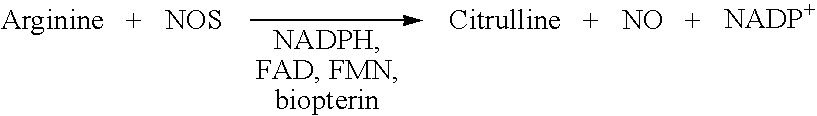Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
50 results about "Guanosine monophosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
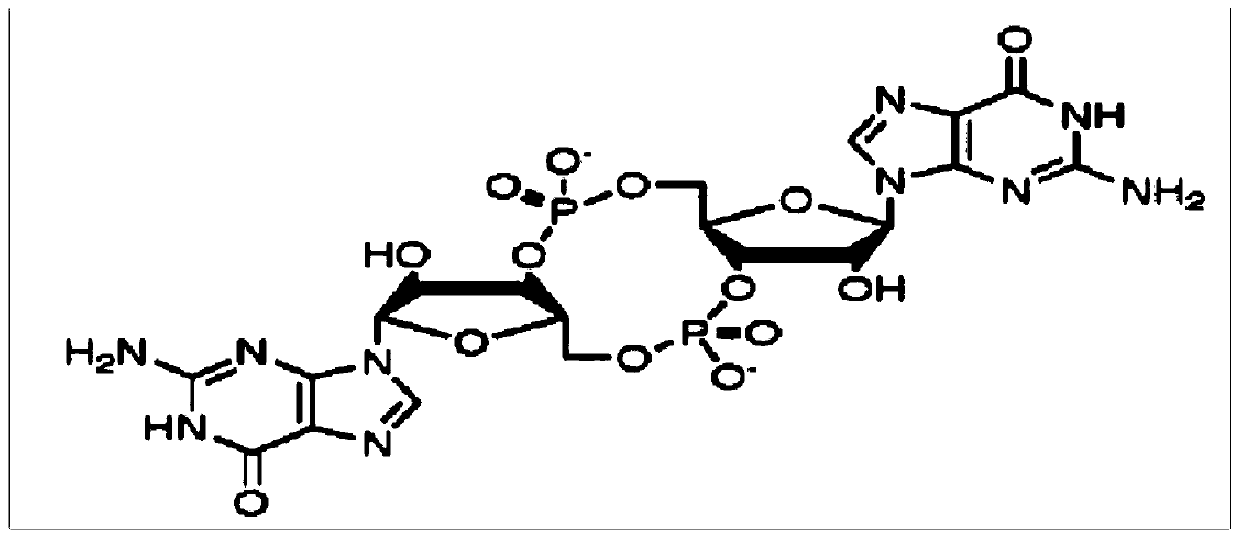
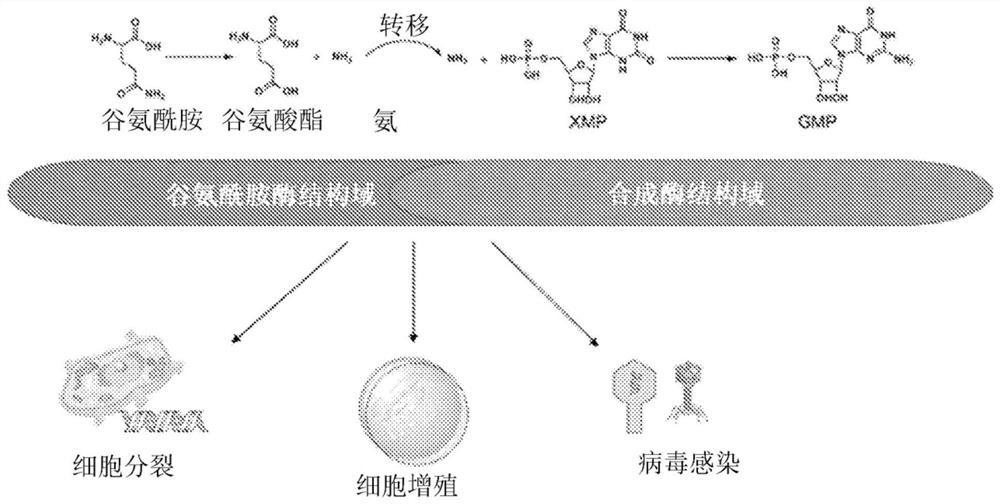
Guanosine monophosphate (GMP), also known as 5'-guanidylic acid or guanylic acid (conjugate base guanylate), is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid with the nucleoside guanosine. GMP consists of the phosphate group, the pentose sugar ribose, and the nucleobase guanine; hence it is a ribonucleoside monophosphate. Guanosine monophosphate is commercially produced by microbial fermentation. It is known as E number reference E626.
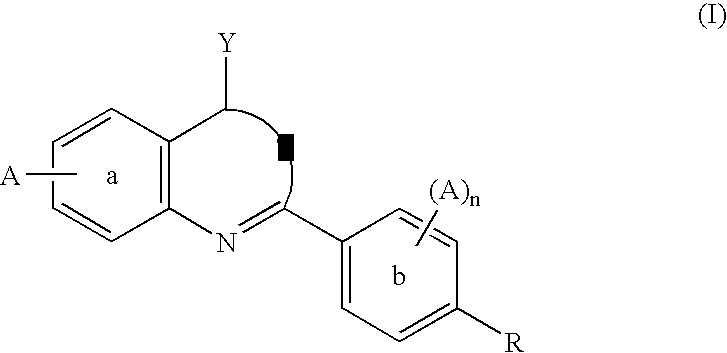
Dihydroorotate dehydrogenase inhibitors for the treatment of viral-mediated diseases
InactiveUS6841561B1Potent activityBiocideOrganic chemistryDiseaseDihydroorotate Dehydrogenase Inhibitor
Flavivirus, rhabdovirus and paramyxovirus infections may be treated by administering an inhibitor of the enzyme dihydroorotate dehydrogenase such as 6-fluoro-2-(2′-fluoro-1,1′-biphenyl-4-yl)-3-methyl-4-quinolinearcarboxylic acid sodium salt (Brequinar). A synergistic effect can be obtained if an interferon such as interferon α2, interferon α8 or interferon β, or an inhibitor of a second enzyme selected from inosine monophosphate dehydrogenase, guanosine monophosphate synthetase, cytidine triphosphate synthetase and S-adenosylhomocysteine hydrolase, is also administered.
Owner:INST OF MOLECULAR & CELL BIOLOGY
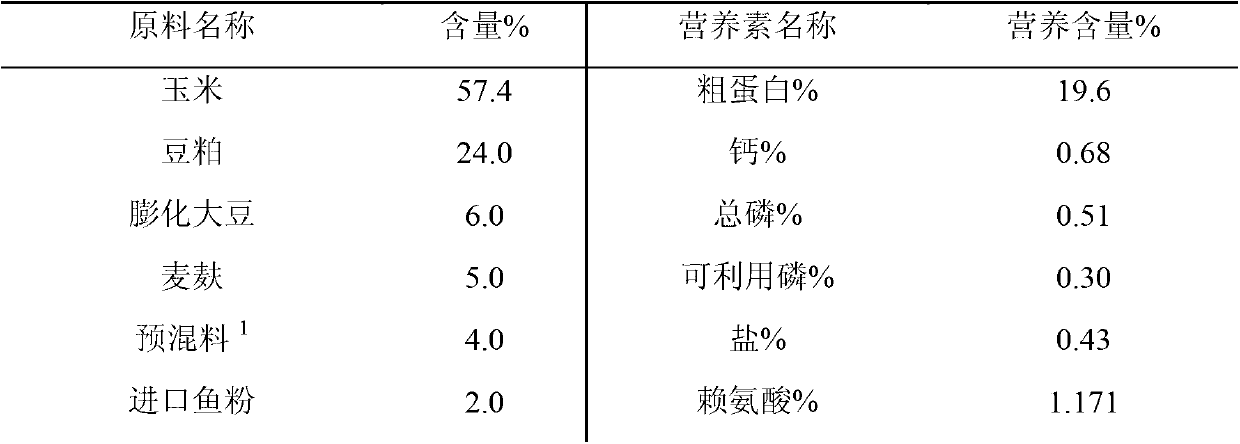
Weaned piglet nucleotide feed additive
InactiveCN102987169AImprove antioxidant capacityEnhance immune functionAnimal feeding stuffBiotechnologyGuanosine monophosphate
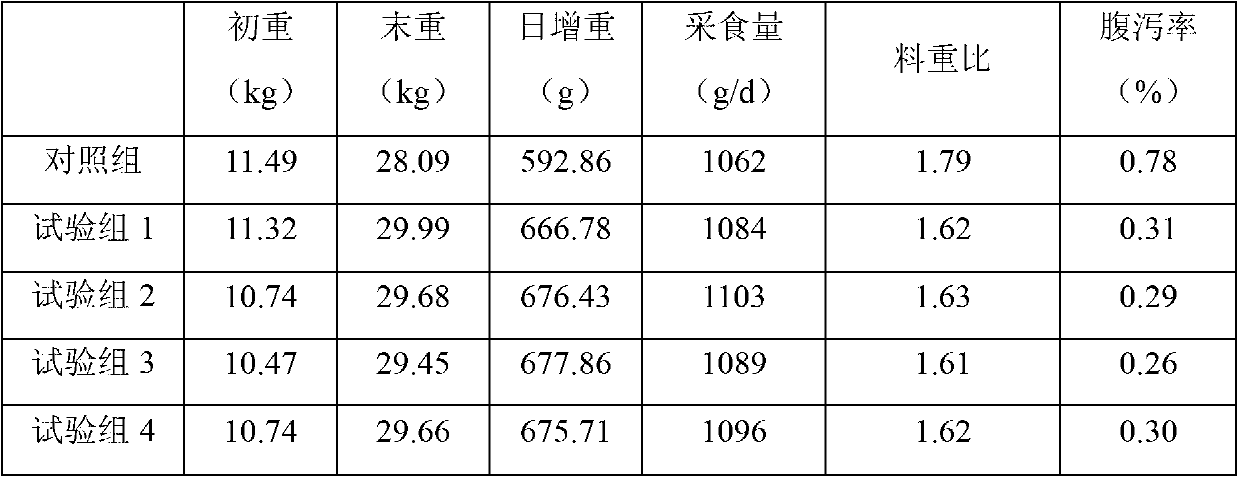
The invention discloses a weaned piglet nucleotide feed additive. The weaned piglet nucleotide feed additive comprises, by weight, 0.8 to 1.3 of adenosine monophosphate 5'-AMP, 0.3 to 0.8 of cytidine monophosphate 5'-CMP, 1.1 to 1.5 of guanosine monophosphate 5'-GMP and 0.7 to 1.4 of uridine monophosphate 5'UMP. The invention further discloses a preparation method and an application of the feed additive. According to the weaned piglet nucleotide feed additive, stress reaction of weaned piglets can be effectively relieved, the piglet grazing rate and the feed utilization efficiency are improved, the oxidation resistance and the immune function of weaned piglets are improved apparently, the organic resistance is enhanced, and accordingly, important significances are provided for modern scientific, intensive and large-scale pig production.
Owner:NANJING BIOTOGETHER
Preparation method and Cu<2+> detection application of rare earth ratiometric fluorescent probe
ActiveCN108517208ABlock deliveryAchieve high sensitivityMaterial analysis by observing effect on chemical indicatorFluorescence/phosphorescenceRare earth ionsGuanosine monophosphate
The invention discloses a preparation method and a Cu<2+> detection application of a rare earth ratiometric fluorescent probe, and belongs to the technical field of environmental detection. The preparation method of the luminol-Tb-GMP fluorescent probe is established through the self-polymerization coordination effect among luminol, GMP and Tb<3+> by using luminol and guanosine monophosphate (GMP)as dual ligands and using a rare earth ion Tb<3+> as a luminescent center ion. The luminol-Tb-GMP fluorescent probe combines the dual fluorescent signals of luminol and Tb<3+>, and simultaneously emits dual fluorescent signals of the luminol and Tb<3+> under the same excitation wavelength. When Cu<2+> exists, the luminol and GMP have a strong coordination effect on the Cu<2+> in order to preventelectrons from being transferred from the amino group of the GMP to Tb<3+>, so fluorescence quenching of the Tb<3+> is caused, and the fluorescence intensity of the luminol is unchanged, thereby a Cu<2+> detection method based on the fluorescence signal ratio of the luminol and Tb<3+> is established. Additionally, the green fluorescence of the Tb<3+> gradually weakens and the blue fluorescence ofthe luminol gradually appears with the increase of the Cu<2+> concentration, so the visual detection of the Cu<2+> can be achieved. The luminol-Tb-GMP fluorescent probe also can be applied to the sensitive detection of the Cu<2+> in an environmental water samples and a biological sample.
Owner:RUIJIN SHENGYUAN METAL NEW MATERIAL CO LTD
Preparation method of ratio-type fluorescent probe and application of ratio-type fluorescent probe in detection of tetracycline antibiotics
ActiveCN113201325AReduce distractionsReliable resultsFluorescence/phosphorescenceLuminescent compositionsAptamerFluoProbes
The invention belongs to the technical field of sensors, and relates to a preparation method and application of a ratio type fluorescent probe. The preparation method comprises the following steps: dissolving fluorescein, zinc acetate and terephthalic acid in DMF (Dimethyl Formamide), carrying out ultrasonic treatment and heating, and after a reactant is cooled, conducting washing and drying to obtain an FSS-coated MOF-5 fluorescent material; and dissolving the FSS-coated MOF-5 fluorescent material in water, adding guanosine monophosphate and europium nitrate under a stirring condition, and conducting washing, centrifuging and drying to obtain the FSS-coated MOF-5-coated GMP / Eu fluorescent probe. The ratio type fluorescent probe prepared by the invention can realize specific detection of tetracycline antibiotics, does not need specific biological materials such as antigens, antibodies or aptamers and the like, and can reduce the time and cost for preparing a specific fluorescent sensor; meanwhile, various color changes can be generated under an ultraviolet lamp, so that semi-quantitative visual detection of tetracycline is realized; and the detection is rapid and accurate, the operation is simple, the probe is not easily influenced by the environment, and the quality and safety of food are guaranteed.
Owner:JIANGSU UNIV
Glycine diagnosis/measuring reagent kit and glycine concentration determination method
InactiveCN101464280AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurements2-ketoglutaric acidEnzymatic Colorimetry
The invention relates to a kit for diagnosing / measuring glycine by utilizing the technologies of the enzymatic doubling amplification method, the enzymic colorimetry and the enzyme linked immunosorbent assay. The invention further relates to a method, a principle and the composition and the components of a reagent for measuring the concentration of the glycine, and belongs to the technical field of medical / food inspection and measurement. The main components of the kit include a buffer solution, coenzyme, a 2-ketoglutaric acid, guanosine monophosphate, glycine aminotransferase, glutamate dehydrogenase, guanosine monophosphate reduced enzyme and a stabilizer. Through mixing a sample and the reagent by a certain volume ratio, a series of enzymatic reactions occur, then the reactant is placed under an ultraviolet / visible light analyzer, and the degree / velocity of the increase in absorbance at 340 nm of the dominant wavelength is detected, thereby measuring the active concentration of the glycine.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Microwave making method of roast beef flavored essence powder
ActiveCN102715487AStrong sense of mellowAftertasteFood preparationMonosodium glutamateFlavoring essences
The invention relates to a microwave making method of roast beef flavored essence powder. The microwave making method of roast beef flavored essence powder includes the steps of adding beef hydrolysate, glucose, xylose, cysteine, glycine, alanine, leucine, beef tallow, vegetable hydrated protein, monosodium glutamate, yeast powder, furanone, I+G (disodium 5'-inosinate and 5'-guanosine monophosphate disodium), and cysteine hydrochloride into a reaction container, mixing to dissolve all amino acid and reducing sugar, keeping the temperature of 100-120 DEG C for reaction for 1-2 hours, cooling to obtain beef reaction spice, adding corn starch and flour into the beef reaction spice, mixing well, and performing microwave drying at 90-120 DEG C to obtain the roast beef flavored essence powder. The made roast beef flavored essence powder is mellow and thick, long in aftertaste, strong in barbecue flavor and particularly flavored unique, and the making method is simple.
Owner:春发生物科技(宁夏)有限公司
Seasoning hot pepper for steaming fish and processing method thereof
InactiveCN101617809AGood colorImprove crispnessFood preparationMonosodium glutamateAdditive ingredient
The invention relates to a seasoning hot pepper for steaming fish and a processing method thereof. The seasoning hot pepper is prepared by the steps: taking the desulfurated pickled capsicum frutescens and the pickled hot green pepper to be matched with other ingredients of ginger, mild water with the temperature of 65 DEG C, citric acid, monosodium glutamate, sodium cyclamate, Acesulfame-K, disodium 5'-Inosine monophosphate, disodium 5'-guanosine monophosphate and ethyl maltol; stirring, standing and sterilizing the substances to obtain the seasoning hot pepper. The seasoning hot pepper has stable quality and low content of sulfur dioxide, better retains the fragility, color and lustre of the hot pepper, as well as the nutrition of the hot pepper, and has longer time and shelf life.
Owner:CHANGSHA TANTANXIANG FLAVORING FOOD +1
Instant sharkskin and preparation method thereof
The invention discloses an instant shark skin, which is produced by mixing, boiling and thickening the following raw materials with the following weight percents: 12 to 15 percent of fishy smell removed sharp skin, 3 to 5 percent of compounding material, and 80 to 85 percent of dripping seasoning. The compounding material is prepared by mushroom, black fungus, white fungus, veiled lady and dried scallop with a wet weight proportion of 3:3:6:6:2 after soaked. The dripping seasoning is prepared by the following dripping materials with the following weight percents and water: 10 to 15 percent of abalone juice, 0.3 to 0.5 percent of fresh chicken powder, 0.3 to 0.5 percent of white spirit, 0.1 to 0.3 percent of inosine monophosphate and guanosine monophosphate, 0.05 to 0.1 percent of salt, 0.01 to 0.05 percent of chicken extract, and 2 to 4 percent of starch. The invention has the advantages of convenient use, high nutritional value and good taste. The invention also discloses a preparation method of the instant sharp skin. The method has the advantage of good fishy smell removing effect; in addition, the compounding material and the dripping seasoning for the preparation of the sharp skin taste delicious and are rich in various nutrients.
Owner:NINGBO UNIV
Glycine diagnosis/measuring reagent kit and glycine concentration determination method
InactiveCN101464272AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsGlycineEnzyme catalysis
The invention relates to a kit for diagnosing / measuring glycine by utilizing the technologies of the doubling amplification method, the enzymic colorimetry and the enzyme linked immunosorbent assay. The invention further relates to a method, a principle and the composition and the components of a reagent for measuring the concentration of the glycine, and belongs to the technical field of medical / food inspection and measurement. The main components of the kit include a buffer solution, coenzyme, inosinic monophosphate, glycine dehydrogenase, guanosine monophosphate reduced enzyme and a stabilizer. Through mixing a sample and the reagent by a certain volume ratio, a series of enzymatic reactions occur, then the reactant is placed under an ultraviolet / visible light analyzer, and the degree / velocity of the increase in absorbance at 340 nm of the dominant wavelength is detected, thereby measuring the active concentration of the glycine.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
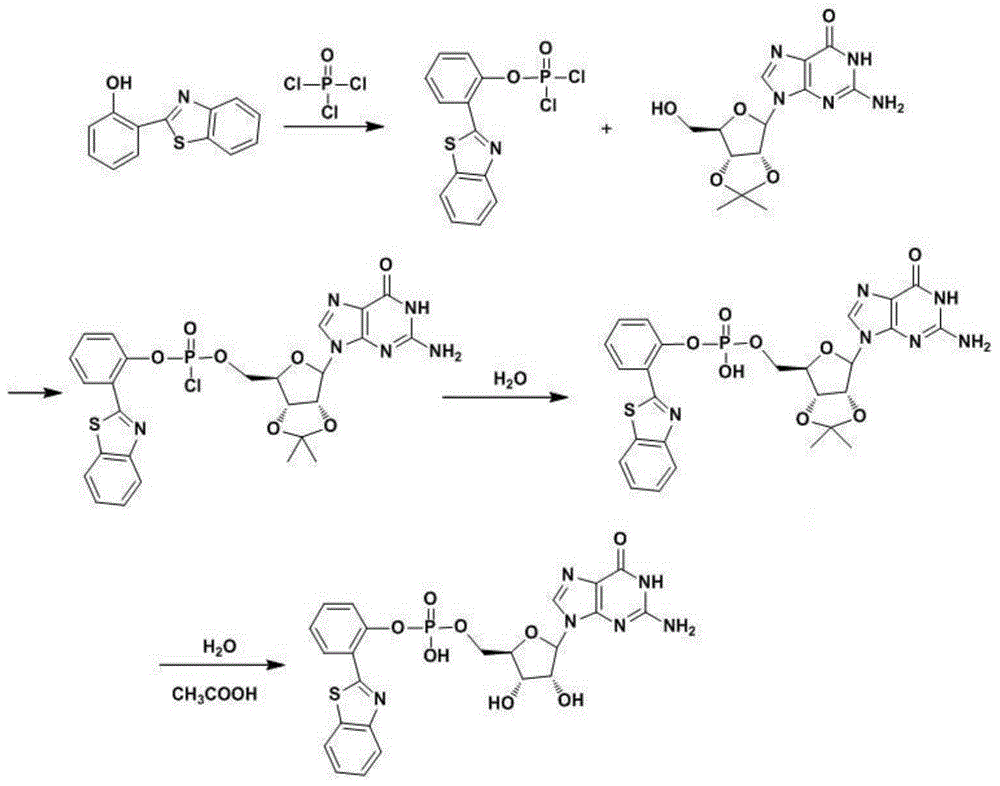
Crystallizing method of 5'-guanosine-monophosphate disodium salt
ActiveCN101863943AEasy to separateImprove solubilitySugar derivativesSugar derivatives preparationAlcoholGuanosine monophosphate
The invention discloses a crystallizing method of a 5'-guanosine-monophosphate disodium salt, which has the advantages of simple operation as well as large crystalline particle, uniform particle, attractive fineness and high purity of products. The method comprises the following steps of: adding sodium hydroxide to a 5'-GMP aqueous solution of which the concentration is 5-40 percent; regulating the PH value of the solution to 7-11 and then adding absolute alcohol which has a PH value of 7-11 and is 0.5-3 times of the solution in volume and a catalyst; stirring at 15-80 DEG C to separate out 5'-GMP, 2Na; and centrifuging, washing and drying to obtain 5'-GMP, 2Na crystals, wherein the catalyst is a mixture of a 60-120 mesh siftage of 5'-guanosine-monophosphate disodium salt crystals and sodium carbonate, the mass ratio of the 60-120 mesh siftage of 5'-guanosine-monophosphate disodium salt crystals to the sodium carbonate is 1:2, and the addition quantity of the catalyst is 0.1-0.2 percent of the mass of the 5'-GMP.
Owner:大连珍奥生物技术股份有限公司
Fluorescent probe GH and preparation method and application thereof
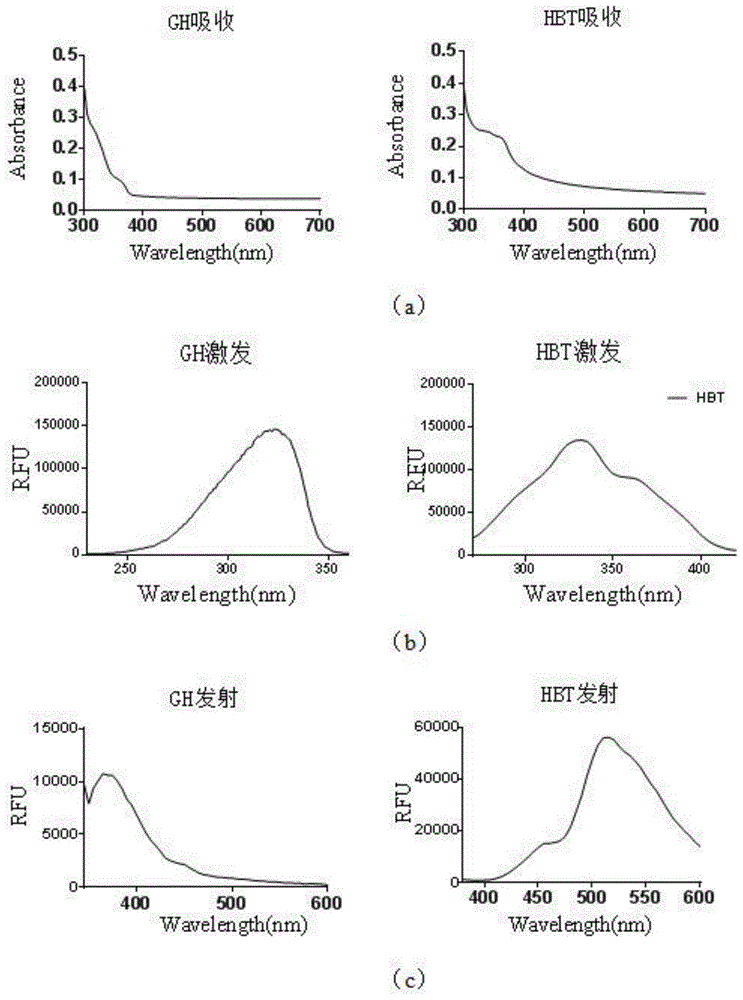
ActiveCN105524609AIncreased sensitivityHigh selectivitySugar derivativesBiological testingFluorescenceGuanosine monophosphate
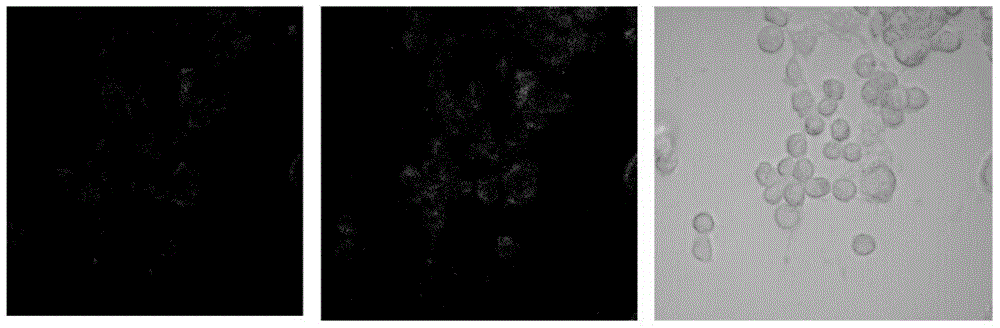
The invention discloses a fluorescent probe GH and a preparation method and application thereof. The fluorescent probe can be used for selectively detecting intracellular alkaline phosphatase (ALP). The main synthetic method includes the steps that 2'2-(hydroxy-phenyl)benzothiazole (HBT) is introduced to micromolecule 5'guanosine monophosphate (GMP) of an analog substrate of the alkaline phosphatase, so that a compound of the structure as shown in the specification is formed. Under the action of the alkaline phosphatase, the HBT is generated. By the aid of the difference of fluorescent properties before and after reacting, selective detection of the alkaline phosphatase can be achieved.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
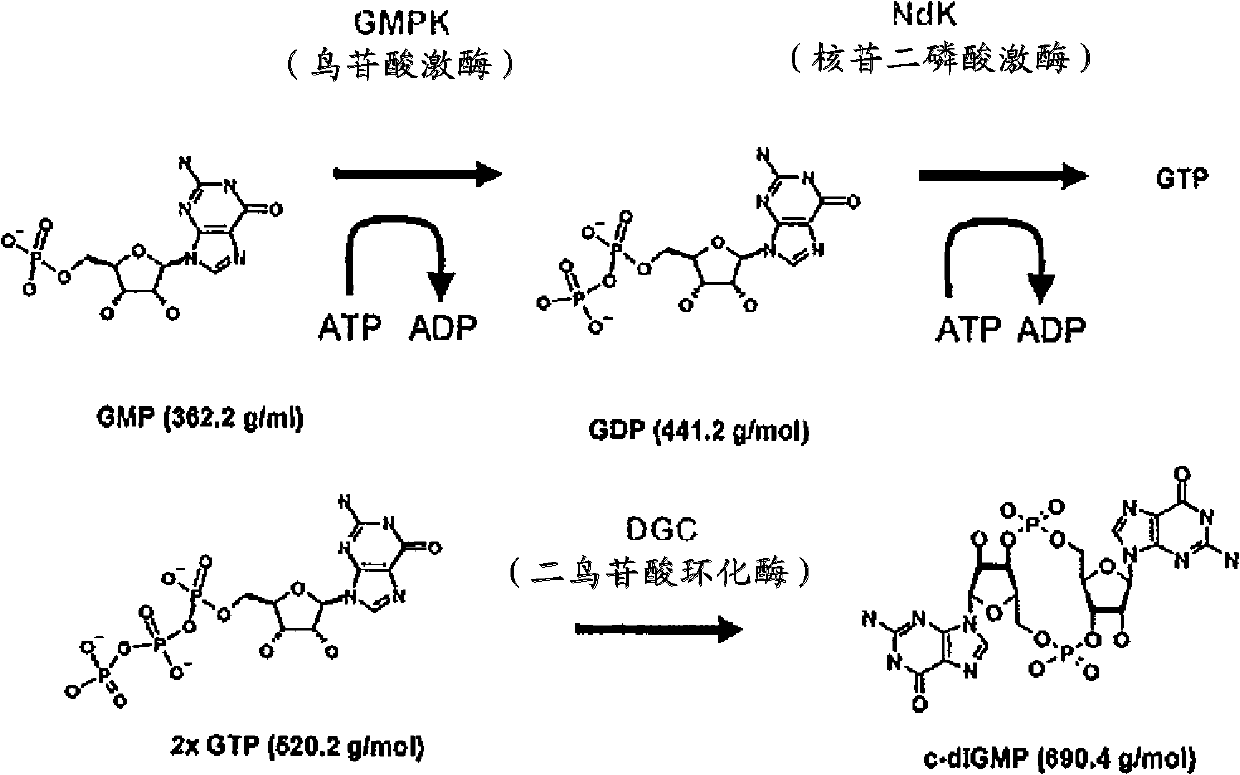
Process for the enzymatic production of cyclic diguanosine monophosphate employing a diguanylate cyclase comprising a mutated rxxd motif
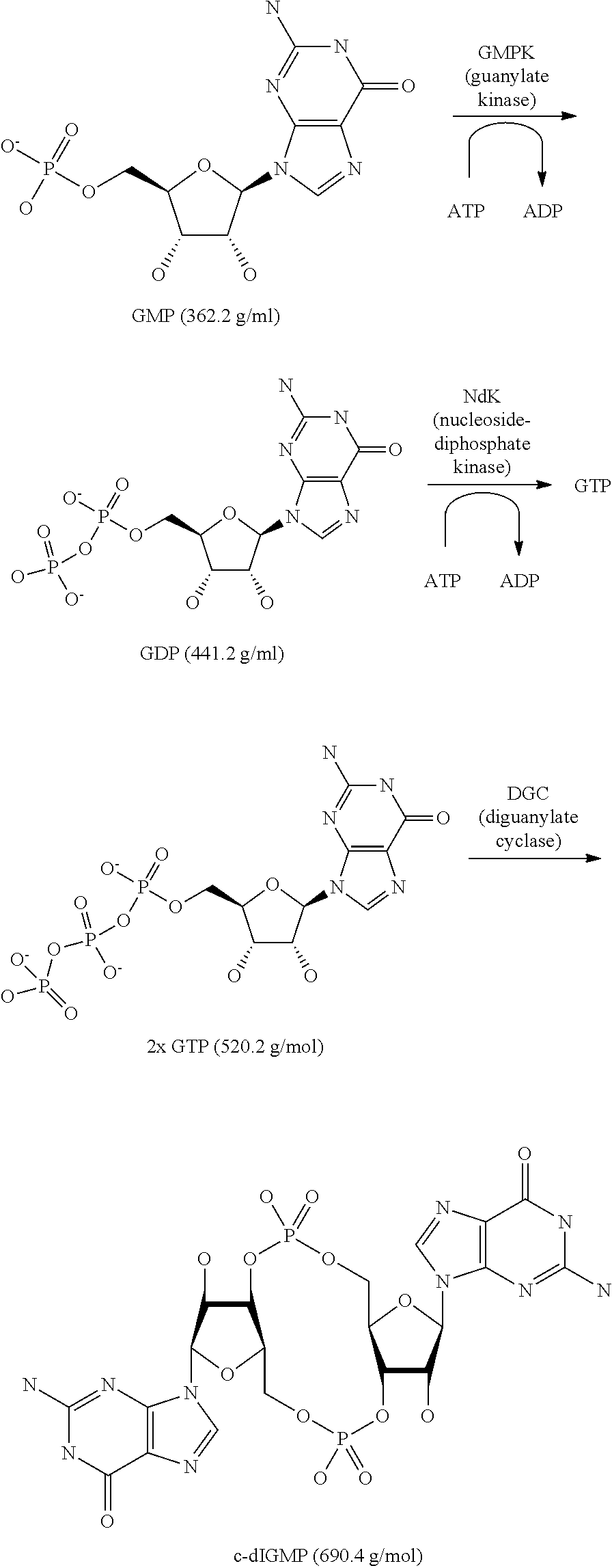
A process is disclosed for the production of cyclic di-guanosine monophosphate (c-di-GMP) without the use of protecting groups by means of an enzymatic synthesis. The process comprises the coupling of two guanosine triphosphate (GTP) molecules so as to form a c-di-GMP molecule. This is done under the influence of a mutant diguanylate cyclase (DGC) comprising the amino acid sequence V153M154G155G156. It has been found that the DGC is obtainable from inclusion bodies, and therewith can be made available in amounts sufficient to improve the c-di-GMP synthesis. Particularly, the latter synthesis can be conducted in a one-pot method starting from commercially available bulk chemicals and allows upscaling to a commercial production scale.
Owner:INTERVET INT BV
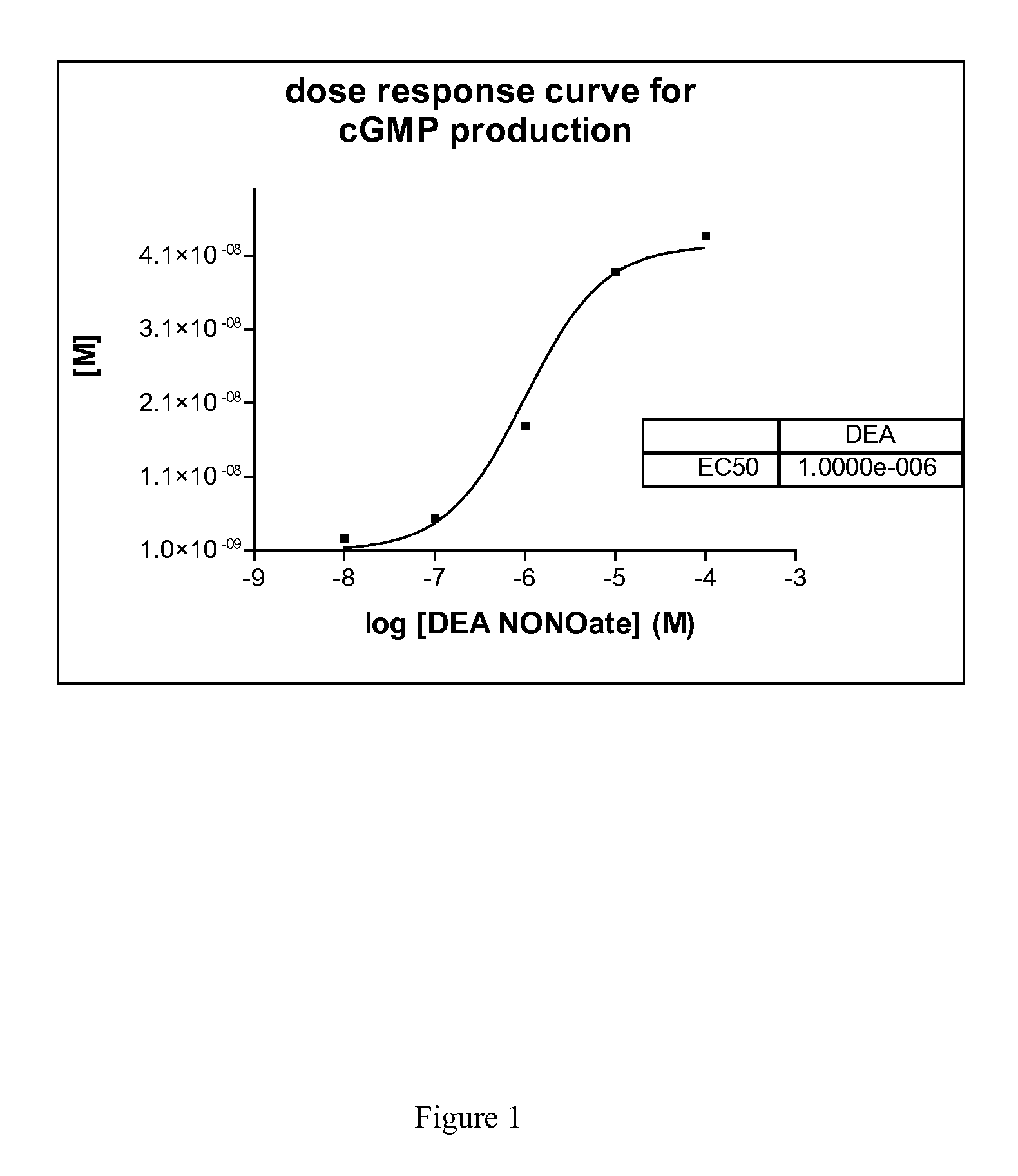
Method for detection of nitric oxide (NO)
The present invention relates to methods for detection of nitric oxide (NO) by measuring guanosine monophosphate (cGMP).
Owner:BOEHRINGER INGELHEIM INT GMBH
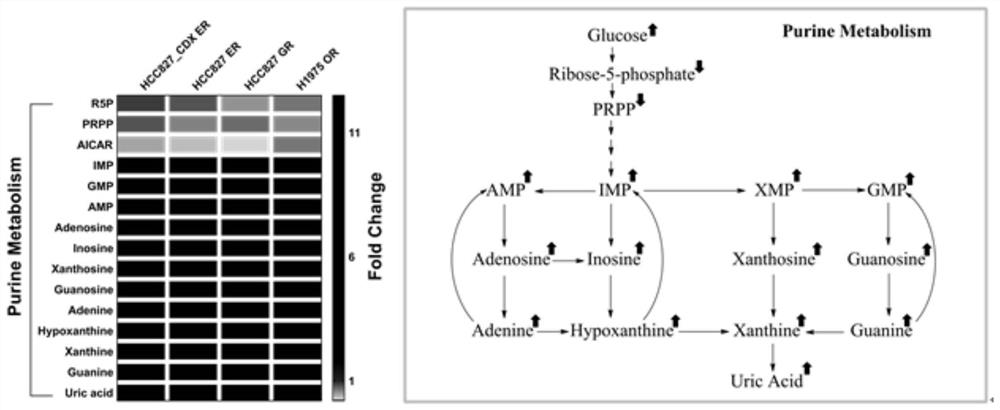
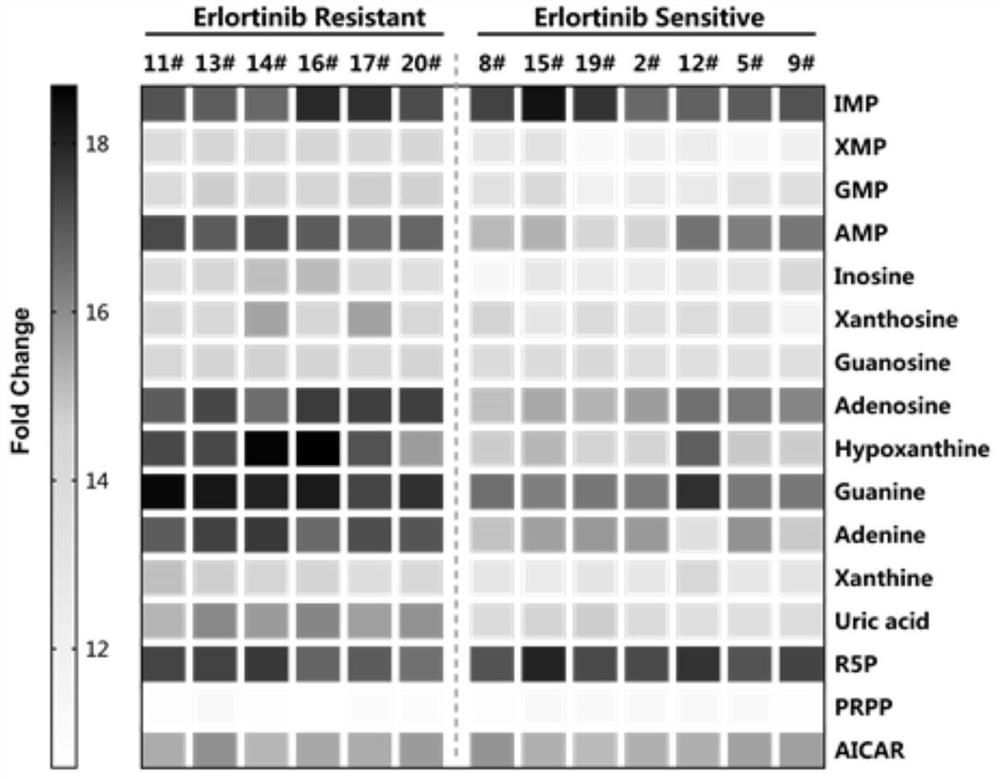
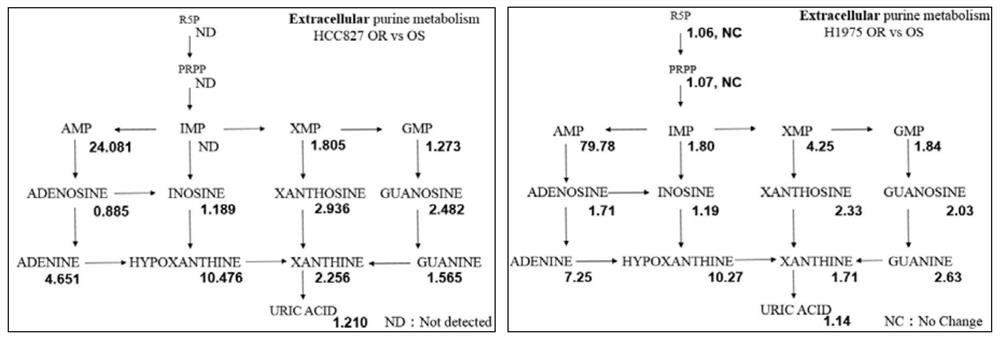
Application of purine metabolism marker in preparation of reagent for screening and diagnosing acquired drug resistance of lung cancer molecular targeted drug
The invention provides application of a purine metabolism marker in preparation of a reagent for screening and diagnosing acquired drug resistance of a lung cancer molecular targeted drug. The purine metabolism marker is one or more of adenosine monophosphate, guanosine monophosphate, inosine monophosphate, xanthosine monophosphate, adenosine, guanosine, inosine, xanthosine, adenine, guanine, hypoxanthine, xanthine and uric acid. The metabolic marker has good diagnostic potential for acquired drug resistance of the lung cancer molecular targeted drug, can be used for diagnosis and curative effect monitoring of acquired drug resistance of the lung cancer molecular targeted drug, and has good guiding significance for development of subsequent clinical application research.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Method for catalytic synthesis of cyclic di-guanosine monophosphate (c-di-gmp) through guanylate cyclase
PendingCN110499323AOptimize synthesis conditionsGuaranteed yieldPhosphorus-oxygen lyasesMicrobiological testing/measurementPurification methodsWild type
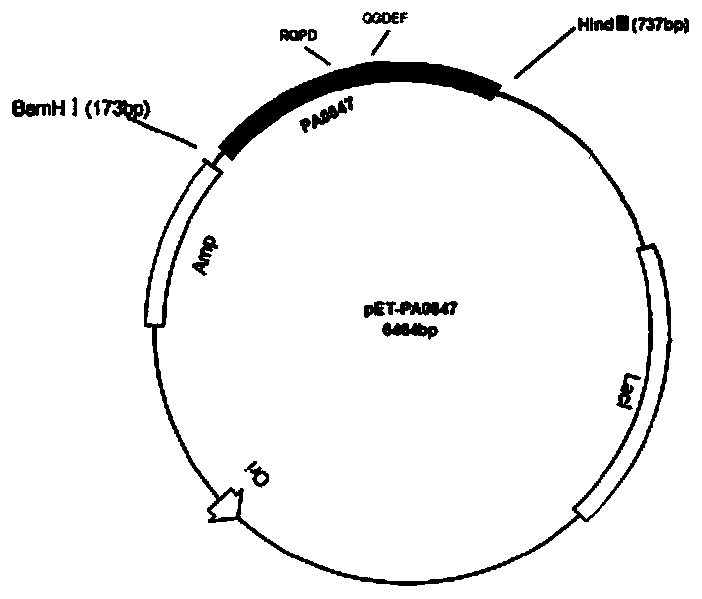
The invention provides a method for catalytic synthesis of cyclic di-guanosine monophosphate (c-di-gmp) through guanylate cyclase. The method specifically comprises the following steps: (1) obtainingof a gene sequence; (2) constructing of a mutation recombinant vector; (3) digestion; (4) conversion; (5) induction expression; (6) degerming for protein collecting; (7) protein purification; (8) protein dialysis; (9) measuring of the protein concentration through a BCA method; and (10) protein in-vitro catalytic reaction. Compared with the prior art, coding protein of a new gene-PA0847 is utilized as c-di-gmp catalytic enzyme; the wild-type PA0847 protein is subjected to point mutation and gene engineering modification; the catalytic enzyme is sufficient in source, and a purification method is stable, simple and convenient; the synthesis process is simple and convenient, and pollution is avoided; and the product yield is stable, and purification is easy.
Owner:NINGXIA MEDICAL UNIV
Process for the enzymatic production of cyclic diguanosine monophosphate employing a diguanylate cyclase comprising a mutated rxxd motif
InactiveUS20110288286A1Better addressSugar derivativesTransferasesEnzymatic synthesisInclusion bodies
A process is disclosed for the production of cyclic di-guanosine monophosphate (c-di-GMP) without the use of protecting groups by means of an enzymatic synthesis. The process comprises the coupling of two guanosine triphosphate (GTP) molecules so as to form a c-di-GMP molecule. This is done under the influence of a mutant diguanylate cyclase (DGC) comprising the amino acid sequence V153M154G155G156. It has been found that the DGC is obtainable from inclusion bodies, and therewith can be made available in amounts sufficient to improve the c-di-GMP synthesis. Particularly, the latter synthesis can be conducted in a one-pot method starting from commercially available bulk chemicals and allows upscaling to a commercial production scale.
Owner:INTERVET INT BV
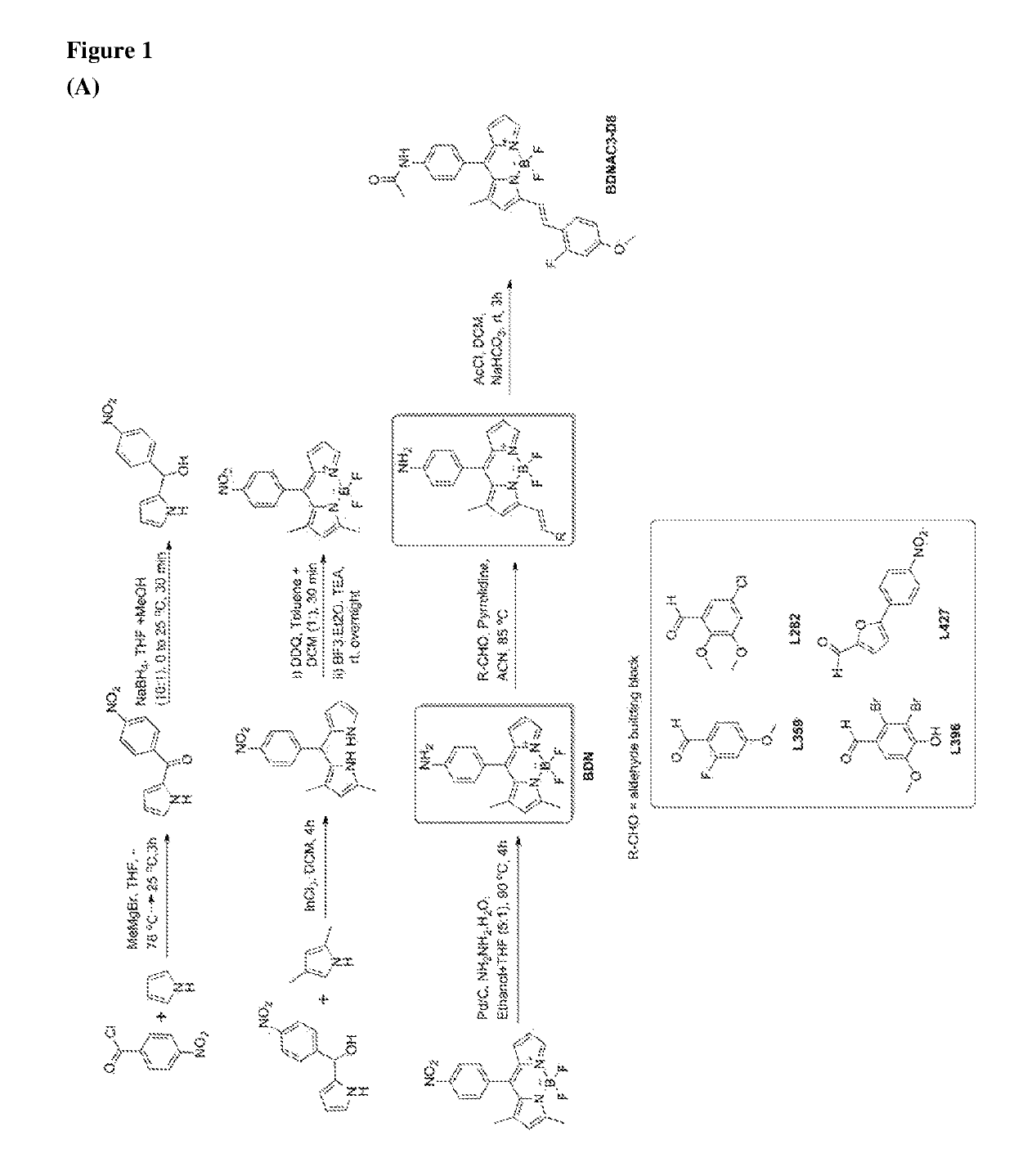
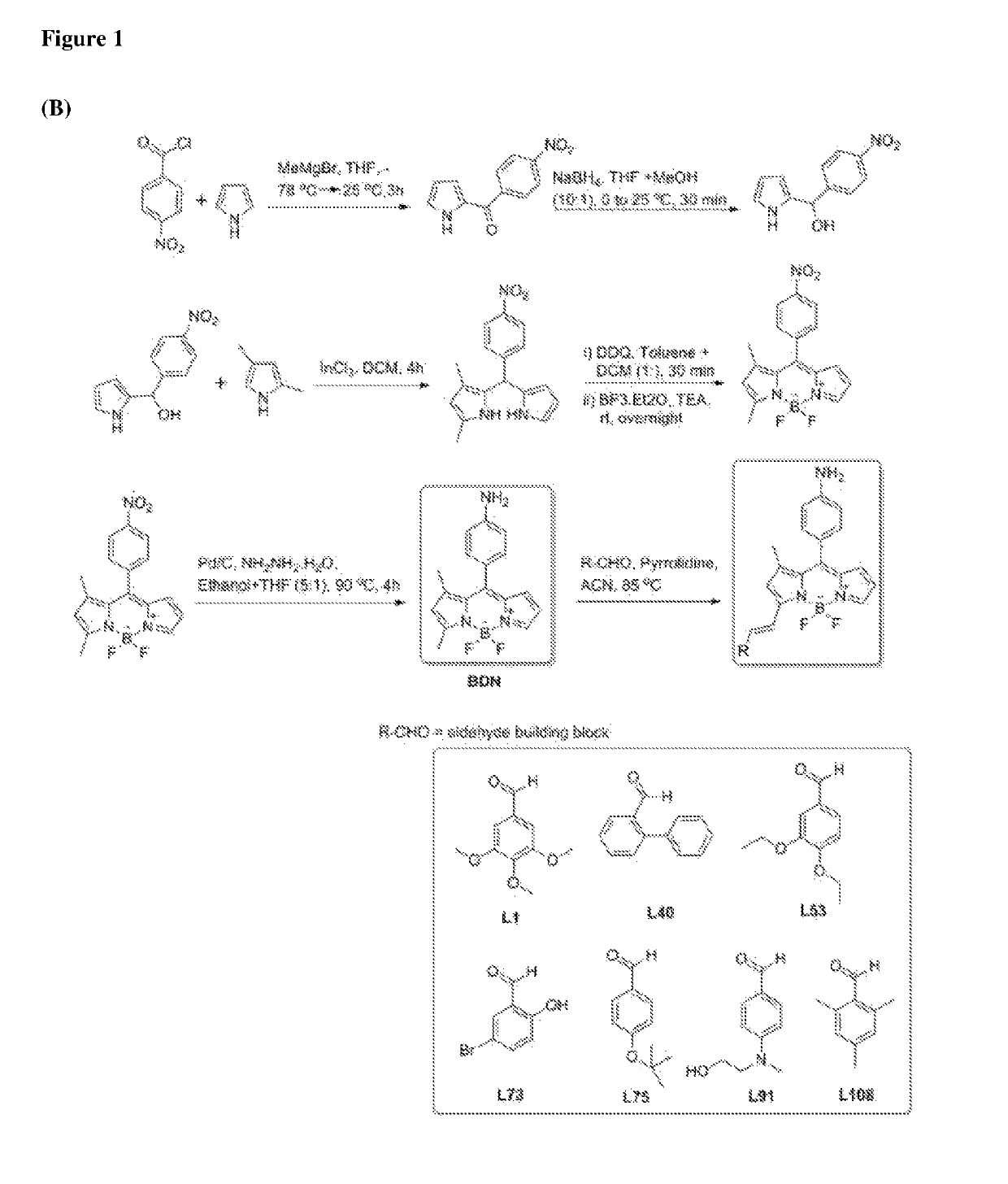
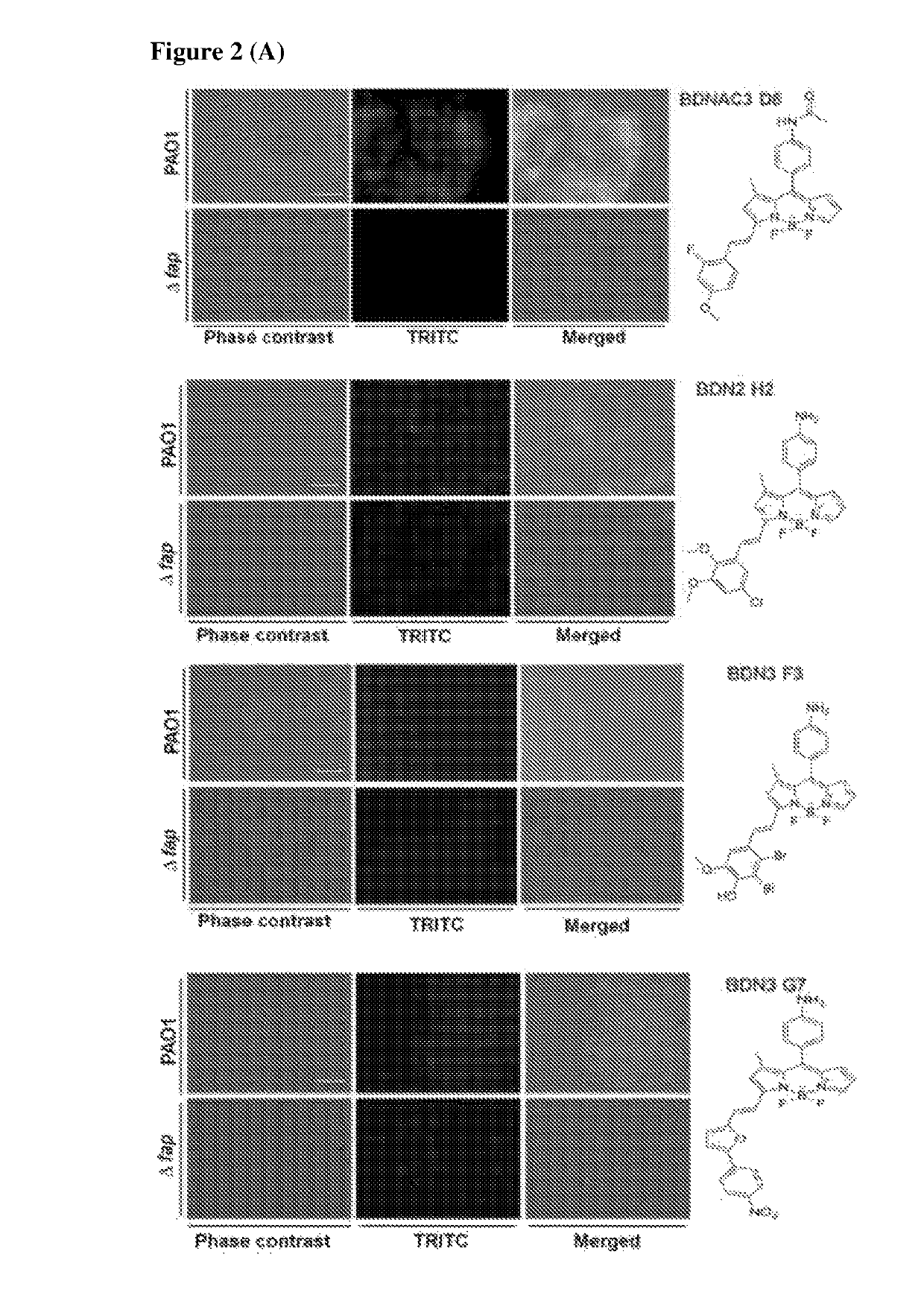
Chemical fluorescent probes for detecting biofilms
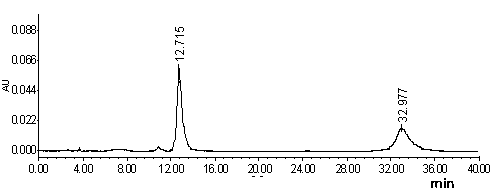
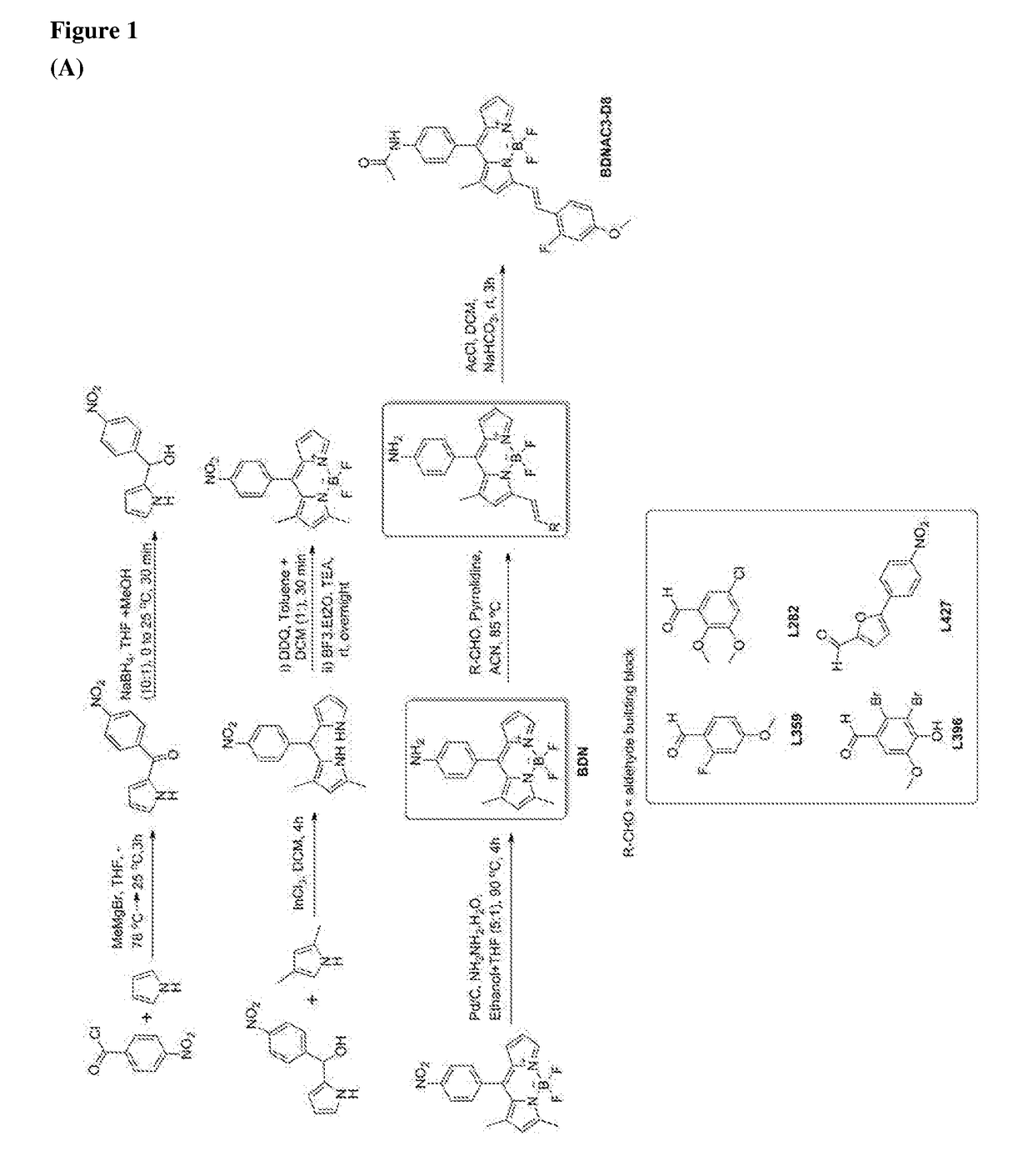
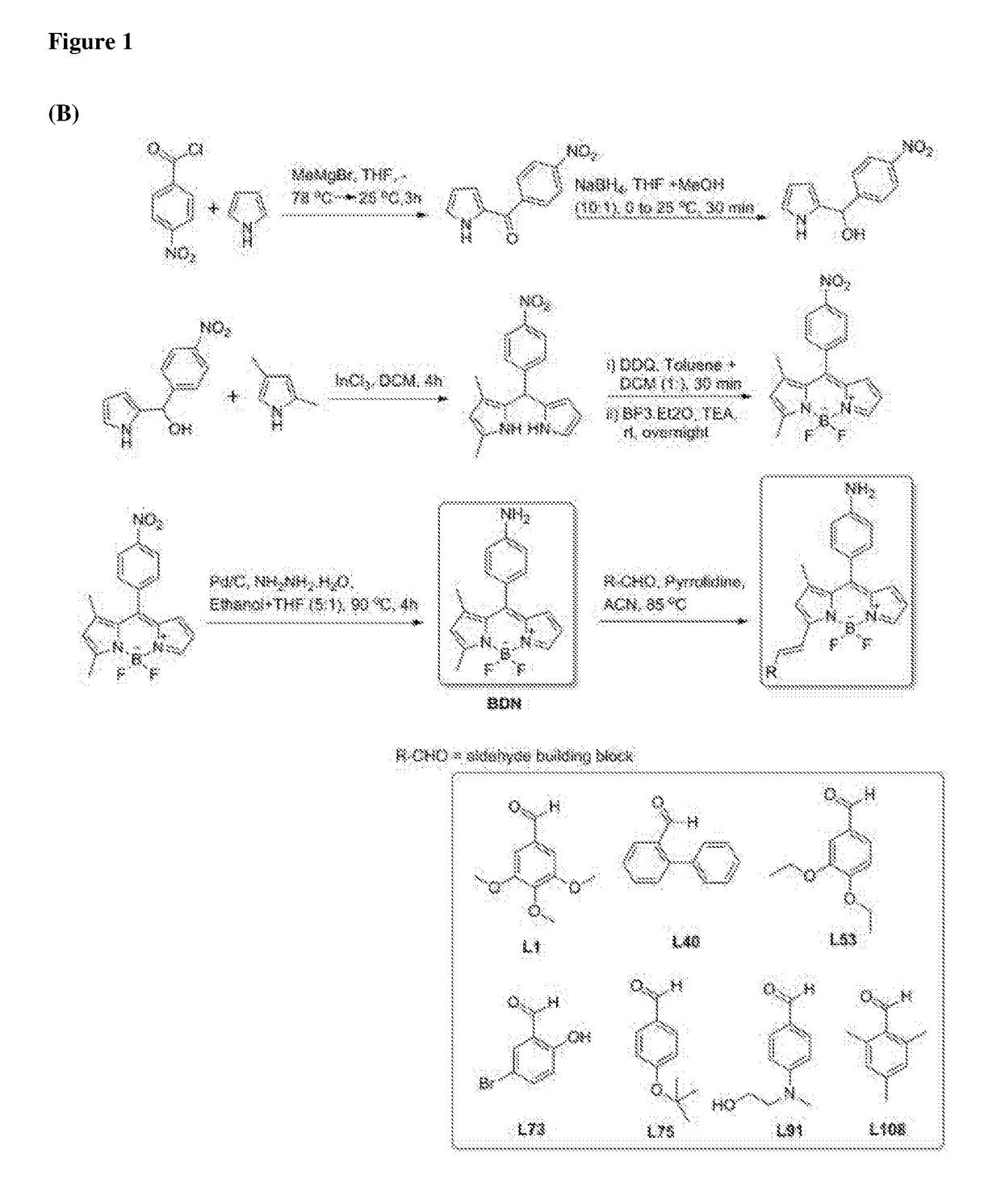
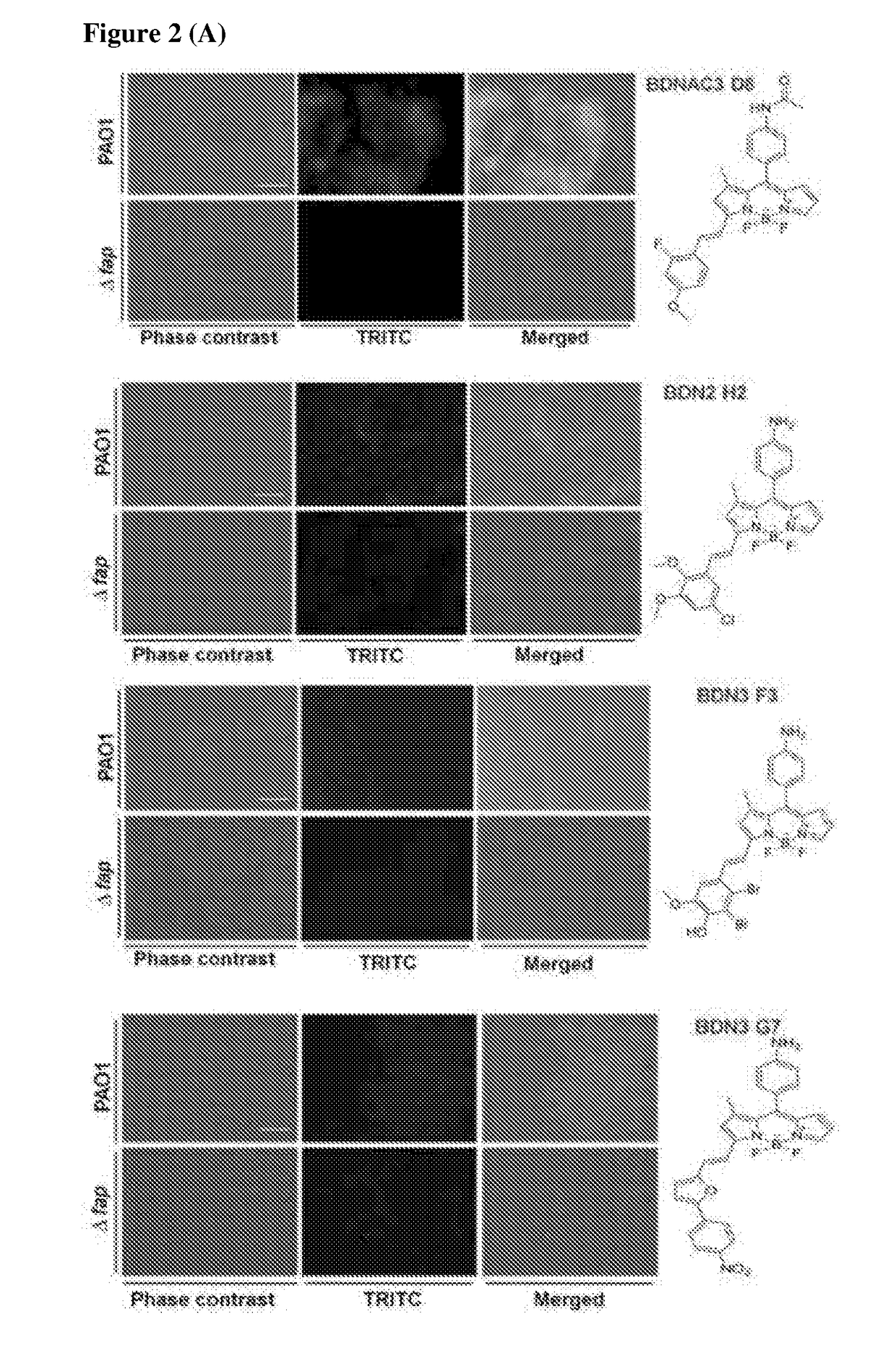
ActiveUS10416153B2Biological material analysisGroup 3/13 element organic compoundsCompound specificBiofilm
The present invention relates to a family of fluorescent compounds based on the BODIPY scaffold, and methods for the preparation of said compounds. The present invention further relates to the use of said compounds for the detection of bacterial biofilms, wherein the bacterial biofilm comprises Pseudomonas aeruginosa and the compound specifically binds to a Fap protein of Pseudomonas aeruginosa, or wherein the compound specifically binds to bacterial cells that contain high levels of cyclic-di-guanosine-monophosphate (c-di-GMP).
Owner:NANYANG TECH UNIV +2
Adenosine deaminase activity determination method and adenosine deaminase diagnosi kit
InactiveCN1769473AStrong specificityLess susceptible to interferenceMicrobiological testing/measurementLength waveGuanosine monophosphate
The invention relates to a method for determining the activity of adenosine deaminase, and also the reagent kit for adenosine deaminase diagnosis. The reagent kit comprises cushioning solution, adenosine, inosine monophosphate, oxidized type coenzyme, guanosine monophosphate reductase, and stabilizer. By mixing sample and reagent of a predetermiend volumetric ratio, generating coupling reaction between them, subjecting the final reactant to biochemiscal analyser, the main wavelength absorbancy variance ratio (speed) can be detected, and the activity of the adenosine deaminase can thus be measured. The method of the invention can be used to obtain the needed measurement result purely through biochemical analytic instruments, and advantages of the method include higher sensibility, better accuracy, less susceptibility to contamination of internal or external materials, and easy application.
Owner:王尔中
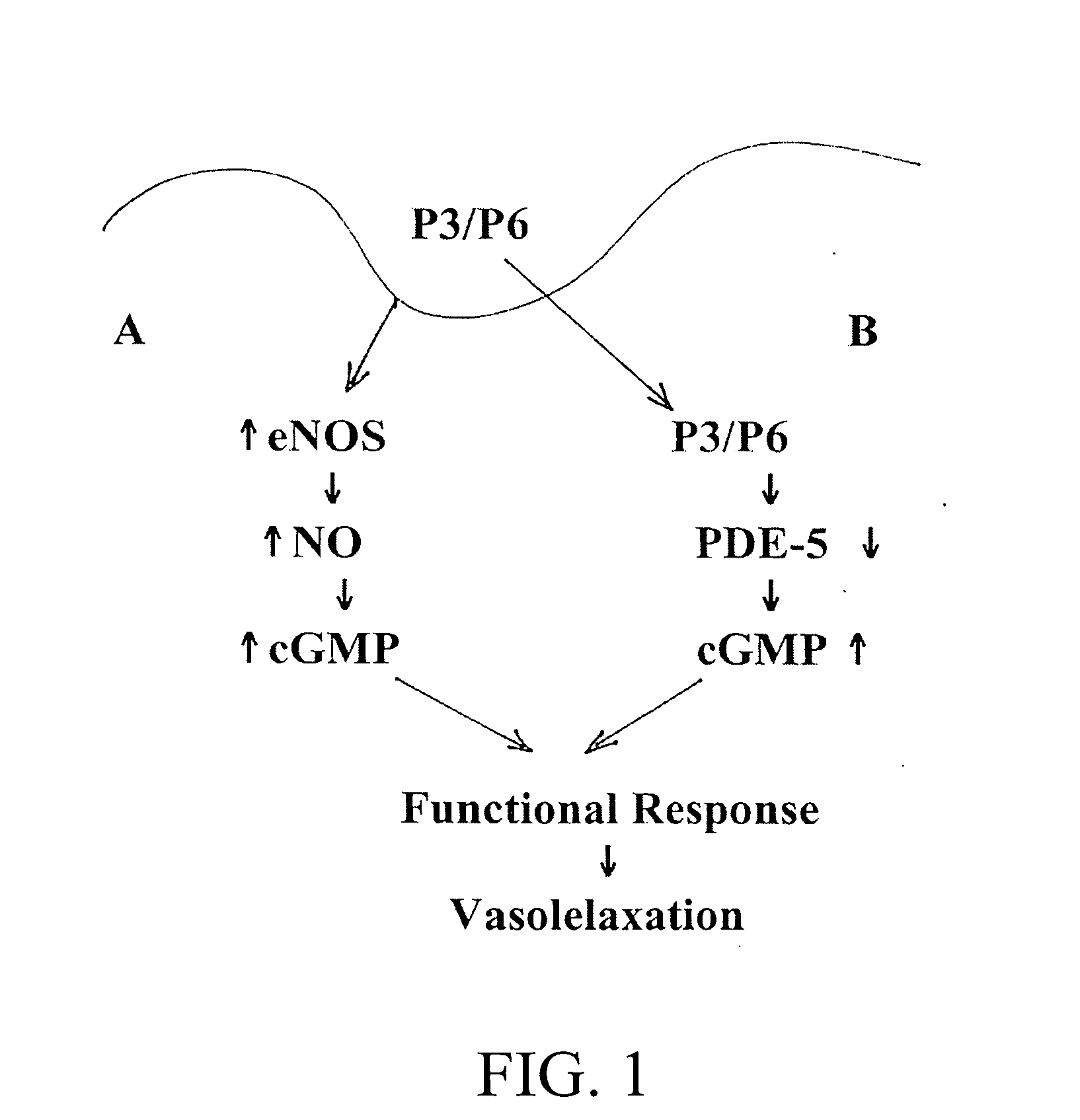
Materials and methods for regulating blood flow
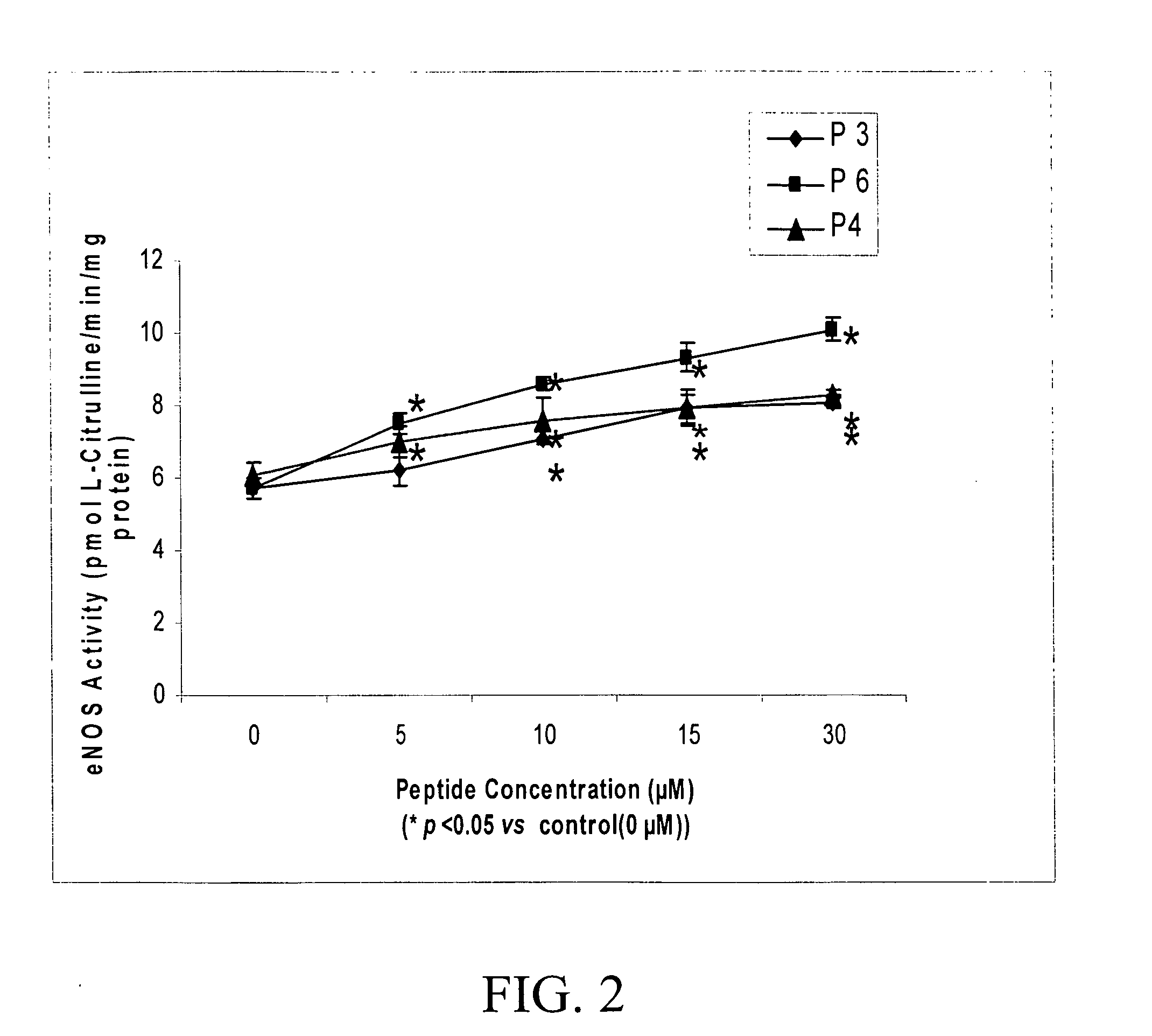
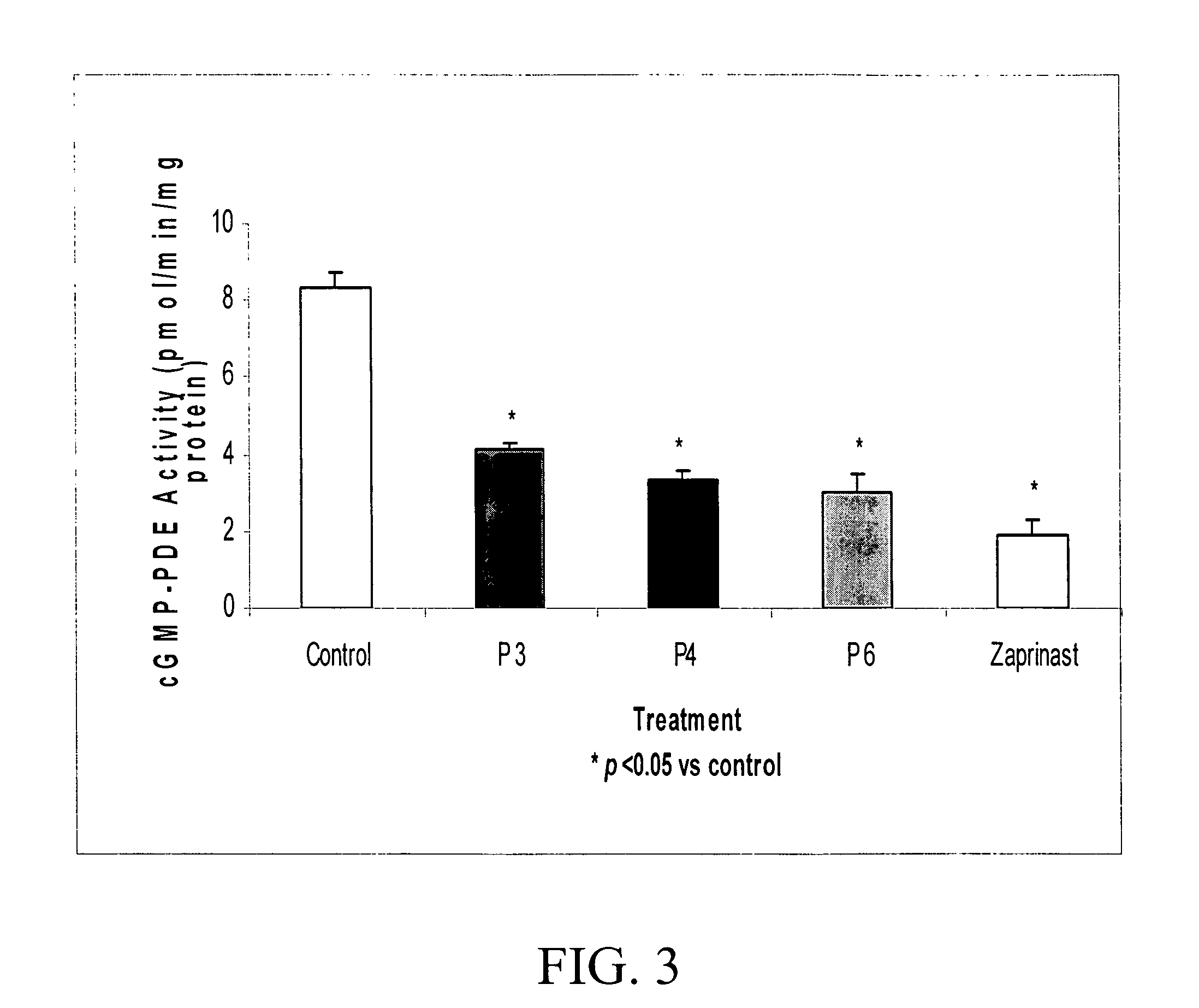
InactiveUS20060258592A1Enhance activity of endothelialReduced activityPeptide/protein ingredientsAntinoxious agentsProviding materialGuanosine monophosphate
The subject invention provides materials and methods useful in regulating blood flow. In a preferred embodiment, the subject invention provides peptides that are able to enhance nitric oxide (NO) activity and / or elevate cellular guanosine monophosphate (cGMP). Through these mechanisms, the subject invention can be used advantageously to effect vasorelaxation, thereby providing therapeutic benefit in a variety of contexts. In a specific embodiment the subject invention provides novel peptides that have been found to enhance the activity of endothelial cell nitric oxide synthase (eNOS). These peptides have also been found to reduce the cGMP-specific phosphodiesterase activity.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Method for extracting cyclic adenosine monophosphate and cyclic guanosine monophosphate from jujube fruits
InactiveCN109651471AImprove extraction efficiencyHigh puritySugar derivativesSugar derivatives preparationAlcoholAdenosine monophosphate.cyclic
The invention discloses a method for extracting cyclic adenosine monophosphate and cyclic guanosine monophosphate from jujube fruits. The method comprises the steps of preparation of crude jujube extracts, alcohol precipitation, macroporous resin chromatographic column separation, membrane separation and preparative liquid chromatography separation. In the method, alcohol is used as an extractingsolution, the three separation ways including macroporous resin chromatographic column separation, membrane separation and preparative liquid chromatography separation are adopted, the cyclic adenosine monophosphate and the cyclic guanosine monophosphate which are obtained by using the method are high in purity, and the purity of the cyclic adenosine monophosphate and the purity of the cyclic guanosine monophosphate reach 90% or above respectively. When the preparative liquid chromatography is applied, on-line monitoring can be achieved, target separation is achieved, and the separation efficiency is high.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Inorganic phosphorus (phosphate radical) diagnosis/determination kit and method for determining inorganic phosphorus (phosphate radical) concentration
InactiveCN101620127AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsInosine kinasePhosphate
The invention relates to an inorganic phosphorus (phosphate radical) diagnosis / determination kit using the technology of an enzyme colorimetric method and an ELISA method, a method for determining inorganic phosphorus (phosphate radical) concentration and composition and components of a reagent, and belongs to the technical field of test and determination of medical science, food, and environment. The kit comprises the following main components: buffer solution, coenzyme, glutamine, adenosine diphoshate, inosine, glutamine synthetase, inosine kinase, guanosine monophosphate reductase and a stabilizer. The method comprises the following steps: mixing a sample and the reagent according to a certain volume ratio to perform a series of enzymic reaction, and then placing reactants under a UV / visible light analyzer to test the ascending speed of absorbance at the position where the dominant wave length is 340nm to determine the inorganic phosphorus (phosphate radical) concentration.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Chemical fluorescent probes for detecting biofilms
ActiveUS20180143185A1Biological material analysisGroup 3/13 element organic compoundsCompound specificBiofilm
The present invention relates to a family of fluorescent compounds based on the BODIPY scaffold, and methods for the preparation of said compounds. The present invention further relates to the use of said compounds for the detection of bacterial biofilms, wherein the bacterial biofilm comprises Pseudomonas aeruginosa and the compound specifically binds to a Fap protein of Pseudomonas aeruginosa, or wherein the compound specifically binds to bacterial cells that contain high levels of cyclic-di-guanosine-monophosphate (c-di-GMP).
Owner:NANYANG TECH UNIV +2
Novel inhibitors of guanosine monophosphate synthetase as therapeutic agents
PendingCN113195470AOrganic active ingredientsOrganic chemistryEnzyme Inhibitor AgentGuanosine monophosphate
Owner:UNIV OF SOUTHERN CALIFORNIA
New human-phosphoguanosine reductase, its coding sequence and application
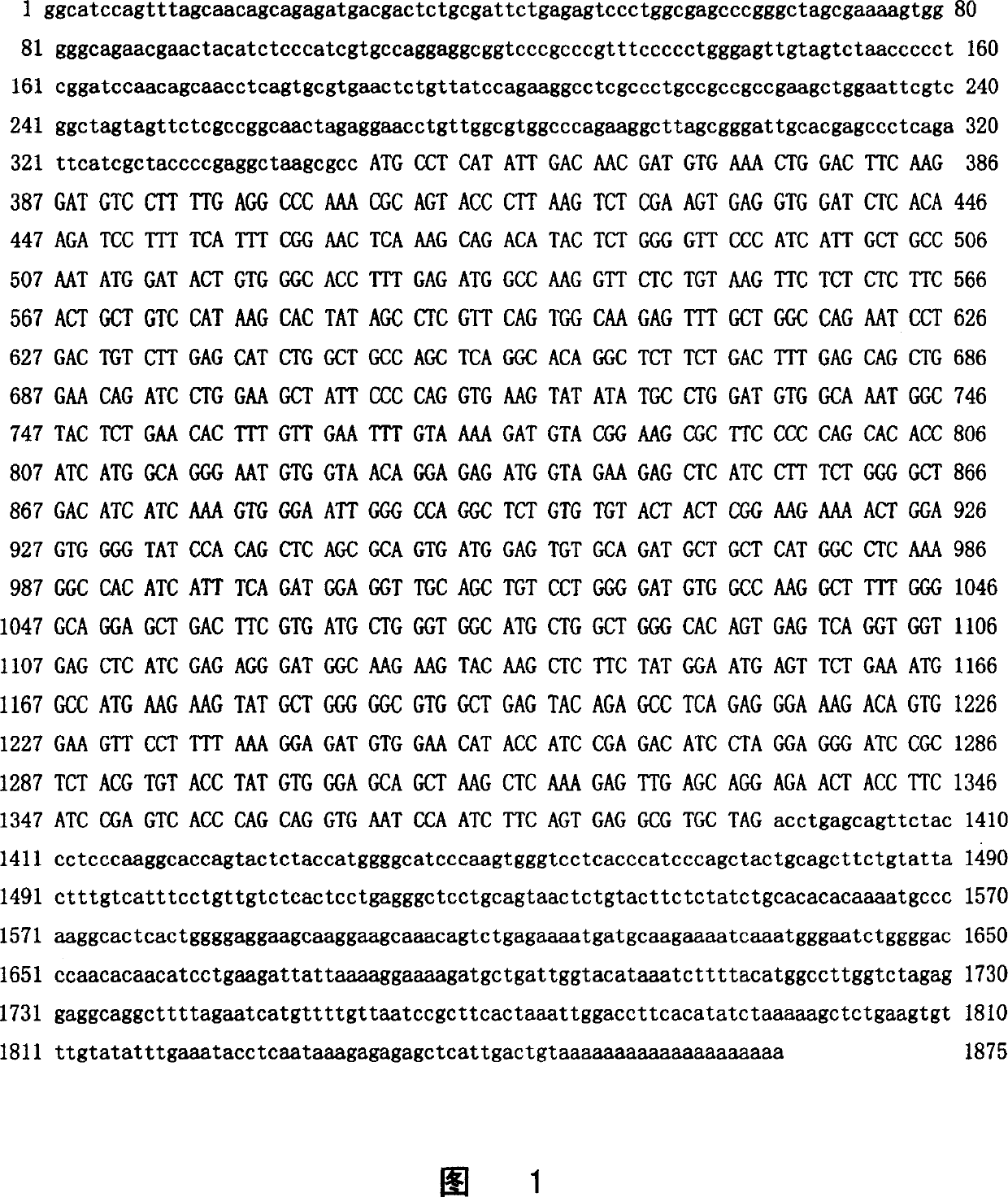
InactiveCN1151251CHigh homologyPromote differentiationSugar derivativesPeptide/protein ingredientsDiseaseProtein molecules
The present invention relates to a novel human guanosine monophosphate reductase GMPR2. Said invention provides the polynucleotide for coding said protein molecule and method for producing this protein molecule by means of DNA recombination technology. It also discloses the application of the polynuleotide coding this novel guanosine monophosphate reductase, dand the zymological acitivity and the relationship of it and tumour cell multiplication and cell differentiation are verified. Said invention also discloses the strategy of resisting said protein molecule for diagnosing and curing diseases, speciallyf ro diagnosing and curing the diseases of tumour, etc.
Owner:第二军医大学免疫学研究所
Homocysteine diagnosis/determination reagent (kit) and homocysteine concentration determination method-982
InactiveCN101762553AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsEnzymatic ColorimetryWavelength
The invention relates to a homocysteine diagnosis / determination reagent (kit) using enzyme-colorimetry and enzyme-linked method technology. Meanwhile, the invention also relates to a homocysteine concentration determination method, reagent compositions and components, which belong to the technical filed of medical test and determination. The reagent (kit) of the invention mainly includes: buffer solution, coenzyme, trimethyl amino acetate salt, inosine monophosphate, betaine-homocysteine methyltransgerase, methionine Gamma-catenase, guanosine monophosphate reductase and stabilizing agent; samples and reagent are mixed according to a certain volume ratio to generate a series of enzymatic reactions; then the reactants are arranged under an ultraviolet / visible light analyzer to detect the increasing degree of absorbance at the 340nm position of a dominant wave so as to measure and calculate the concentration of the homocysteine.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Homocysteine diagnosis kit and method for determining homocysteine concentration
InactiveCN101620174AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsLyaseElisa method
The invention relates to a homocysteine diagnosis kit using the technology of an enzyme colorimetric method and an ELISA method, a method for determining homocysteine concentration, and composition and components of a reagent, and belongs to the technical field of medical test and determination. The kit comprises the following main components: buffer solution, coenzyme, inosine monophosphate, homocysteine-alpha, gamma-lyase, guanosine monophosphate reducase and a stabilizer. The method for determining homocysteine concentration comprises the following steps: mixing a sample and the reagent according to a certain volume ratio to perform a series of enzymic reaction, and then placing reactants under a UV / visible light analyzer to test the ascending degree of absorbance at the position where the dominant wave length is 340nm to determine the concentration of homocysteine.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Seasoning hot pepper for steaming fish and processing method thereof
InactiveCN101617809BGood colorImprove crispnessFood preparationMonosodium glutamateAdditive ingredient
The invention relates to a seasoning hot pepper for steaming fish and a processing method thereof. The seasoning hot pepper is prepared by the steps: taking the desulfurated pickled capsicum frutescens and the pickled hot green pepper to be matched with other ingredients of ginger, mild water with the temperature of 65 DEG C, citric acid, monosodium glutamate, sodium cyclamate, Acesulfame-K, disodium 5'-Inosine monophosphate, disodium 5'-guanosine monophosphate and ethyl maltol; stirring, standing and sterilizing the substances to obtain the seasoning hot pepper. The seasoning hot pepper has stable quality and low content of sulfur dioxide, better retains the fragility, color and lustre of the hot pepper, as well as the nutrition of the hot pepper, and has longer time and shelf life.
Owner:CHANGSHA TANTANXIANG FLAVORING FOOD +1
Determination method for ammonia (ammonia ions) and ammonia (ammonia ion) diagnostic/assay kit
InactiveCN102565364AMicrobiological testing/measurementColor/spectral properties measurementsGuanylate kinaseGuanosine monophosphate
The invention relates to a determination method for the content of ammonia (ammonia ions) by using an enzymatic colorimetric technology and an enzymatic couple reaction and to composition and components of a reagent. A technical principle used in the method is that determination is realized through series reactions of ammonia kinase, guanylate kinase and guanosine monophosphate reductase. The invention also relates to an ammonia (ammonia ion) diagnostic / assay kit. The determination method provided in the invention has high sensitivity and a small error; the determination method and the kit in the invention can be widely applied in clinical medicine / food inspection.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Homocysteine diagnosing/measuring reagent (kit) and homocysteine concentration measuring method
InactiveCN101750302AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsAdditive ingredientLyase
The invention relates to a homocysteine diagnosing / measuring reagent (kit) using the technologies of an enzyme colorimetric method and an enzyme linkage method. At the same time, the invention also relates to a homocysteine concentration measuring method, the reagent composition and reagent ingredients, which belong to the technical field of medical detection and measurement. The reagent (kit) mainly comprises the following ingredients: a buffer solution, cozymase, dimethyl sulfonium-based acetate, inosine monophosphate, thetine-homocysteine methyltransferase, methionine gama-lyase, guanosine monophosphate reductase and stabilizing agents. The method has the following steps: mixing samples and the reagent by the certain volume percent for making the samples and the reagent take a series of enzymatic reaction, and then, placing reactants under an ultraviolet / visible light analyzer to detect the rising degree of the light absorbancy in the position with the main wave length of 340 nm, so the concentration of the homocysteine can be measured and calculated.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Crystallizing method of 5'-guanosine-monophosphate disodium salt
ActiveCN101863943BEasy to separateImprove solubilitySugar derivativesSugar derivatives preparationAlcoholGuanosine monophosphate
The invention discloses a crystallizing method of a 5'-guanosine-monophosphate disodium salt, which has the advantages of simple operation as well as large crystalline particle, uniform particle, attractive fineness and high purity of products. The method comprises the following steps of: adding sodium hydroxide to a 5'-GMP aqueous solution of which the concentration is 5-40 percent; regulating the pH value of the solution to 7-11 and then adding absolute alcohol which has a pH value of 7-11 and is 0.5-3 times of the solution in volume and a catalyst; stirring at 15-80 DEG C to separate out 5'-GMP, 2Na; and centrifuging, washing and drying to obtain 5'-GMP, 2Na crystals, wherein the catalyst is a mixture of a 60-120 mesh siftage of 5'-guanosine-monophosphate disodium salt crystals and sodium carbonate, the mass ratio of the 60-120 mesh siftage of 5'-guanosine-monophosphate disodium salt crystals to the sodium carbonate is 1:2, and the addition quantity of the catalyst is 0.1-0.2 percent of the mass of the 5'-GMP.
Owner:大连珍奥生物技术股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com