amino acid injection. The preparation method comprises the following steps: respectively washing a preparation container and a pipeline with alkaline water, a metal ion chelating agent solution and water for injection; putting the water for injection into a liquid preparation tank, adding sodium hydroxide, and stirring while adding until the sodium hydroxide is dissolved; introducing N2 into the liquid preparation tank, then sequentially adding tyrosine, isoleucine, leucine, lysine hydrochloride, methionine, threonine, valine, alanine,arginine hydrochloride, aspartic acid, glutamic acid, proline, serine, glycine, phenylalanine, histidine hydrochloride, xylitol and cysteine hydrochloride, and stirring and dissolving one by one; continuously introducing N2 into the liquid preparation tank, ensuring that the oxygen content is within 3%, cooling the materials, adding water for injection, adding tryptophan, stirring, dissolving, andsupplementing water to a full amount to obtain a mixed solution; and filtering and sterilizing the mixed solution, injecting the mixed solution into an infusion bottle, capping and sealing the bottle, and performing sterilizing and performing light inspection to obtain the compound amino acid injection.
Owner:武汉久安药业有限公司
Methods for improving health in animals
A method of improving health in an animal includes administering to the animal a nutritional supplement comprising an amino acid secretagogue composition, which stimulates the pituitary gland in the animal to produce growth hormone. The nutritional supplement may be administered orally. The nutritional supplement may comprise L-arginine hydrochloride, L-pyroglutamic acid, L-lysine hydrochloride, and cysteine. When desired, the nutritional supplement may consist essentially of L-arginine hydrochloride, L-pyroglutamic acid, L-lysine hydrochloride, N-acetyl-L-cysteine, L-glutamine, and schizonepeta powder.
Owner:IP INVESTMENT
Composite health food composition for alleviating physical fatigue and preparation method thereof
The invention discloses a composite health food for alleviating the physical fatigue and a preparation method of the composite health food. The composite health food comprises maca powder, paecilomyces hepialid, extract of the fruit of Chinese wolfberry and L-arginine hydrochloride. The invention further discloses the preparation method of the composite health food. Animal experiments prove that the composite health food has the function of alleviating the physical fatigue without any toxic and side effects.
Owner:中科乐仁(北京)科技发展有限公司
Complex vitamin and amino acid oral liquid as well as preparation method and application thereof
ActiveCN105708829AReasonable compositionMaintain nutritional balanceDispersion deliveryMetabolism disorderThreonineTryptophan
The invention belongs to the field of a health care product, and in particular relates to a complex vitamin and amino acid oral liquid as well as a preparation method and application thereof. The complex vitamin and amino acid oral liquid provided by the invention mainly consists of isoleucine, threonine, valine, aspartic acid, arginine hydrochloride, lysine hydrochloride, phenylalanine, methionine, leucine, tryptophan, nicotinamide, vitamin B1, vitamin B2, vitamin B6 and water. The complex vitamin and amino acid oral liquid provided by the invention is reasonable in compatibility of various components, and is capable of effectively keeping nutrient balance in human bodies; and meanwhile, the oral liquid also has functions of boosting immunity of organism and relieving fatigue of the human bodies. At the same time, upon tests, the complex vitamin and amino acid oral liquid has a certain effect on improving idiopathic pulmonary fibrosis.
Owner:GUANGDONG JIABO PHARM CO LTD
Methods for improving health in canines
Owner:IP INVESTMENT
Protein-free serum-free medium suitable for diploid cell culture and application
The invention provides a protein-free serum-free medium suitable for diploid cell culture and a preparing method thereof. The medium contains calcium chloride, magnesium sulfate, potassium chloride, sodium dihydrogen phosphate, L-arginine hydrochloride, L-asparaginate, L-cystine dihydrochloride, L-histidine hydrochloride, L-leucine, L-isoleucine and the like. The medium can meet the requirement of the growth or maintenance stage of diploid cells and prevents contact with exogenous viruses possibly existing in protein and serum to reduce potential safety hazards; as the components are simple and definite, standard products can be purchased in the market, the difference between different product batches is small, and the step of purifying down-stream products is simplified; a scientific basis is provided for large-scale standard production. In addition, the protein-free serum-free medium can be applied in the growth or maintenance stage of the diploid cells.
Owner:SICHUAN BAINUOJI TECH CO LTD
Solubilization technology for producing human fibrinogen
ActiveCN103405754AImprove utilizationEffective inactivationPowder deliveryFibrinogenSolubilityLipid formation
The invention relates to a solubilization technology for producing human fibrinogen. The solubilization technology comprises following main steps: (1) source plasma treatment; (2) dissolving and centrifuging of cryoprecipitate; (3) inactivation of viruses; (4) chromatography purification; (5) centrifugal separation; (6) dissolving of sediments; (7) degerming and filtering; (8) split charging and freeze drying. The solubilization technology has the advantages that the purity of the extracted product can be up to over 95%; the content of foreign protein is low; a lipid envelope virus and a non-lipid envelope virus can be effectively activated; the safety of the product is ensured; arginine hydrochloride and glycine are adopted as stabilizers; the product can be fully protected in a freeze-drying process; the temperature change of the product in the freeze-drying process is stable by prolonging the freeze-drying time (about 4-8 days, and about 3 days for general factories); a freeze-dried product with a uniform structure can be obtained; the solubility of the human fibrinogen is increased; the product is quick to dissolve after being re-dissolved; no highly visible denatured protein is generated.
Owner:WUHAN ZHONGYUAN RUIDE BIOLOGICAL PROD CO LTD
Capsule containing ginseng saponin, radix notoginseng saponin and amino acids and preparation method thereof
ActiveCN101618044AQuality improvementEasy to transportOrganic active ingredientsMetabolism disorderDiseaseThreonine
The invention provides a compound capsule containing ginseng saponin Rb1, ginseng saponin Re, radix notoginseng saponin R1, tryptophan, threonine, leucine, valine, methionine, isoleucine, phenylalanine and lysine hydrochloride, wherein the compound capsule can also contain arginine hydrochloride and / or histidine hydrochloride. The preparation method of the capsule comprises the step of preparing various amino acids and saponin into coating micro-pills respectively. The capsule has stable quality, convenient transportation, storage and use and good absorbing effect. An advanced micro-pill coating preparation technique is adopted, the amino acids, the pseudo-ginseng saponin and the ginseng saponin are respectively prepared into the coating micro-pills with various colors, the quality is more stable by respective packaging, and the absorption of an organism is further promoted, thereby retaining the high potency of the capsule. The capsule has definite curative effect and wide adaptive disease, and clinical observation shows that the capsule can effectively enhance the immunity of the organism, can quicken the healing of wounds and has a very good curative effect on various leukopenia and gastrointestinal tract malabsorption diseases.
Owner:LIVZON PHARM GRP INC
Preparing method for lauroyl arginine monohydrochloride
InactiveCN105037216AOvercome the conditionsOvercome temperatureOrganic chemistryOrganic compound preparationFatty amineOrganosolv
The invention provides a preparing method for lauroyl arginine monohydrochloride. The preparing method includes the steps that L-arginine monohydrochloride, alkali and lauroyl chloride are weighed according to a weight ratio, the molar ratio of L-arginine monohydrochloride to alkali to lauroyl chloride is 1: 1-100: 1-100, and the alkali is carbonate, or bicarbonate, or acetate, or nitrogen heterocyclic amine or C3-C20 fatty amine; under the action of the alkali, the L-arginine monohydrochloride and the lauroyl chloride react in a condensation mode in first organic solvent for 1-24 h under the stirring condition, the temperature is controlled at minus 60 DEG C-100 DEG C, suction filtration, concentration and rotary evaporation are carried out on obtained reaction liquid to remove the organic solvent, and an obtained crude product is recrystallized with second organic solvent to obtain the lauroyl arginine monohydrochloride. The preparing method is moderate in reaction condition and easy to operate.
Owner:SHANGHAI INST OF TECH
Serum-free culture medium for VERO serum-free cell culture and corresponding virus production
InactiveCN110628697ADoes not increase the percentage of apoptoticReduce manufacturing costCulture processMicroorganism based processesApoptosisTryptophan
The invention discloses a serum-free culture medium for VERO serum-free cell culture and corresponding virus production. The culture medium comprises the following components: amino acids, vitamins, inorganic salts, auxiliary components and proteins. The amino acids comprise glycine, alanine, arginine hydrochloride, cystine dihydrochloride, glutamic acid, glutamine, histidine hydrochloride monohydrate, isoleucine, leucine, lysine hydrochloride, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine disodium salt dihydrate, valine, hydroxyproline, ornithine and taurine. Theproteins comprise human transferrin and recombinant insulin. When being used for VERO cell culture, the culture medium does not depend on the use of animal serum, does not introduce animal-derived protein, greatly lowers the production cost, sufficiently ensures the stable product quality, and can implement long-time culture and passage without increasing the apoptosis ratio.
Owner:山东巨山能源科技有限公司
Pre-filled recombinant human interferon injection
The invention relates to a pre-filled recombinant human interferon injection which is prepared from recombinant human interferon, arginine hydrochloride or lysine hydrochloride, acetic acid-acetate buffer solution with the pH value of 4.5 to 5.5 and tween-80; the injection prepared by the formula of the invention can be immediately filled into a small glass bottle for injection, or can be directly filled into a prefilled syringe; the injection of the invention is filled into the small glass bottle, the problems of a large amount of protein aggregation and greatly reduced interferon titer caused by the surface absorption of the small glass bottle are solved, and after being stored for a long time, interferon still has quite high titer.
Owner:BEIJING KAWIN TECH SHARE HLDG +1
Sodium aescinate preparation curing haemorrhoids and preparation method for sodium aescinate preparation
ActiveCN104288167ALess irritatingImprove complianceOrganic active ingredientsAerosol deliverySodium aescinateARGININE HYDROCHLORIDE
The invention discloses a sodium aescinate preparation curing haemorrhoids and a preparation method for the sodium aescinate preparation. The sodium aescinate preparation comprises the effective components of sodium aescinate and arginine hydrochloride; a gelata can be prepared by adding corresponding auxiliary materials. According to the invention, the arginine hydrochloride is adopted to reduce the thrill of the sodium aescinate to rectum mucosa; the sodium aescinate preparation directly acts on haemorrhoids part through part external administration, is fast to play function, enhances compliance of patients, and provides a novel administration route for the patients.
Owner:MAYINGLONG PHARMA GROUP
Composite amino acid freeze dried powder injection and its preparation process
InactiveCN1839813AImprove bioavailabilityImprove malabsorptionPowder deliveryOrganic active ingredientsActivated carbon filtrationTryptophan
The invention relaets to a compound amino acid freeze-dried powder injection, which comprises L-arginine hydrochloride, L-histidine hydrochloride, L-leucine, L-isoleucine, L-lysine hydrochloride, L-phenylalanine, L-threonine, L-valine, L-methionine, L-tryptophan, glycine, L-alanine, L-proline, L-tyrosine, L-serine, L-cysteine hydrochloride, L- aspartic acid, L-glutamic acid and sodium bisulphate. Its preparing process is also disclosed in the invention.
Owner:武汉同源药业有限公司
Method for extracting human fibrinogen from component I through column chromatography
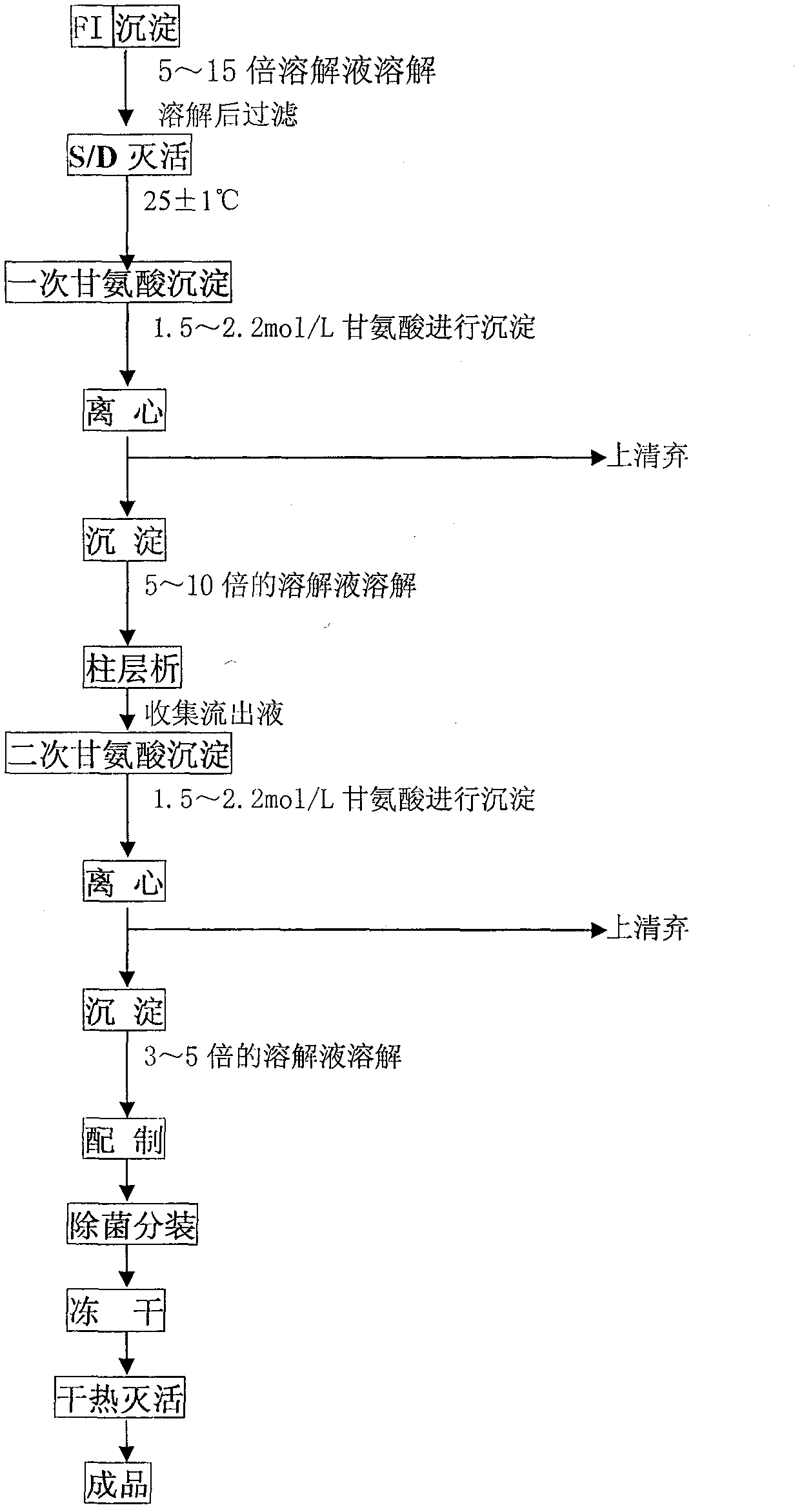
The invention relates to a method for extracting human fibrinogen from a component I through column chromatography. The problem of low purity, high stability and high yield of the human fibrinogen can be effectively solved. The method comprises the following steps of: (1) dissolving and filtering FI precipitate; (2) inactivating S / D ; (3) precipitating glycine for the first time; (4) dissolving and filtering the precipitate obtained in the precipitation for the first time; (5) performing column chromatography; (6) precipitating glycine for the second time; (7) dissolving and filtering the precipitate obtained in the precipitation for the second time; (8) precipitating; (9) packing the product in bags and freeze-drying the product; and (10) inactivating the product through hot air. In the method, glycine serves as a precipitator, so the finally obtained product contains about 0.5 percent of glycine; and the glycine and arginine hydrochloride are combined to serve as a stabilizer of the product, so the stability of freeze-dried product can be improved and the quality, the purity and the yield of the final product are improved. Therefore, the method is an innovation for preparing the human fibrinogen.
Owner:BANGHE PHARMA CO LTD
Highly concentrated stabilized igm solution
ActiveUS20090285802A1Aggregation of IgM in solutions may be suppressedOrganic active ingredientsDispersion deliveryChlorideARGININE HYDROCHLORIDE
Owner:CHUGAI PHARMA CO LTD
A kind of bactericidal pollution-free pesticide
The invention discloses a bactericidal pollution-free pesticide, which is prepared from leucine, valine, proline, methionine, glycocoll, threonine, tyrosine, serine, acetylcysteine, lysine hydrochloride, arginine hydrochloride, histidine monohydrochloride, tryptophan, alanine, glutamic acid, isoleucine, phenylalanine, sorbierite, sodium hydrogensulfite and water. The bactericidal pollution-free pesticide is used for preventing and controlling diseases such as gold diseases, black gold diseases, red gold diseases and the like of cereals, fruit trees and vegetables, does not pollute crops and environment, does not have residual toxicity, is long in lasting time of pesticidal potency, has the effects of prevention and control, and can prevent and control the diseases of the cereals, the fruit trees and the vegetables; and when the bactericidal pollution-free pesticide is applied, the application time is reduced, labors are saved, the drought tolerance and waterlogging tolerance of the cereals, the fruit trees and the vegetables are enhanced, so that root systems are developed, branches and leaves are flourish, healthy and strong, fruits are quick in coloring and early in maturity, the crops grow perfectly, excessive growth is inhibited, and the quality and yield can be improved.
Owner:张文吉
Compound amino acid dipeptide injection and preparation method and application thereof
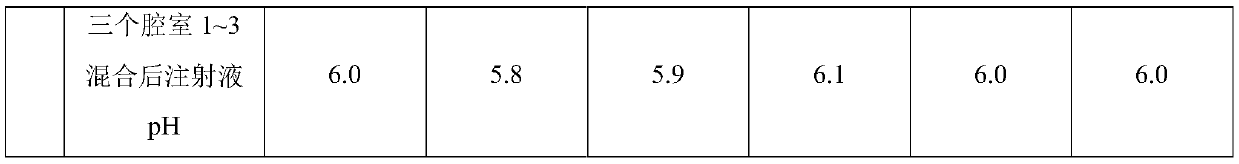
InactiveCN110404048AAvoid decompositionImprove material and energy metabolismDipeptide ingredientsHydroxy compound active ingredientsDipeptideL-alanyl-l-glutamine
The invention belongs to the field of medicine, and provides a compound amino acid dipeptide injection, a preparation method thereof and an application thereof, the injection comprises three cavity bags, the three cavity bags are respectively separated by three bags and filled with a branched-chain amino acid solution, a L-alanyl-L-glutamine solution, and a mixed solution containing arginine hydrochloride and vitamin B6; wherein, in every 1000 mL of the injection solution, the total mass of the amino acid is 50 g to 130 g; and the branched chain amino acid mass accounts for 10 to 80% of the total mass of the amino acid; the mass of L-alanyl-L-glutamine is 5 to 70% of the total mass of the amino acid; the mass of arginine hydrochloride is 0 to 50% of the total mass of the amino acid; and the mass of the vitamin B6 is 0 to 1 g. The injection of the invention improves the stability and effectiveness of the product while providing product safety, and the technical scheme is simple and convenient for industrial production.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Cell culture medium without animal component
The invention provides an animal-source-free cell culture medium, which is prepared by proportionally mixing the components including calcium chloride, potassium chloride, anhydrous magnesium sulfate, sodium chloride, anhydrous sodium dihydrogen phosphate, L-arginine hydrochloride, L-cystine hydrochloride, L-glutamine, L-Leucine, L-isoleucine, L-histidine hydrochloride, L-tyrosine, D glucose, choline chloride, folic acid, nicotinamide, pyridoxal hydrochloride, riboflavin, thiamine hydrochloride and the like. Cells can be directly cultivated on the cell culture medium provided by the invention, rather than gradual domestication and cultivation, so that the density and the harvesting frequency of the cultured cells are increased and the expression quantity is increased so as to increase the vaccine yield in the field of medicine, thereby effectively avoiding the interference of animal-originated characteristics to the cultivated animal or human cells. The animal-source-free cell culture medium solves the problems that the cells cultivated by the prior serum-free medium have premature deaths and unsaturated forms.
Owner:CHANGCHUN MEINENG BIOLOGICAL ENG
Preparation for tonifying Yang, strengthening body and improving immunity and preparation method of preparation
InactiveCN105055492ASolve the problem of difficult powder compressionSolving Difficult Tablet ProblemsOrganic active ingredientsPharmaceutical delivery mechanismMale infertilityAging male
The invention discloses a preparation for tonifying Yang, strengthening body and improving immunity and a preparation method of the preparation, belongs to the technical field of health food. The preparation for tonifying Yang, strengthening body and improving immunity comprises tablet cores, wherein the tablet cores comprise the following components in percentage by mass: 75-85% of L-arginine monohydrochloride Maca particles, 8-15% of sorbitol, 3-7% of microcrystalline cellulose, 0.5-1.5% of croscarmellose sodium and 0.5-1.5% of magnesium stearate; the invention further discloses the preparation method of the preparation. The preparation provided by the invention is suitable for young, middle-aged and elderly men, and has obvious improving effects on the male infertility, sexual dysfunction, sexual capacity decline and prostatic gland problems and people with kidney deficiency and body weakness.
Owner:JIANGSU ALAND NOURISHMENT
Method for preparing L-ornithine by utilizing enzyme method
InactiveCN102041282ASimple processStrong specificityHydrolasesFermentationManganese sulphateManganous chloride
The invention discloses a method for preparing L-ornithine by utilizing an enzyme method. Reaction materials mainly comprise L-arginine hydrochloride, L-arginase, manganese sulfate and concentrated hydrochloric acid; raw materials for extracting the L-arginase mainly comprise beef liver, Tris-HCl buffer solution and a small amount of manganese chloride; and the reaction materials are finally prepared into the L-ornithine through the steps of adding the materials, reacting, regulating the pH value, concentrating, precipitating, filtering and the like. The invention has the characteristics of simple process, short period, low energy consumption, strong specificity, high yield, no pollution and the like.
Owner:XINYI HANLING BIO ENG
Applications of L-arginine and derivative of L-arginine in preparation of cyclododecanoneoxime, and method used for preparing cyclododecanoneoxime
ActiveCN110498748AReduce interfacial tensionReduce decomposition rateOximes preparationCyclododecanoneDecomposition
The invention discloses applications of L-arginine and a derivative of L-arginine in preparation of cyclododecanoneoxime, and a method used for preparing cyclododecanoneoxime. The derivative of L-arginine comprises one or a plurality of components selected from L-arginine-L-glutamic acid, an L-arginine hydrochloride, and an L-arginine succinate. According to the preparation method, one or more than one components selected from L-arginine and the derivative of L-arginine are taken as auxiliary agents, cyclododecanone is reacted with hydroxylamine sulphate to prepare cyclododecanoneoxime. The auxiliary agents are capable of controlling iron ion content in the reaction system, inhibiting hydroxylamine sulphate decomposition, inhibiting cyclododecylamine generation, and increasing reaction selectivity, and are convenient to separate and recycle.
Owner:WANHUA CHEM GRP CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com