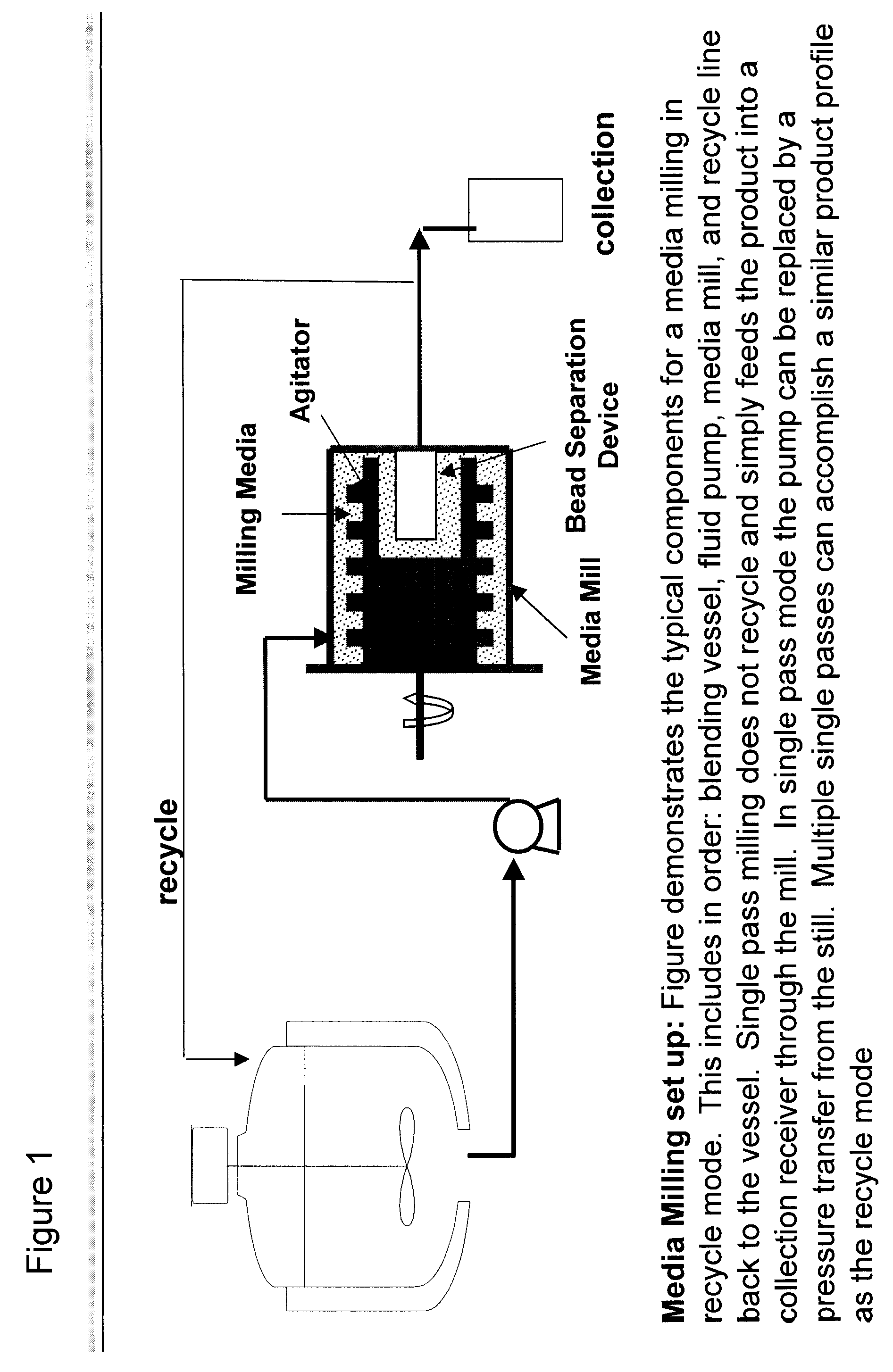
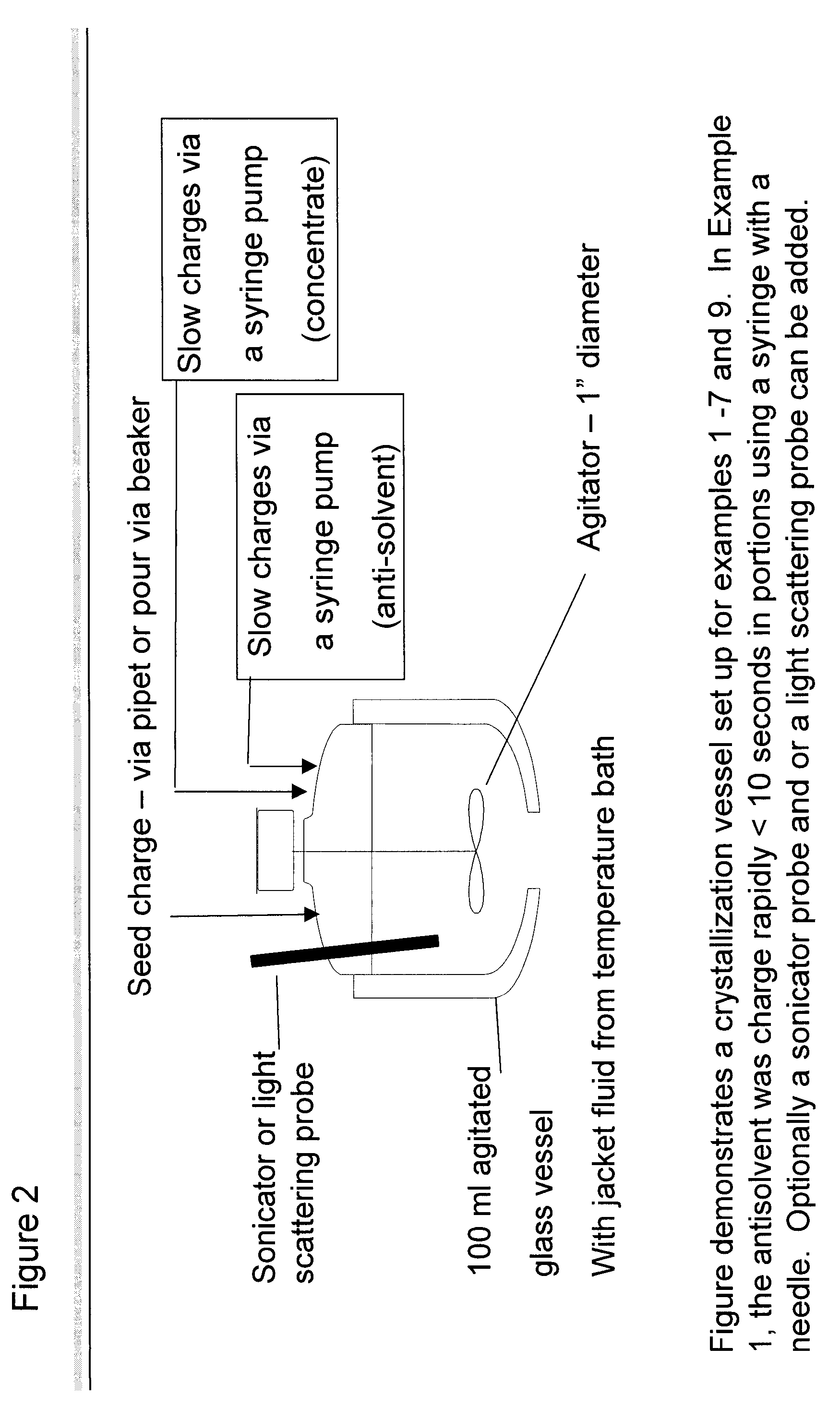
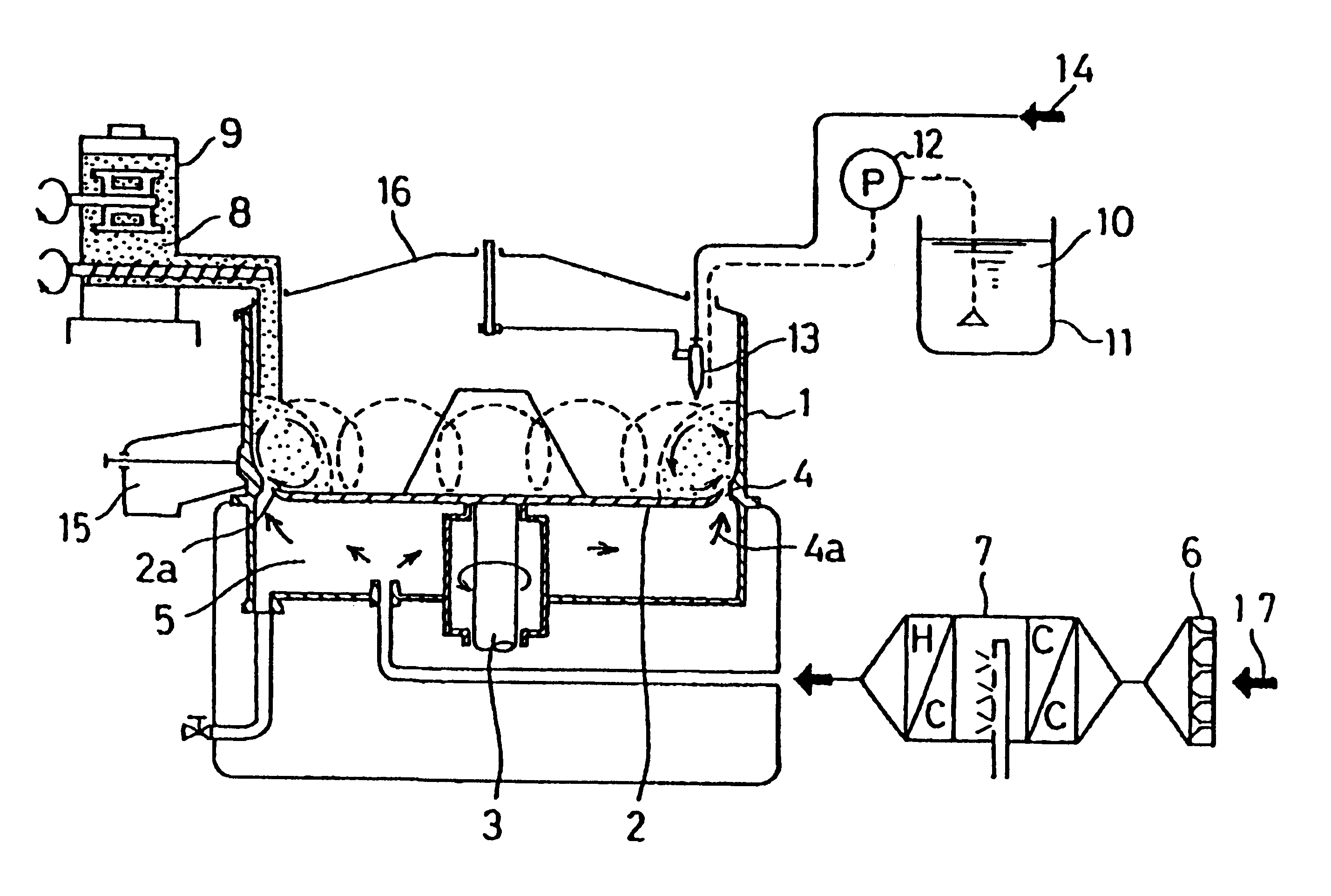
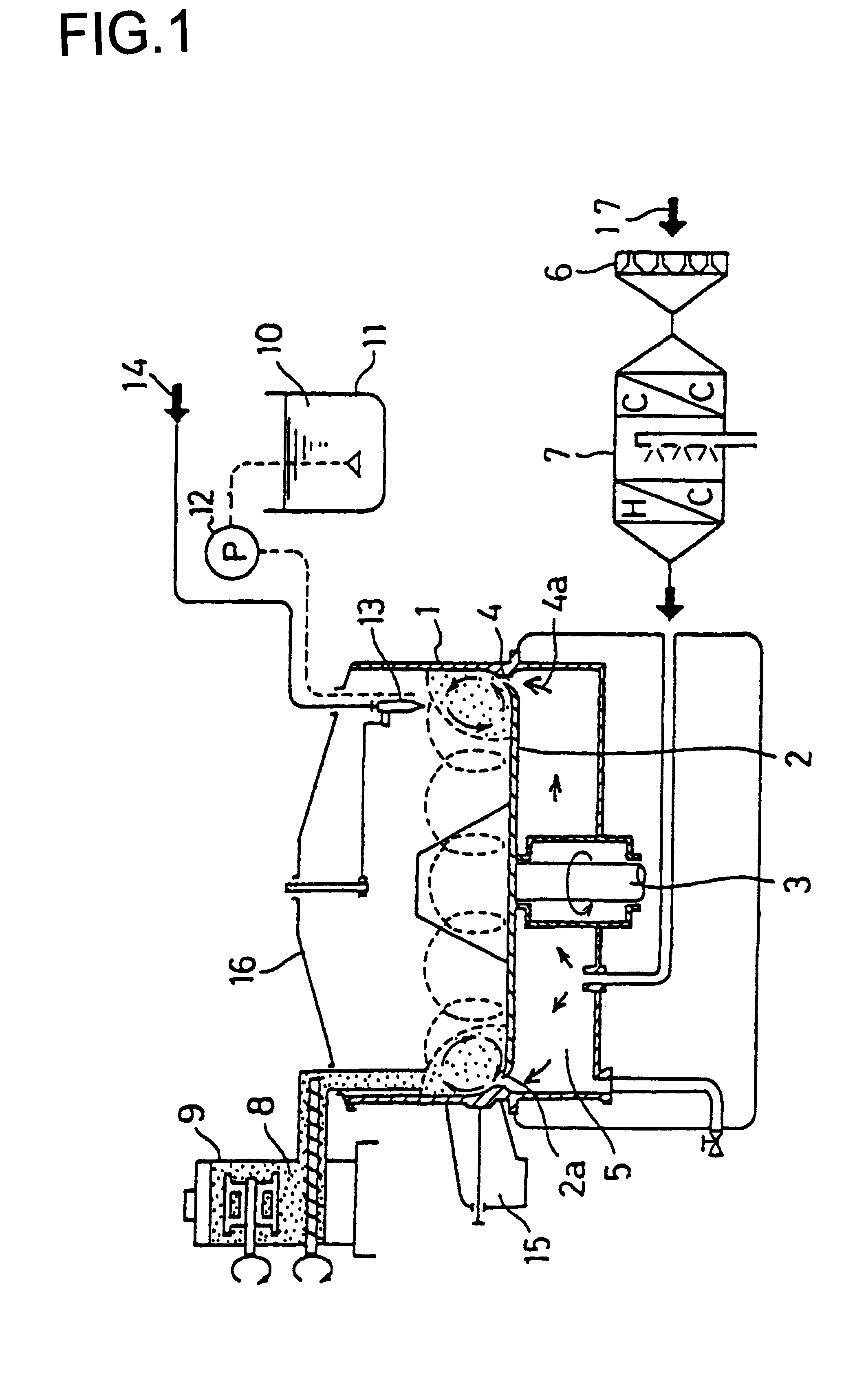
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
580 results about "Crystalline particle" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
: completely crystalline : made up wholly of crystals or crystalline particles —used of a rock (as granite)
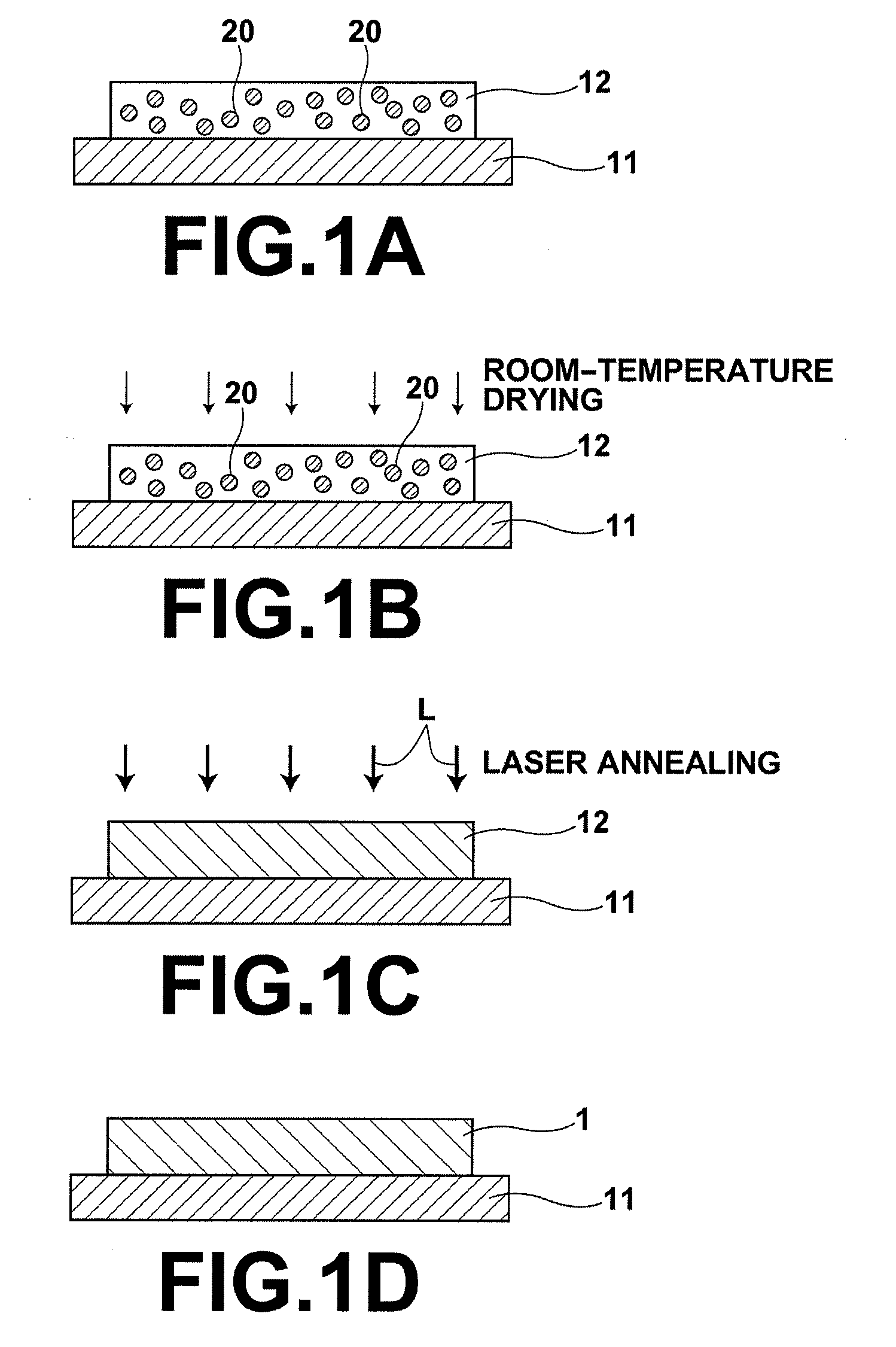
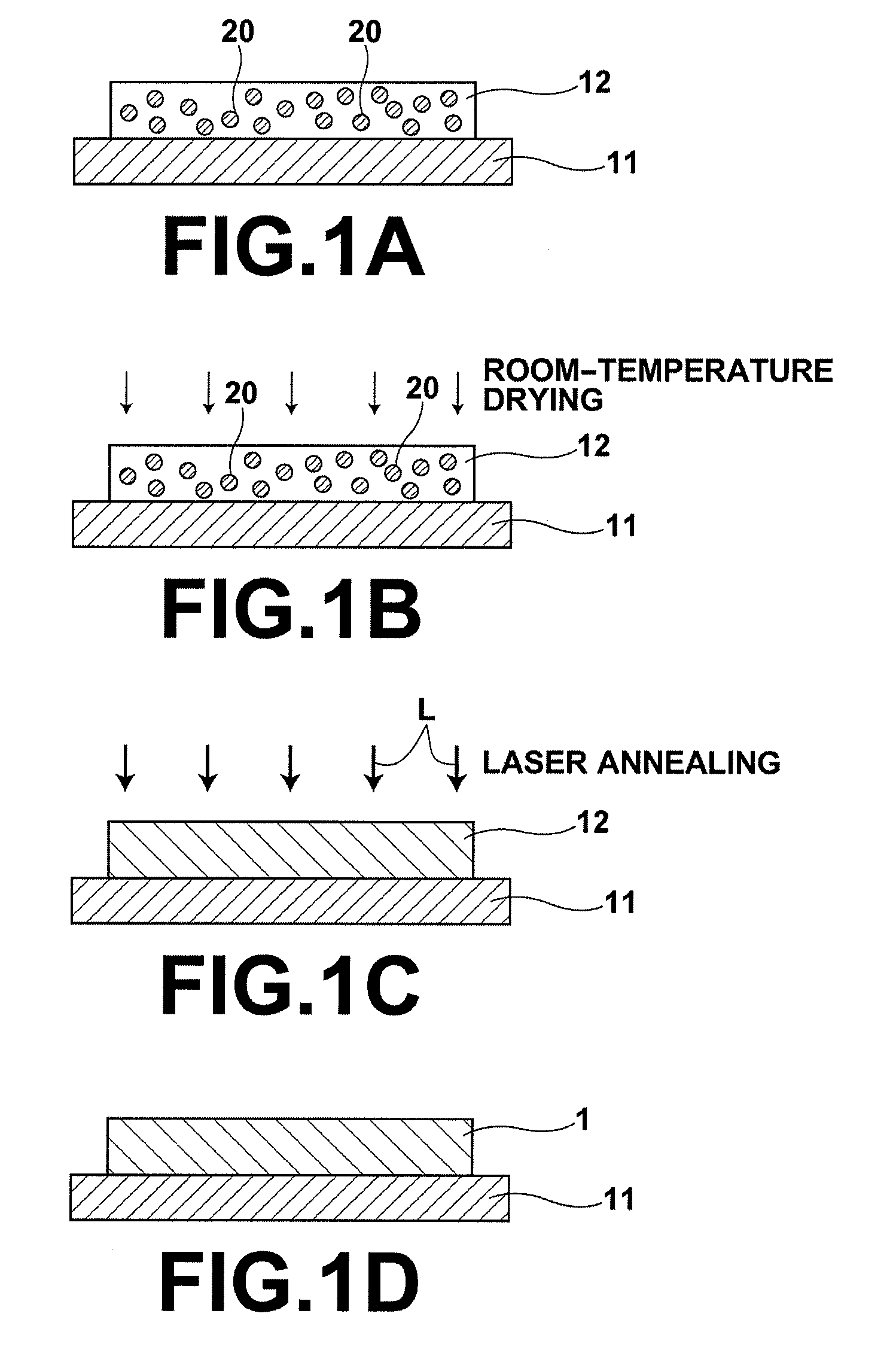
Process for producing oriented inorganic crystalline film, and semiconductor device using the oriented inorganic crystalline film
ActiveUS20090152506A1Orientation can be controlledLow costFrom gel stateFrom solid stateOrganic solventDevice material
In a process for producing an oriented inorganic crystalline film, a non-monocrystalline film containing inorganic crystalline particles is formed on a substrate by a liquid phase technique using a raw-material solution which contains a raw material and an organic solvent, where the inorganic crystalline particles have a layered crystal structure and are contained in the raw material. Then, the non-monocrystalline film is crystallized by heating the non-monocrystalline film to a temperature equal to or higher than the crystallization temperature of the non-monocrystalline film so that part of the inorganic crystalline particles act as crystal nuclei.
Owner:FUJIFILM CORP
Process for producing oriented inorganic crystalline film, and semiconductor device using the oriented inorganic crystalline film
ActiveUS8202365B2Orientation can be controlledLow costFrom gel stateFrom solid stateOrganic solventCrystal structure
In a process for producing an oriented inorganic crystalline film, a non-monocrystalline film containing inorganic crystalline particles is formed on a substrate by a liquid phase technique using a raw-material solution which contains a raw material and an organic solvent, where the inorganic crystalline particles have a layered crystal structure and are contained in the raw material. Then, the non-monocrystalline film is crystallized by heating the non-monocrystalline film to a temperature equal to or higher than the crystallization temperature of the non-monocrystalline film so that part of the inorganic crystalline particles act as crystal nuclei.
Owner:FUJIFILM CORP
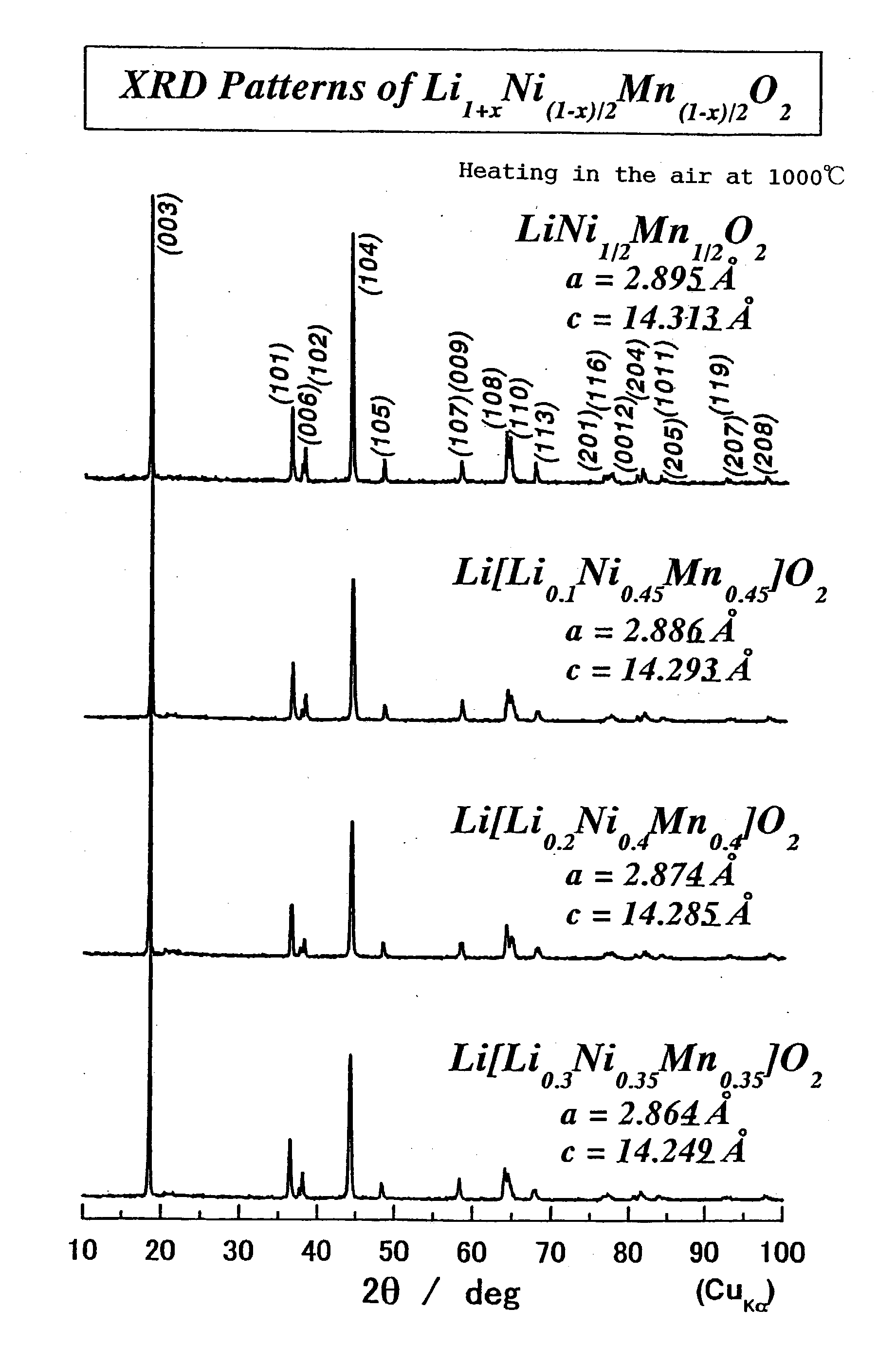
Positive-electrode active material and nonaqueous-electrolyte secondary battery containing the same
InactiveUS20030170540A1Improve the immunityLarge ion permeabilityIron oxides/hydroxidesElectrode thermal treatmentCrystal structureOxygen
The present invention provides a high-capacity and low-cost non-aqueous electrolyte secondary battery, comprising: a negative electrode containing, as a negative electrode active material, a ssubstance capable of absorbing / desorbing lithium ions and / or metal lithium; a separator; a positive electrode; and an electrolyte, wherein the positive electrode active material contained in the positive electrode is composed of crystalline particles of an oxide containing two kinds of transition metal elements, the crystalline particles having a layered crystal structure, and oxygen atoms constituting the oxide forming a cubic closest packing structure.
Owner:PANASONIC CORP +1
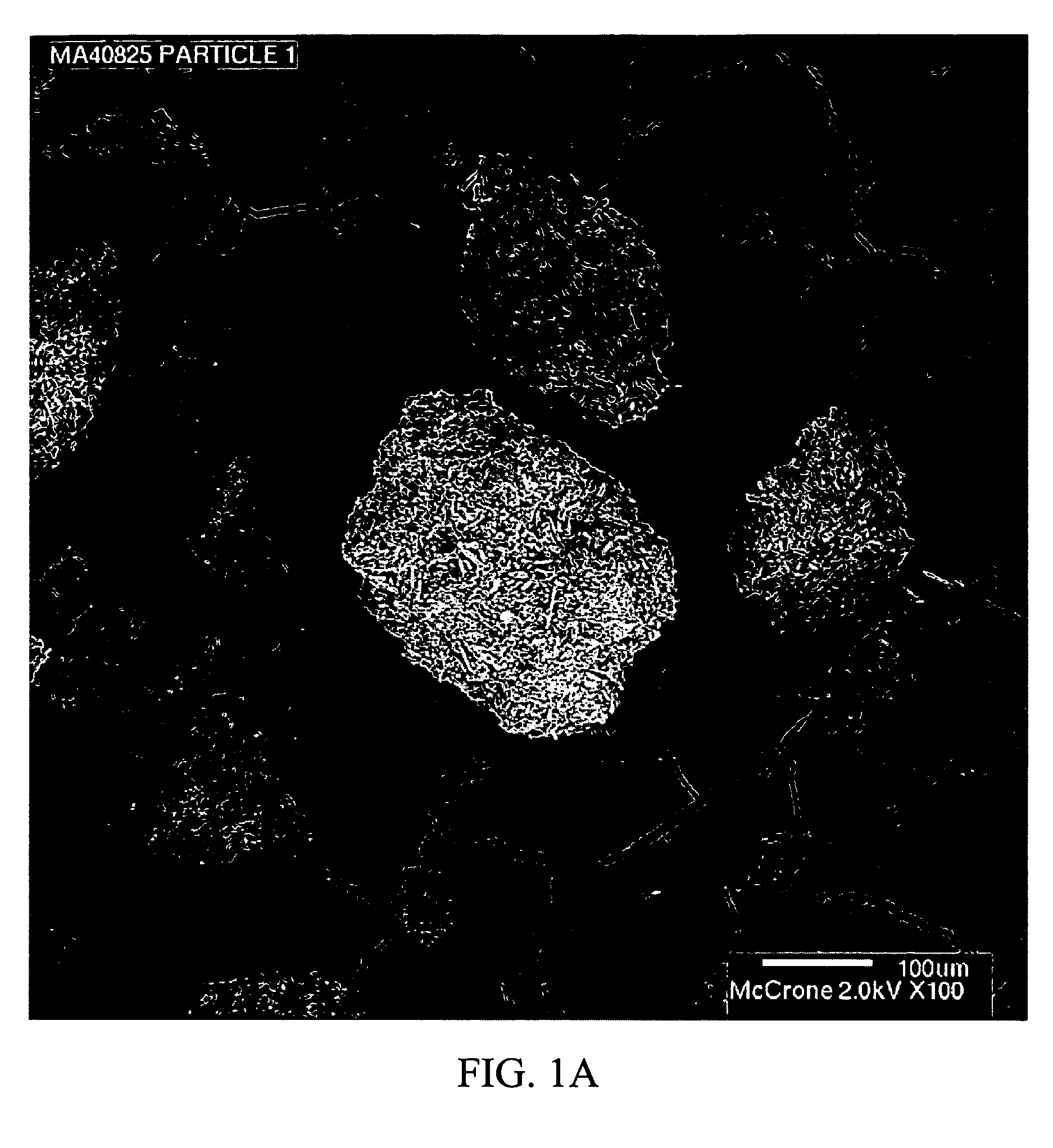
Processes and Apparatuses for the Production of Crystalline Organic Microparticle Compositions by Micro-Milling and Crystallization on Micro-Seed and Their Use
InactiveUS20090087492A1Large specific surface areaIncrease ratingsBiocideNervous disorderMicroparticleOrganic compound
The present invention relates to a process, for the production of crystalline particles of an active organic compound The process includes the steps of generating a micro-seed by a wet-milling process and subjecting the micro-seed to a crystallization process. The resulting crystalline particles have a mean particle size of less than about 100 μm. The present invention also provides for a pharmaceutical composition which includes the crystalline particles produced by the method described herein and a pharmaceutically acceptable carrier.
Owner:MERCK SHARP & DOHME CORP
Spherical single-substance particles, medicines and foodstuffs containing the particles, and method of production thereof
The present invention relates to a process for producing a spherical particle comprising an aggregate of particles containing at least 95% of a water-soluble single substance having a viscosity of 10 mPa.s or less as determined in the form of a saturated aqueous solution, the process comprising: preparing moist spherical particles of the single substance by charging, as cores, crystalline particles or granulated particles of the single substance on a rotary disc in a processing vessel of a centrifugal tumbling granulating apparatus, wherein the granulated particles are prepared by granulating a powder of the single substance, and dispersing over the cores a powder of the single substance and simultaneously spraying on the cores a liquid such as water or the like while supplying slit air to provide a fluidized condition; and then fixation treating the moist spherical particles by drying them while spraying an aqueous solution of the single substance or the like on the spherical particles in a fluidized bed apparatus; to the spherical particle produced by the process; and to a pharmaceutical preparation and a food containing the spherical particle.
Owner:FREUNT IND +1
Use of Nanocrystals for Drug Delivery from a Balloon
ActiveUS20110008260A1Increases drug carrying capacityIncrease capacityUltrasonic/sonic/infrasonic diagnosticsBiocideReady to useNanocrystal
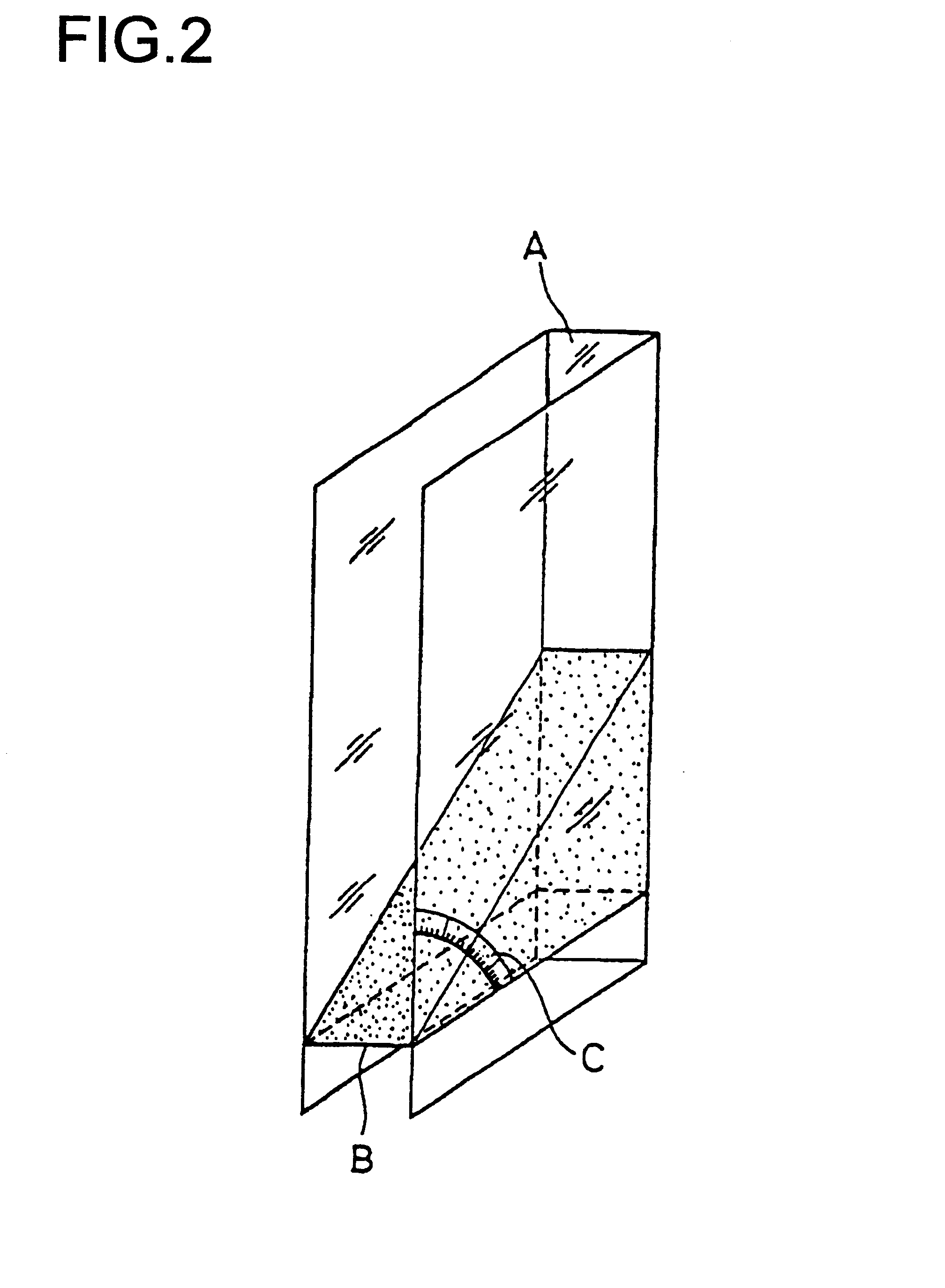
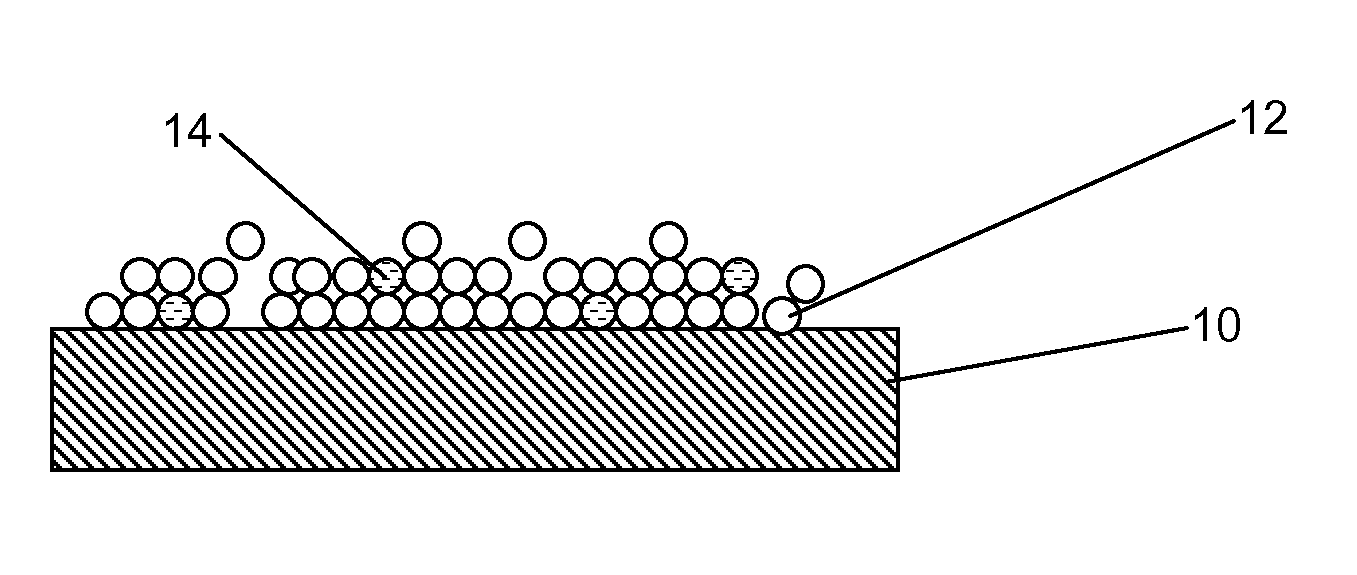
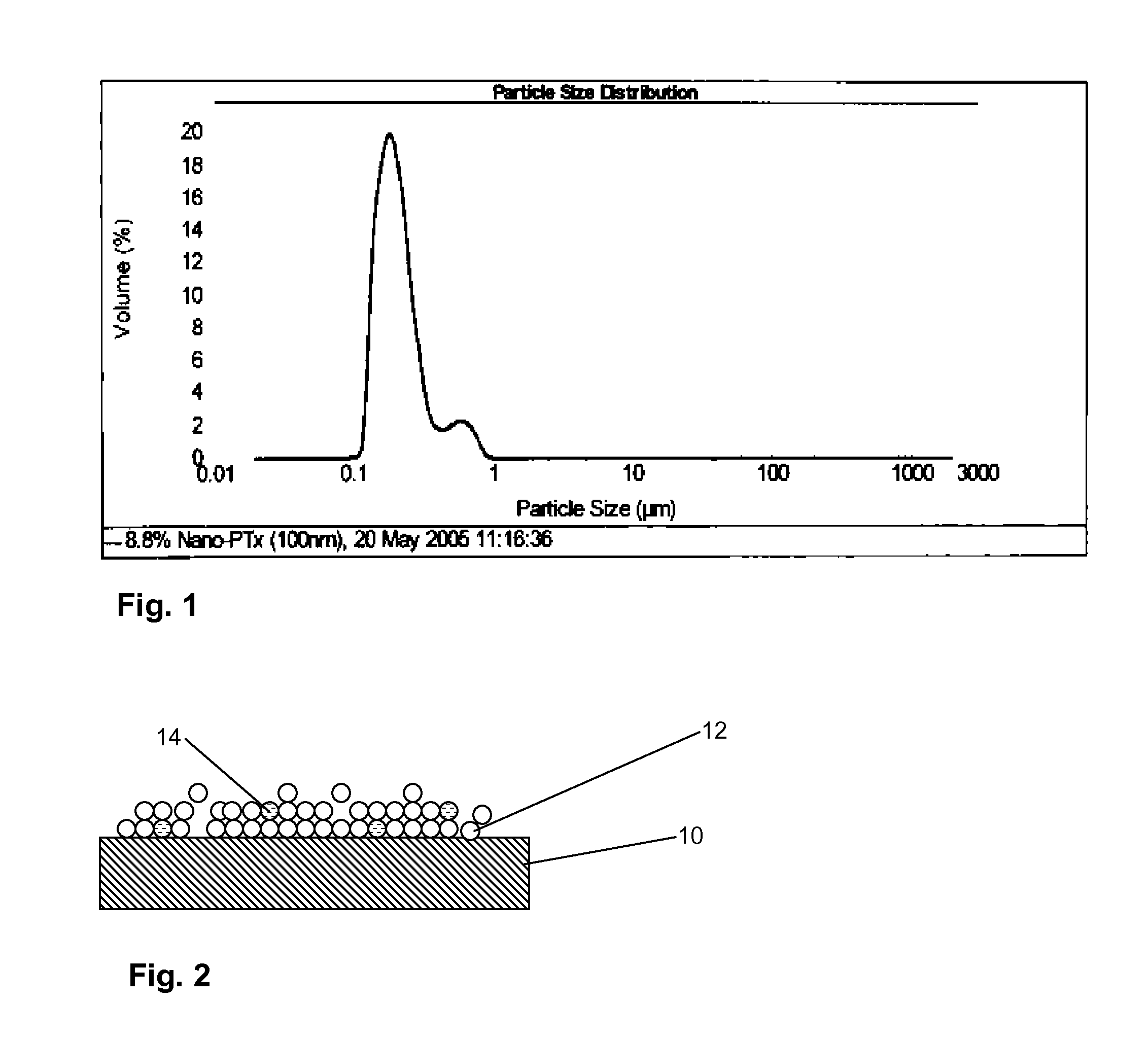
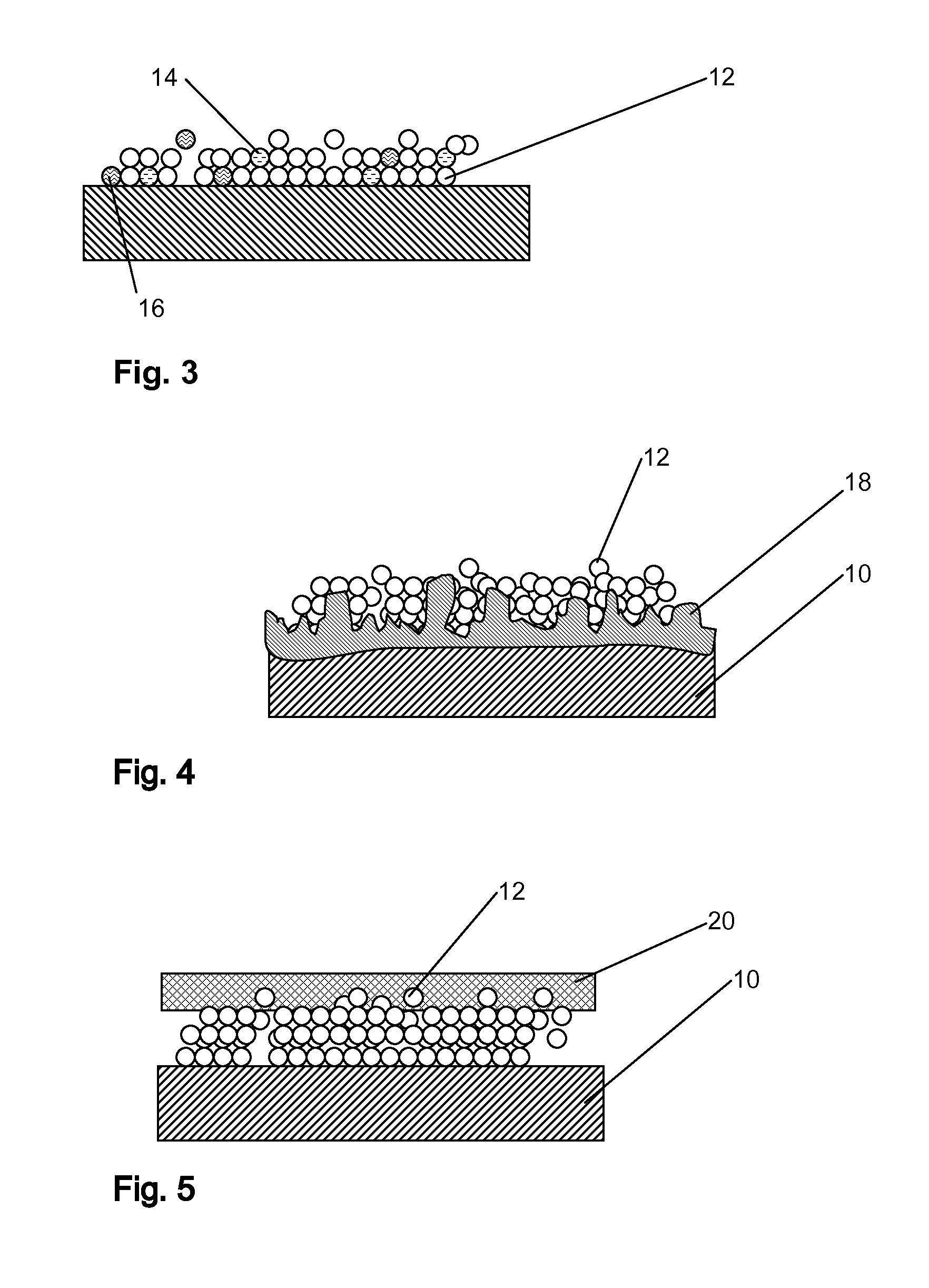
A drug delivery balloon (10) has a drug thereon in the form of crystalline particles (12), the drug having a predetermined size distribution. Optionally marker particles (14, 16) are also provided. A texturized coating (18), a cap layer (20) and / or other methods may be used to increase particle loading capacity of the balloon.
Owner:BOSTON SCI SCIMED INC
Long-cycle and high-safety power lithium ion battery positive electrode material and preparation method thereof
InactiveCN105406056AUniform dispersion and depositionEvenly dispersedCell electrodesSecondary cellsElectrolytic agentElectrical battery
The invention discloses a long- cycle and high-safety power lithium ion battery positive electrode material and a preparation method thereof. The positive electrode material can be shown as a general formula LiNi<(1-a-b-c)>CoMnM<c>O2.xLiM<1>O<y>, wherein a is greater than 0 and less than 1, b is greater than 0 and less than 1, a+b+c is greater than 0 and less tan 1, x is greater than 0 and less than 0.1, and y is greater than 1 and less than 5; LiNi<(1-a-b-c)>CoMnM<c>O2 is a primary active crystalline particle of the positive electrode material, and is a lithiated composite oxide composed of nickel cobalt manganese and doping element M; and the LiM<1>O<y> is a coating layer coating crystal boundary of the primary active crystalline particle and surface of a polycrystalline secondary particle. The positive electrode material provided by the invention has high stability of crystalline main body, surface and crystal boundary, has good compatibility with electrolyte and is not liable to generate side reaction. The material provided by the invention is used for batteries, has long cycle life, high over-charging resisting capability, good high temperature and high voltage performance and high integral safety, and is particularly suitable for power batteries.
Owner:HUNAN SOUNDDON NEW ENERGY
Thin film transistor and method for manufacturing the same
InactiveUS8119468B2High purityImprove featuresTransistorSolid-state devicesAmorphous siliconCrystalline particle
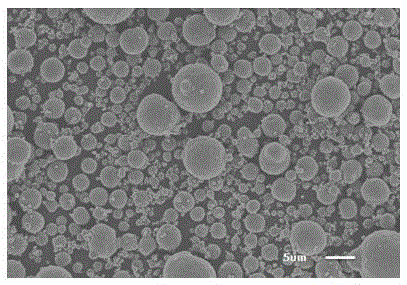
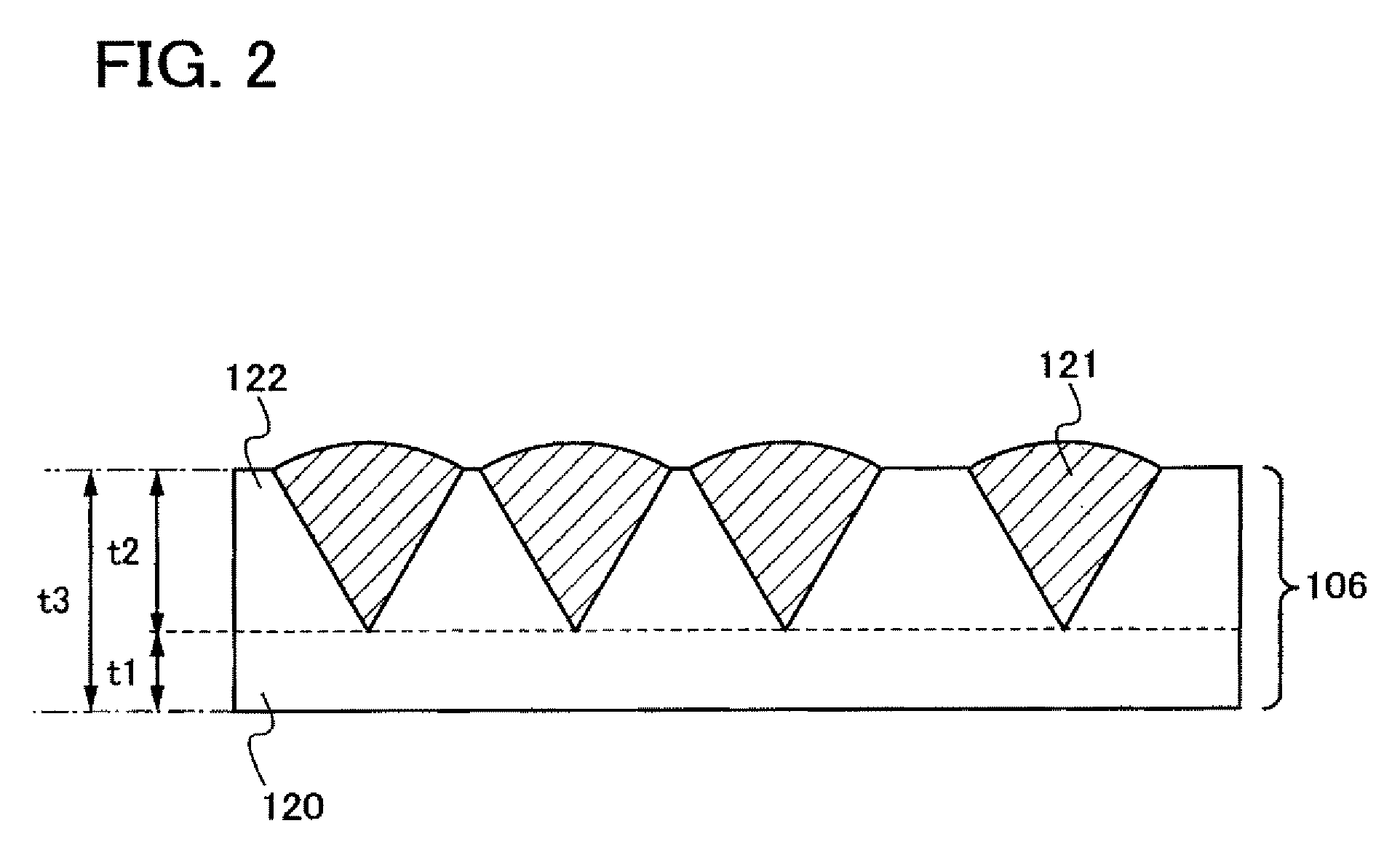
Disclosed is a thin film transistor which includes, over a substrate having an insulating surface, a gate insulating layer covering a gate electrode; a semiconductor layer which functions as a channel formation region; and a semiconductor layer including an impurity element imparting one conductivity type. The semiconductor layer exists in a state that a plurality of crystalline particles is dispersed in an amorphous silicon and that the crystalline particles have an inverted conical or inverted pyramidal shape. The crystalline particles grow approximately radially in a direction in which the semiconductor layer is deposited. Vertexes of the inverted conical or inverted pyramidal crystal particles are located apart from an interface between the gate insulating layer and the semiconductor layer.
Owner:SEMICON ENERGY LAB CO LTD
Luminescence crystal particle, luminescence crystal particle composition, display panel and flat-panel display
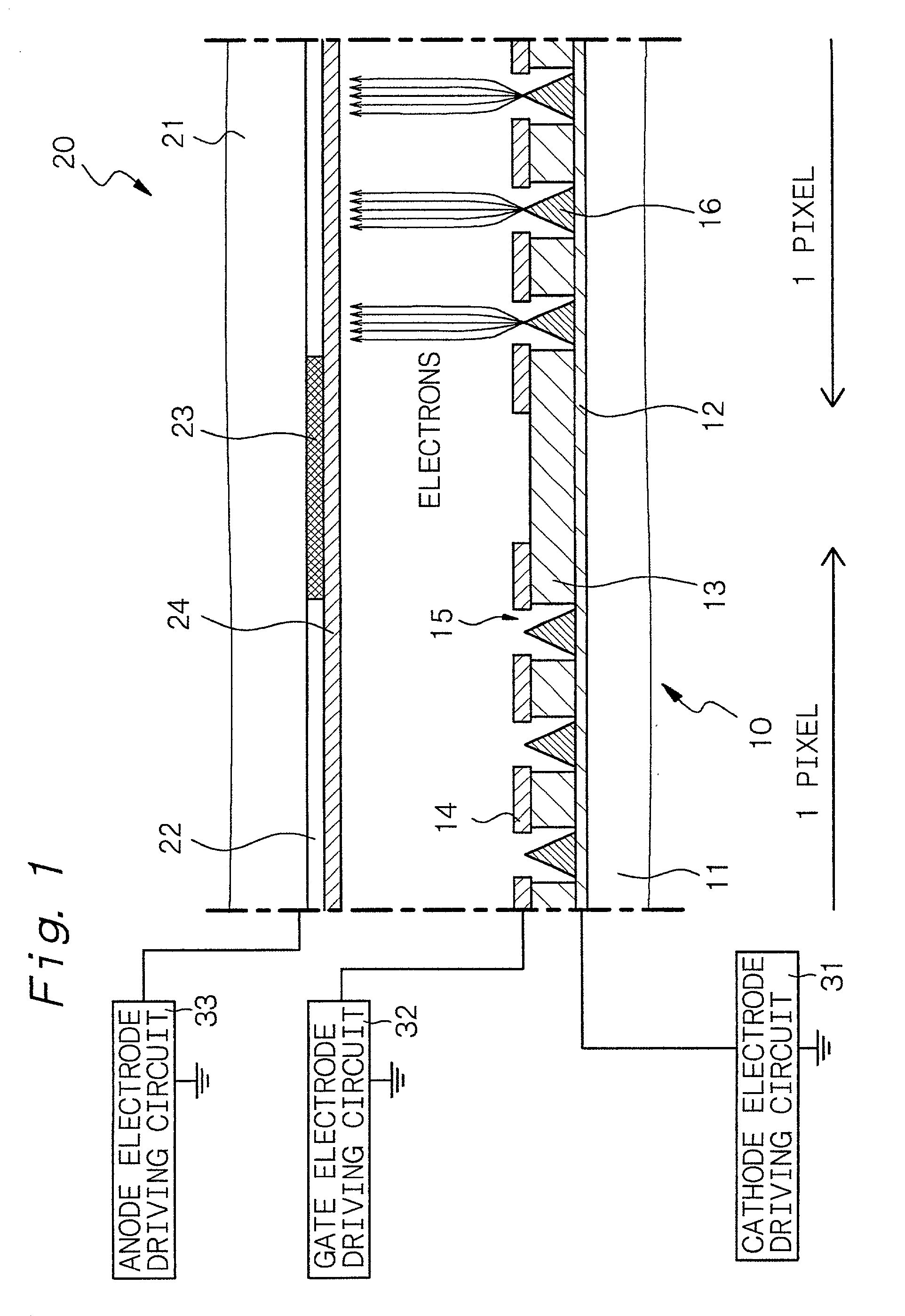
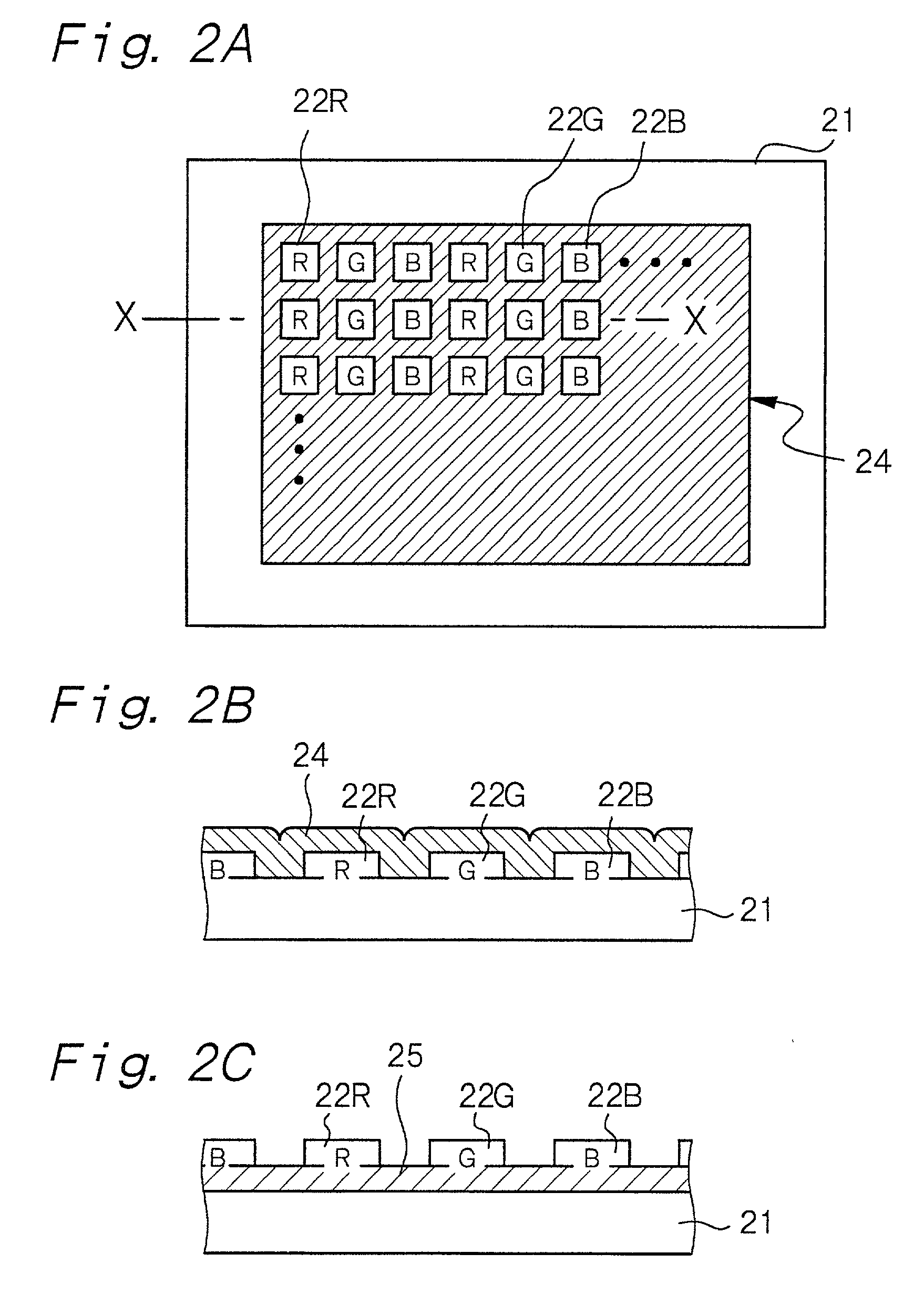
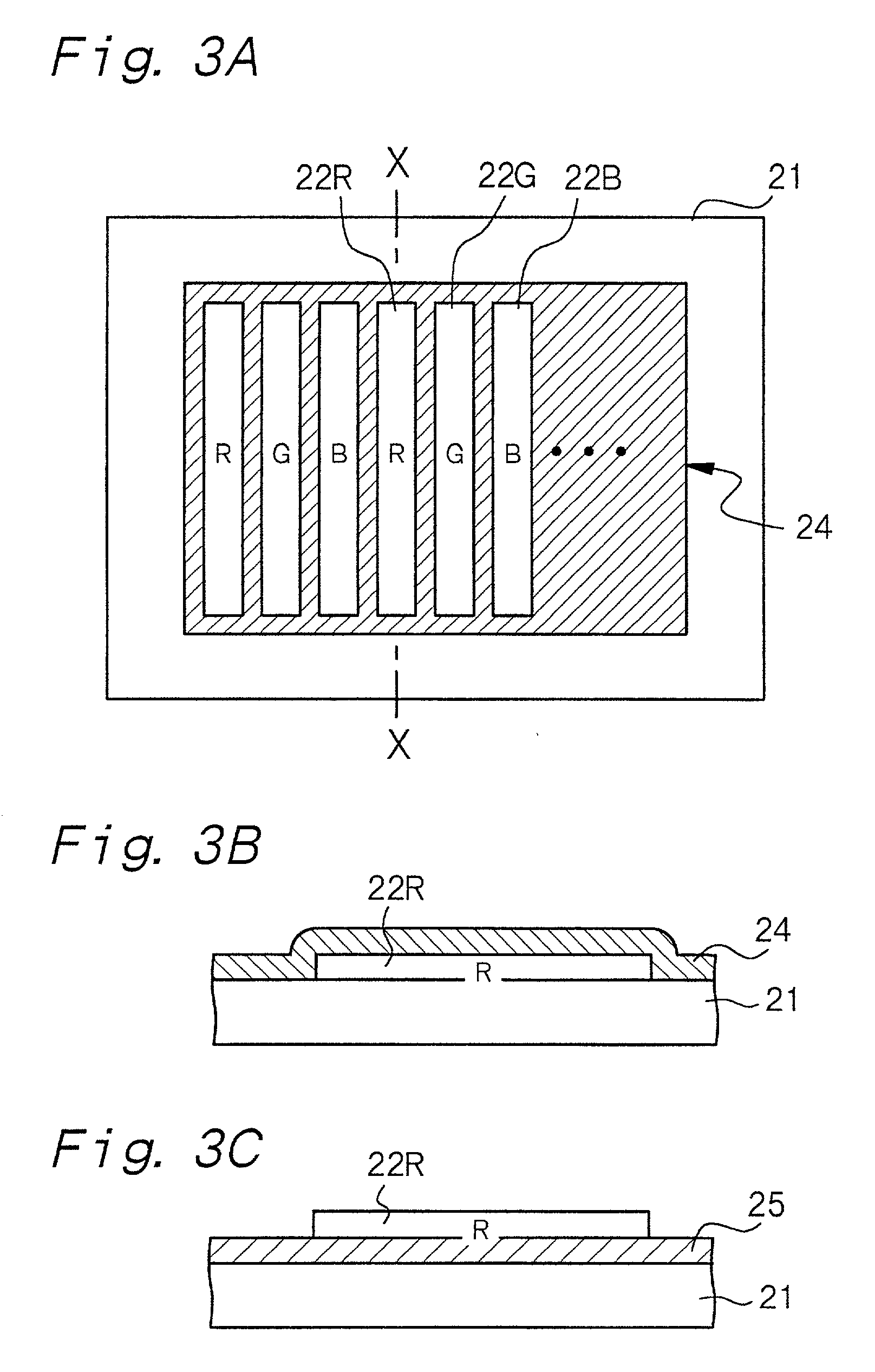
InactiveUS20010024084A1Uniform electron-emitting propertyImprove adhesionDischarge tube luminescnet screensGas discharge electrodesDisplay deviceVolumetric Mass Density
A luminescence crystal particle which emits light upon irradiation with an energy beam and which has a crystal defect density of 5x107 defects / cm2 or less in a region located from the surface of the luminescence crystal particle to a portion as deep as the energy beam reaches.
Owner:SONY CORP
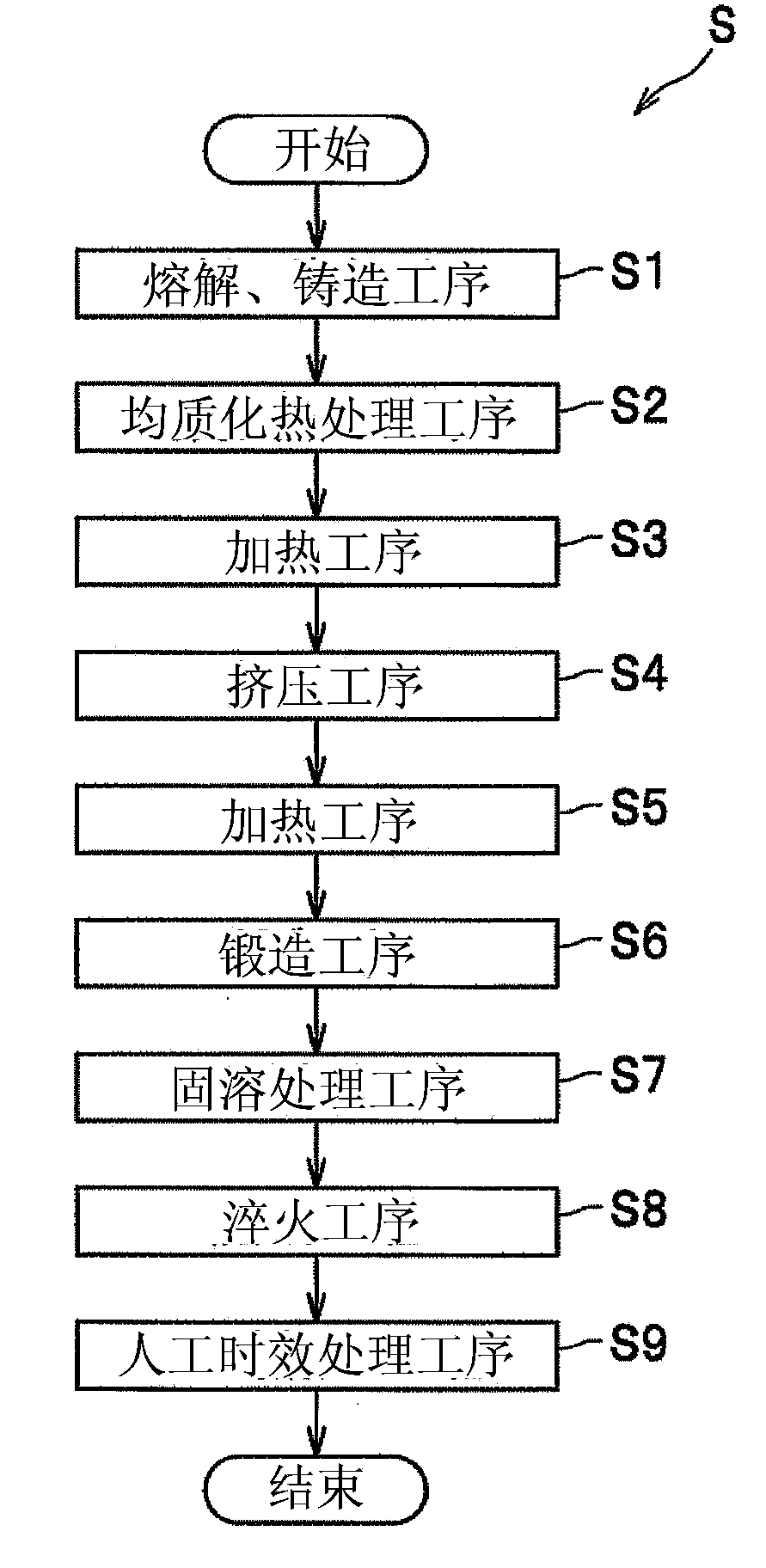
Aluminum alloy forged material for automotive vehicles and production method for the material
InactiveCN103361519AGuaranteed corrosion resistanceHigh tensile strengthCrystalline particleImpurity
An aluminum alloy forged material for automotive vehicles comprises 0.6ˆ¼1.2 mass% of Mg, 0.7ˆ¼1.5 mass% of Si, 0.1ˆ¼0.5 mass% of Fe, 0.01ˆ¼0.1 mass% of Ti, 0.3ˆ¼1.0 mass% of Mn, at least one of 0.1ˆ¼0.4 mass% of Cr and 0.05ˆ¼0.2 mass% of Zr, a restricted amount of Cu that is less than or equal to 0.1 mass%, a restricted amount of Zn that is less than or equal to 0.05 mass %, a restricted amount of H that is less than or equal to 0.25 ml in 100g A1 and a remainder of A1 and inevitably contained impurities, and the material includes precipitated crystalline particles among which the largest one has a maximum equivalent circle diameter equal to or less than 8 µm and an area ratio of the precipitated crystalline particles is equal to or less than 3.6%.
Owner:KOBE STEEL LTD
Sintered porous metal body and a method of manufacturing the same
InactiveUS20110020662A1Improve scalabilityFluctuation in qualityLayered productsThin material handlingSemiconductor materialsAbsorbed energy
A sintered porous metal body, which has a sintered structure having a volumetric porosity of 10 to 90%, wherein there are at least one powder particles selected from the group consisting of dielectric material powders and semiconductor material powders that absorb energy of electromagnetic wave having a frequency of 300 MHz to 300 GHz among the metal crystalline particles constituting the sintered body, wherein the particles are substantially homogeneously dispersed in the sintered body, and wherein the metal particles are sintered to bond each other to be united to constitute pores. The invention discloses a method of manufacturing the sintered porous metal body.
Owner:HITACHI LTD
Multilayer ceramic capacitor and method for production thereof
ActiveUS20090225494A1Prolong lifeStable temperatureFixed capacitor dielectricStacked capacitorsRare-earth elementBarium titanate
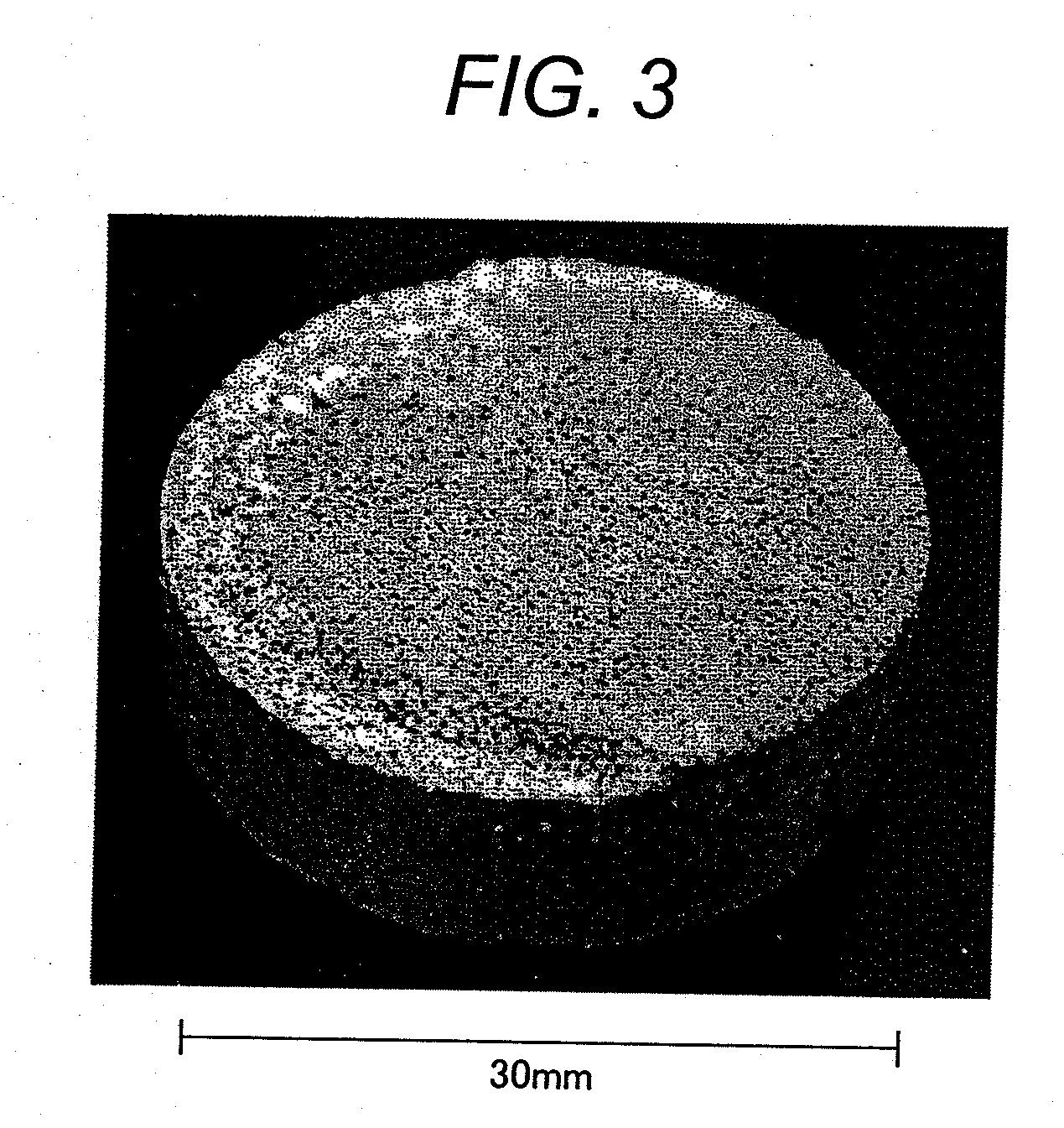
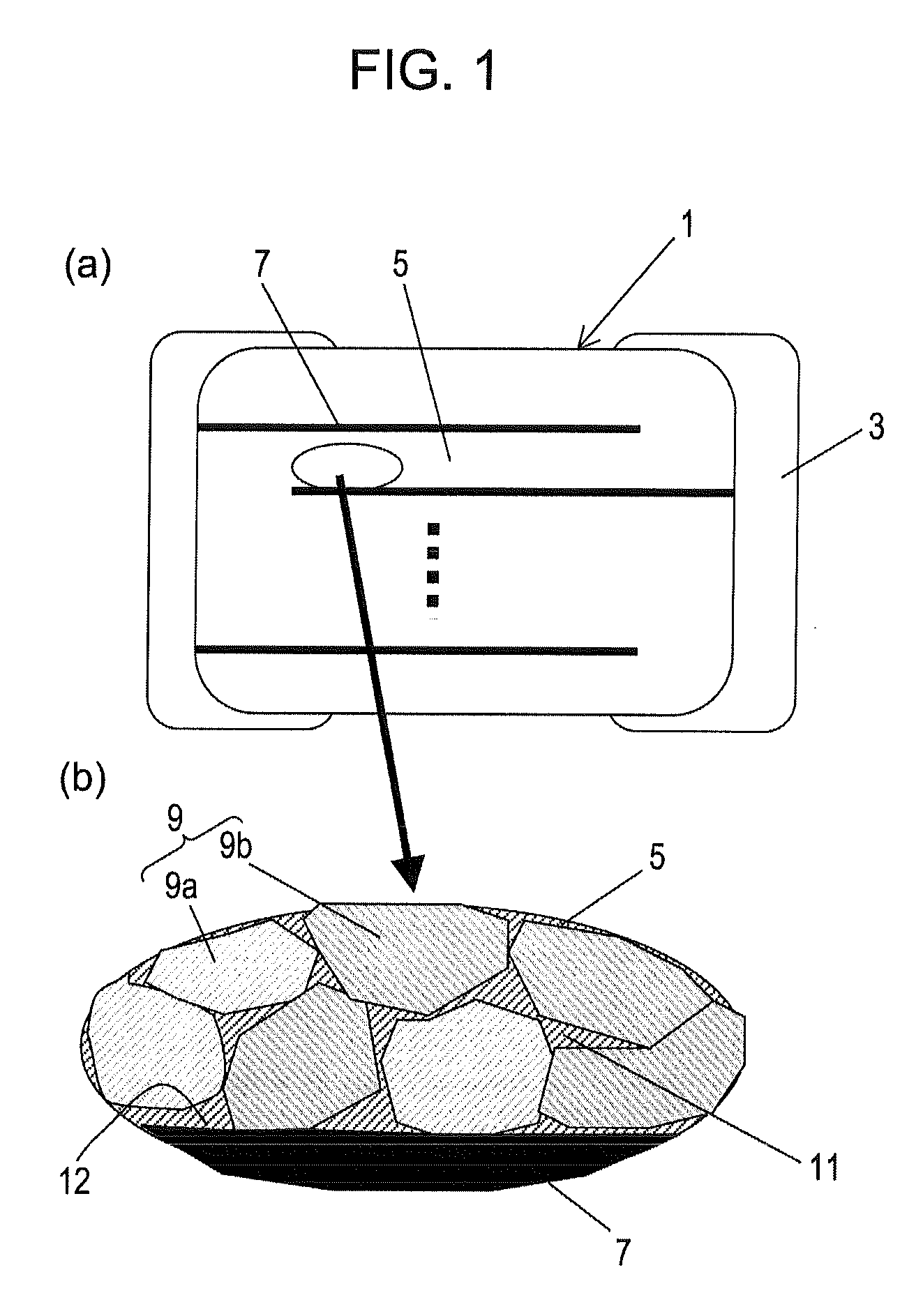
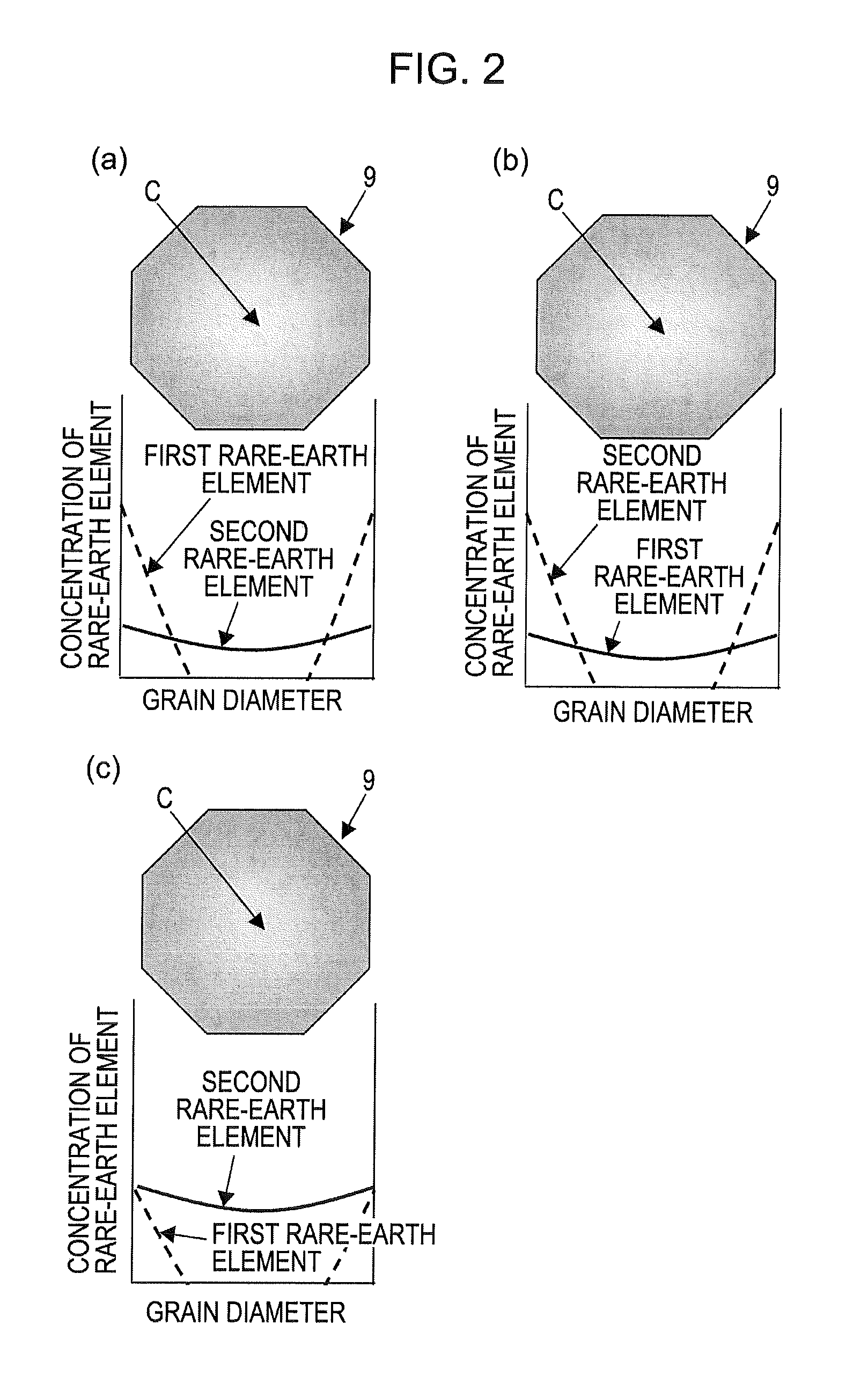
Disclosed is a multilayer ceramic capacitor which has a dielectric ceramic having a dielectric layer, wherein the dielectric layer mainly comprises barium titanate, contains a crystalline particle having an average crystal diameter of 0.15 to 0.3 μm, and contains Mg in an amount of 0.5 to 2 parts by mol in terms of MgO, Mn in an amount of 0.2 to 0.5 part by mol in terms of MnO, and a first rare earth element (RE) selected from Ho, Y, Er, Tm, Yb and Lu and a second rare earth element (RE) selected from Sm, Eu, Gd, Tb and Dy in a total amount of 0.7 to 3 parts by mol in terms of RE2O3 relative to 100 parts by mol of barium titanate, the crystalline particle contains the first rare earth element and the second rare earth element in such a manner that the amount of the first rare earth element is larger than that of the second rare earth element, and the density gradients of the first rare earth element and the second rare earth element in the crystalline particle as determined from the particle boundary toward the center of the crystalline particle are −0.005 to −0.05 atm % / mm and −0.0005 to −0.005 atm % / mm, respectively. Also disclosed is a method for producing the multilayer ceramic capacitor.
Owner:KYOCERA CORP
Porous clusters of silver powder promoted by zirconium oxide for use as a catalyst in gas diffusion electrodes, and method for the production thereof
ActiveUS8142938B2Long lastingRobust structureCellsFuel and primary cellsZirconium hydrideSilver particles
A catalyst including: a plurality of porous clusters of silver particles, each cluster including: (a) a plurality of primary particles of silver, and (b) crystalline particles of zirconium oxide (ZrO2), wherein at least a portion of the crystalline particles of ZrO2 is located in pores formed by a surface of the plurality of primary particles of silver.
Owner:BAR ILAN UNIV REASERCH & DEV
Cerium oxide abrasive and slurry containing the same
InactiveUS20060162260A1Dispersion force can be reducedSlow polishing rateMaterial nanotechnologyPigmenting treatmentCeriumCrystal structure
Disclosed are a cerium oxide abrasive for selectively polishing various SiO2 films and SiO2—Si3N4 films; and a slurry containing the same. The cerium oxide abrasive and the polishing slurry of the present invention have a high polishing rate and are also free from microscratches in a polished surface upon polishing since polycrystalline cerium oxide having a mean crystalline particle size of 5 nm or less is synthesized by using hexagonal cerium carbonate having a hexagonal crystal structure as a raw material of cerium.
Owner:LG CHEM LTD
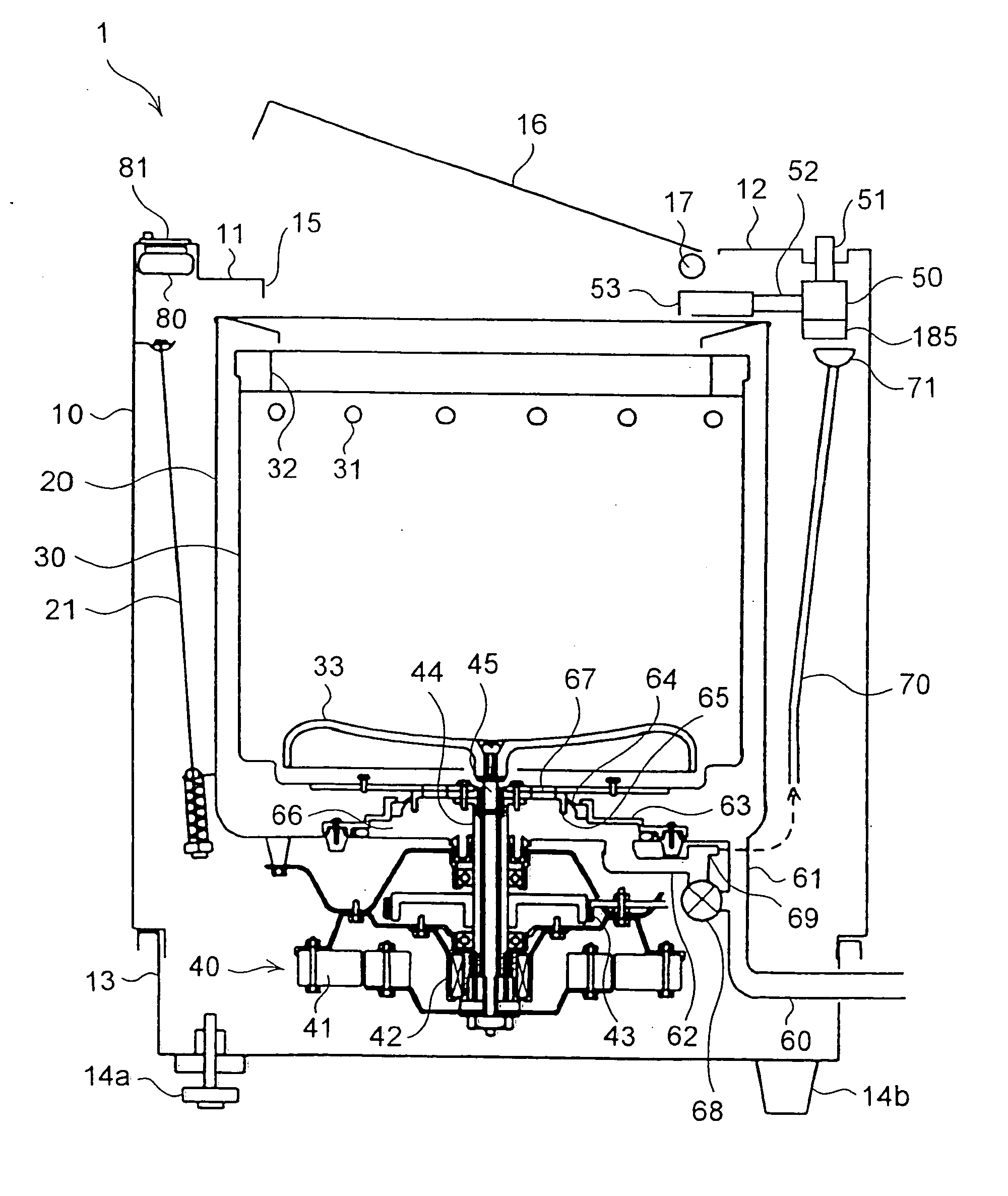
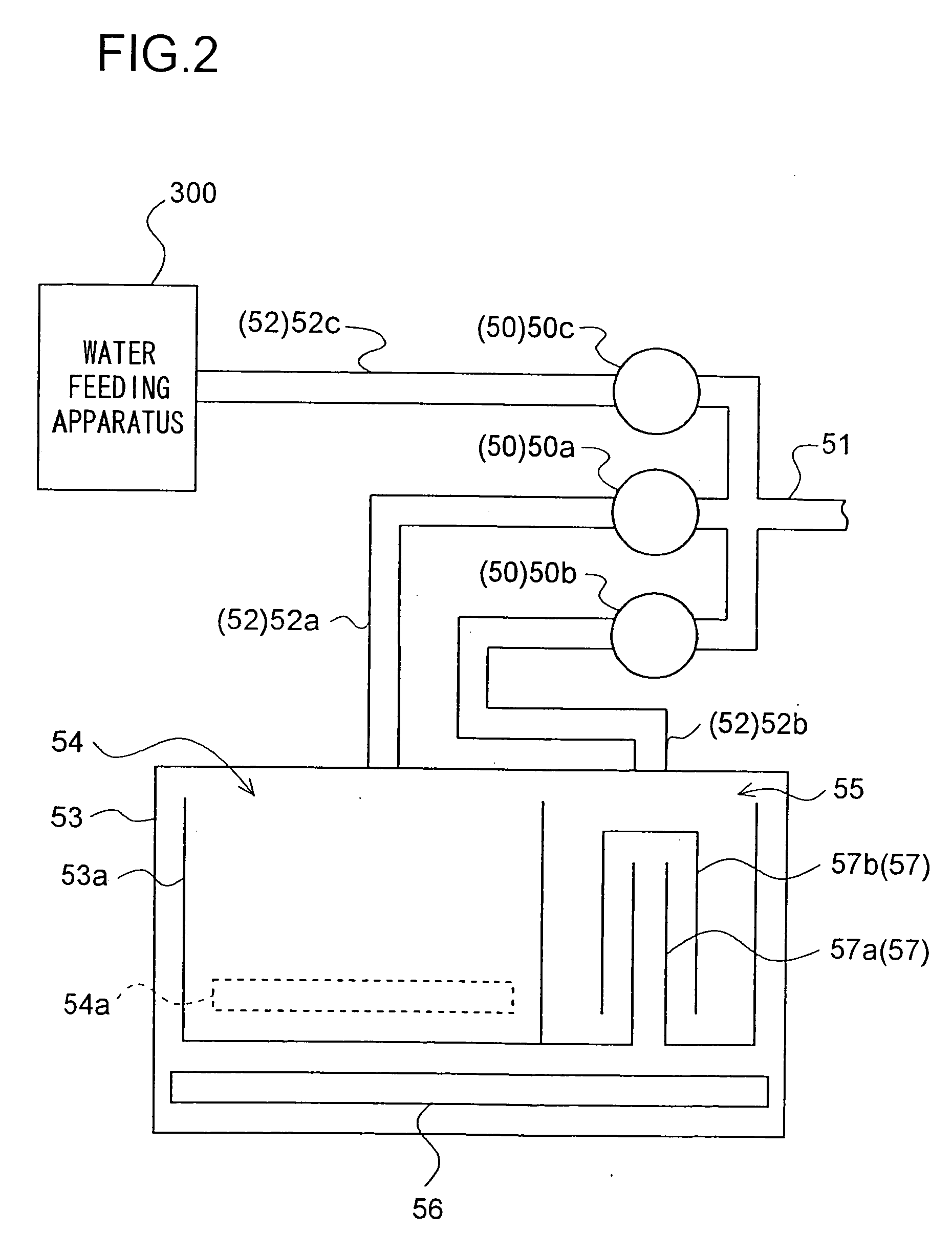
Water supply device, water supply method, and washing machine having water supply device
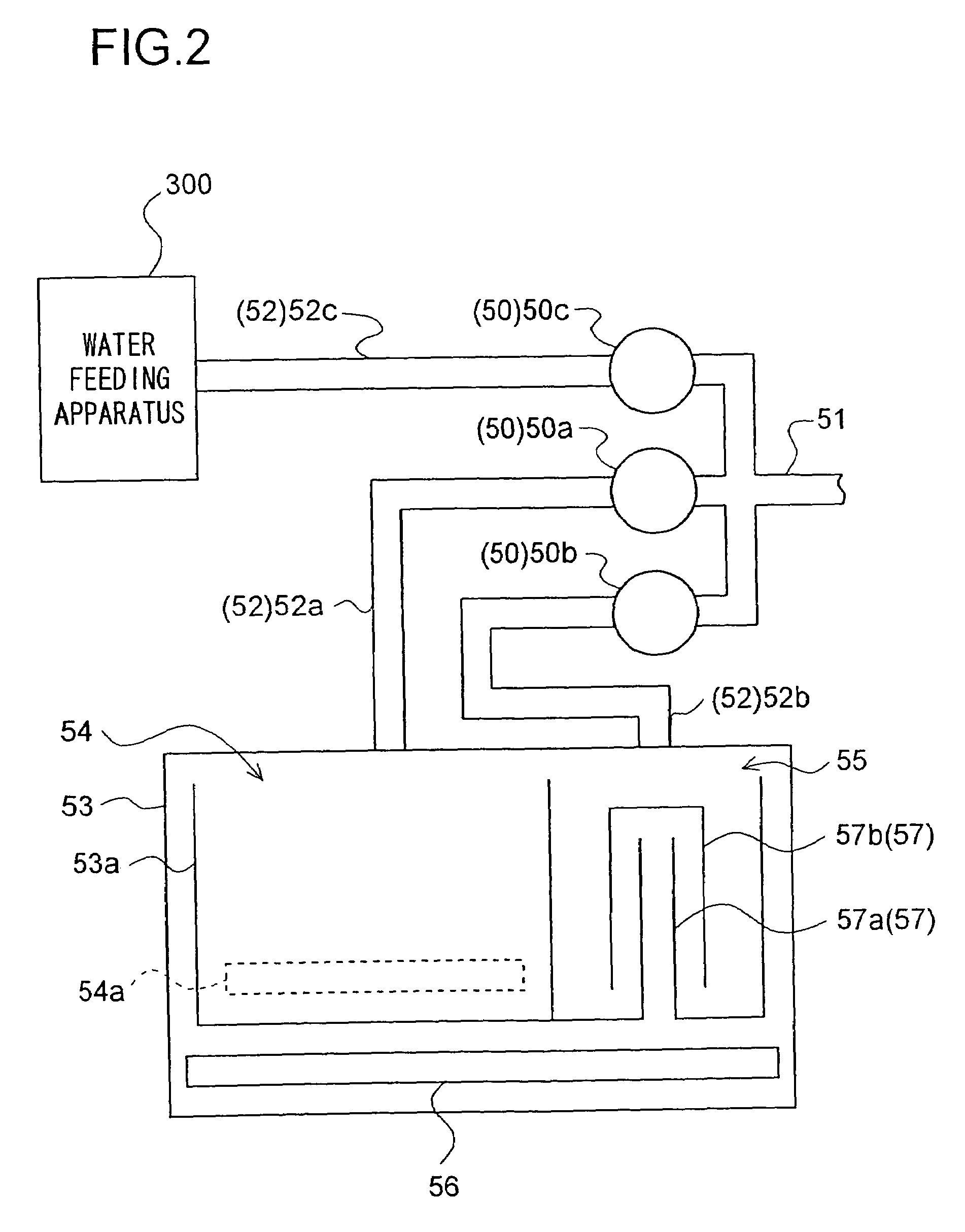
InactiveUS7624601B2Effect is exertedBiocideWater/sewage treatment by electrochemical methodsLattice defectsEngineering
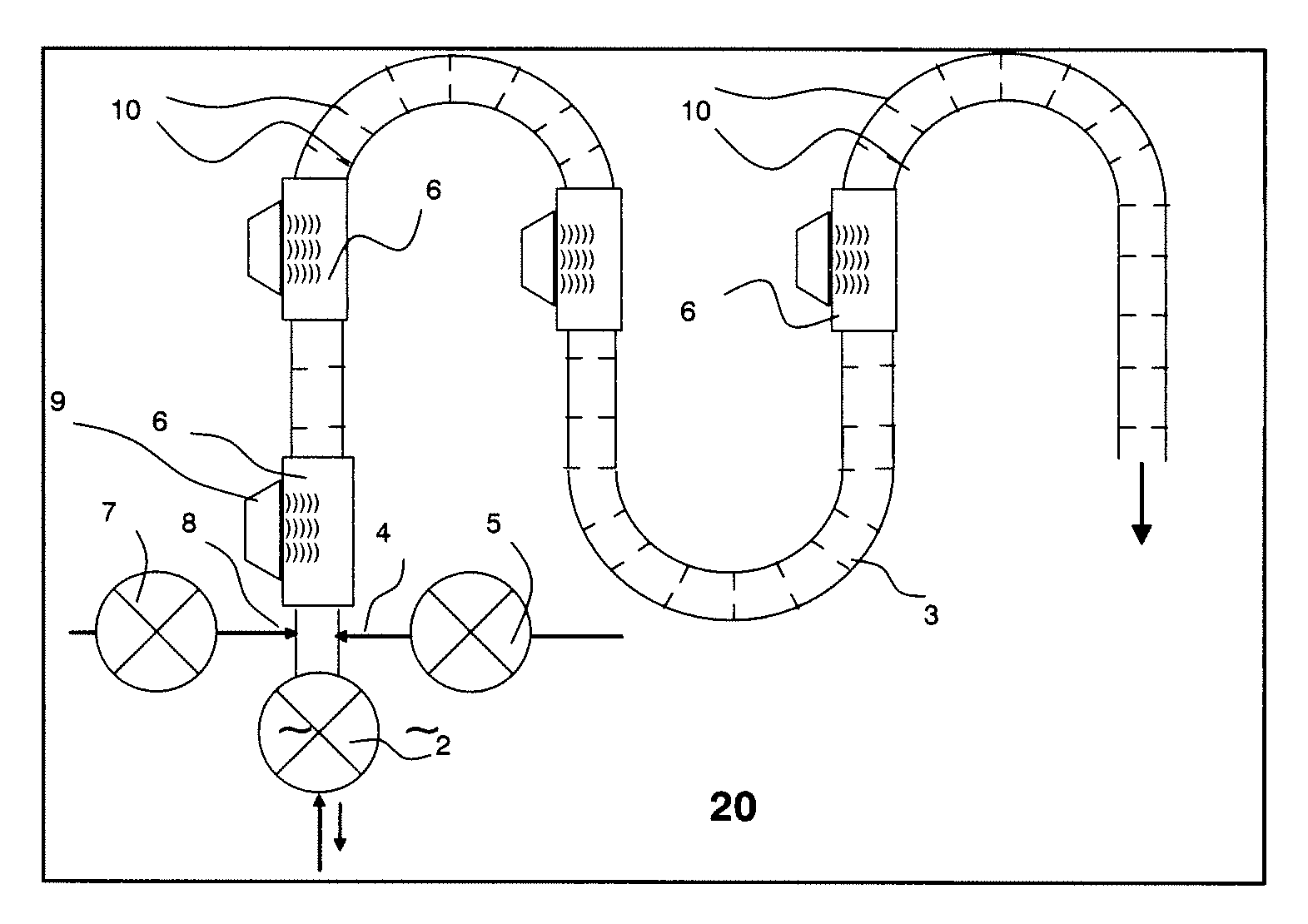
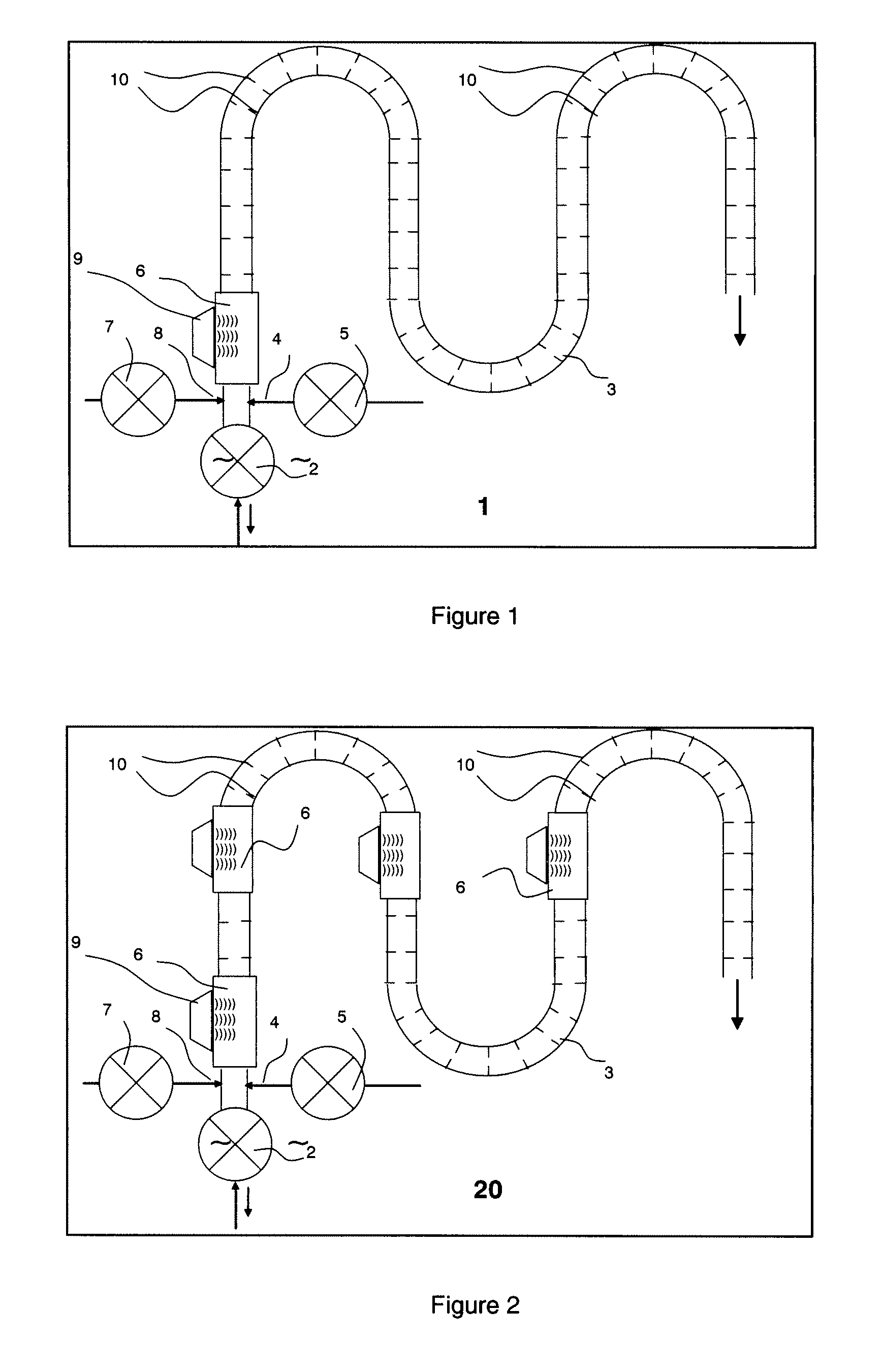
A water feeding apparatus (300) has an ion eluter (100) and a shower emitter (200). The shower emitter (200) receives water via a coupling pipe (250) from the ion eluter (100), and sprays the water, in the form of a shower, onto laundry. Liquid droplets in the form of a shower are small and easy to dry, and thus produce crystals having smaller particles (with large surface areas), having more lattice defects, and easier to dissolve. With these crystals attached to the laundry, when the crystals make contact with moisture next time, the silver ion in the liquid droplets easily dissolves. Even when the laundry is made of water-repellent or hydrophobic cloth, the solution dries up on the surface of the cloth before water is repelled. Thus, even this type of laundry can benefit from the antimicrobial effect of the silver ion.
Owner:SHARP KK
Combination particles for the treatment of asthma
InactiveUS7172752B2More controlled deliveryGood dispersibilityOrganic active ingredientsBiocideRough surfaceDisease
Inhalation particles incorporating, in an individual particle, a combination of a βhd 2-agonist and a glucocorticosteroid in a predetermined and constant ratio, a process for the preparation thereof and pharmaceutical compositions comprising the inhalation particles. The particles have a narrow particle size distribution and are preferably in the form of spherical crystalline particles with a rough surface. The particles are particularly useful in the treatment of asthma and other respiratory disorders.
Owner:ORION CORPORATION
Packing material for liquid chromatography and process for producing the same
InactiveUS6306297B1Excellent in dissolution resistanceEasy to separateIon-exchange process apparatusPhosphatesCalcium biphosphateFilling materials
Column packing materials useful in applications such as liquid chromatography, as well as a process for producing such a packing material are described. A first aspect of the invention concerns a column packing material composed of a spherical substrate having a coating of a calcium phosphate based compound on the surface thereof. A second aspect of the invention concerns a packing material comprising porous calcium phosphate based granules having open pores with an average pore size of from 0.01 to 20 .mu.m, said granules being composed of crystalline particles with an average size of from 0.1 to 10 .mu.m. A third aspect of the invention concerns a spherical packing material for liquid chromatography comprising spherical particles of at least one material selected from the group consisting of Ca.sub.10 (PO.sub.4).sub.6 (OH).sub.2, Ca.sub.3 (PO.sub.4).sub.2, Ca.sub.2 P.sub.2 O.sub.7, Ca(PO.sub.3).sub.2, Ca.sub.10 (PO.sub.4).sub.6 F.sub.2 and Ca.sub.10 (PO.sub.4).sub.6 Cl.sub.2, said packing material having a dense structure with a porosity of no more than 5%. A fourth aspect of the invention concerns a packing for liquid chromatography comprising particles having at least on the surface thereof a fluoroapatite represented by formula (I): wherein x is a number of from about 0.1 to 1.
Owner:ASAHI KOGAKU KOGYO KK
Rotating electrical machine
InactiveUS20080012445A1Reduce iron lossReduce eddy-current lossMagnetic circuit rotating partsMagnetic circuit stationary partsElectric machineConductor Coil
A rotating electrical machine comprises a stator and a rotor; the stator comprising a stator core having teeth and slots, and stator windings disposed in the slots, wherein the stator core is made of laminated steel sheets, teeth and slots of the steel sheet are made by etching, and the thickness of the steel sheet is between 0.05 mm and 0.30 mm. Specifically, it is preferable that the steel sheet used herein be a silicon steel sheet containing crystalline particles.
Owner:HITACHI LTD
Method for preparing magic color crystallite ultra-large particle polishing brick
The invention discloses a preparation method of glittering microcrystal polished tile with super large particles. The tile comprises porcelain tile green body and particle decoration layer. The invention is characterized in that: the particle decoration layer comprises large particles with diameter of 30mm-80mm, medium particles with diameter of 10mm-30mm and small particles with diameter less than 10mm. The specific technique comprises the following steps: (1) large, medium or small crystalline particles are prepared; (2) blank is prepared; (3) crystalline frit is prepared; (4) the crystalline particles, blank and crystalline frit are combined, mixed and press molded; (5) drying, sintering, edging, polishing, antifouling treatment and separation test are carried out. The porcelain tile prepared by the technique has the advantages of simple technique, low cost, various and rich colors, similarity to jade, etc.
Owner:GUANGDONG BODE FINE IND CONSTR MATERIAL
Semiconductor memory device and method of manufacturing thereof
A semiconductor memory device comprises a field effect transistor including a source / drain region, an interlayer insulation film burying the field effect transistor, a ferroelectric capacitor including a lower electrode, a ferroelectric film and an upper electrode, the lower electrode with a concave-convex surface, and a plug electrically connecting between the source / drain region and the ferroelectric capacitor. A height and a size in an in-place direction of each convex portion in the concave-convex surface is 1 to 50 nm. The ferroelectric film includes a lower ferroelectric film with a predetermined height from the lower electrode and an upper ferroelectric film formed on the lower ferroelectric film as being formed from the same material as the lower ferroelectric film. The lower ferroelectric film includes a part of which at least one of composition, crystallizing orientation and size of a crystalline particle being different from a crystalline particle in the upper ferroelectric film.
Owner:KK TOSHIBA
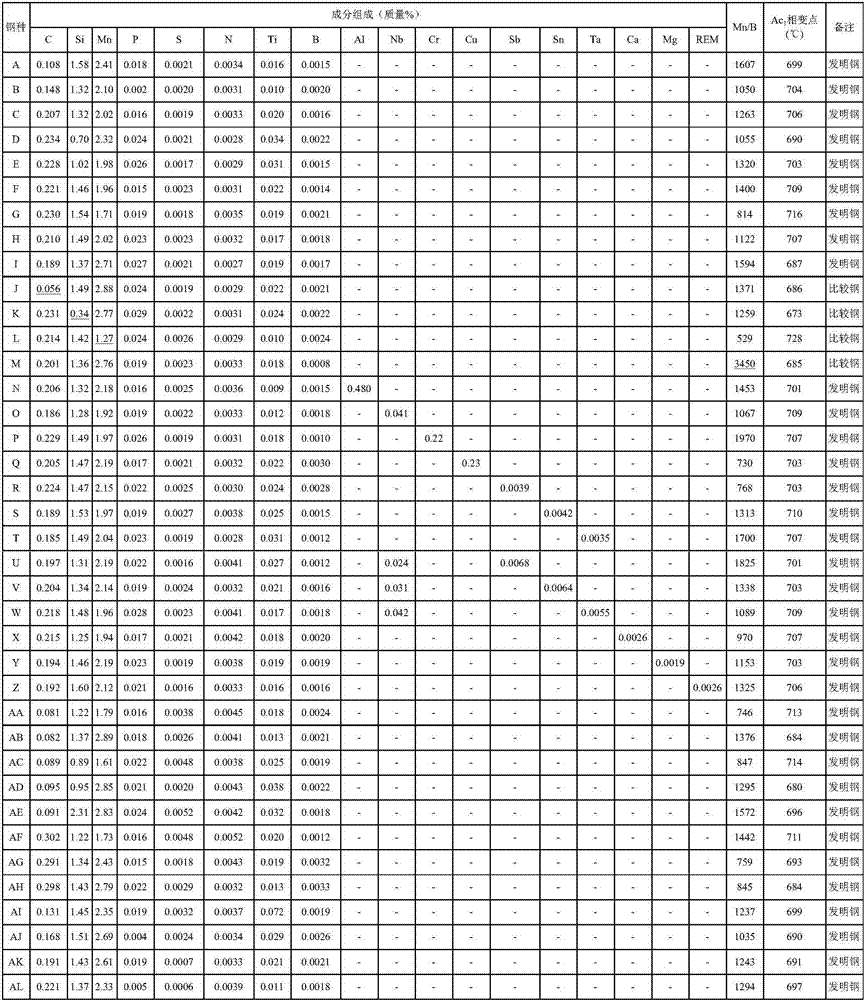
High-strength steel sheet and production method for same, and production method for high-strength galvanized steel sheet
InactiveCN107075627AImprove ductilityExcellent stretch flangeabilityHot-dipping/immersion processesFurnace typesSheet steelMartensite
The present invention provides a high-strength steel sheet having a tensile strength (TS) of at least 780 MPa, excellent ductility and stretch flangeability, and excellent material-quality stability, achieved by: having a specific component composition; the Mn volume divided by the B volume being not more than 2100; the steel structure having, in terms of area ratio, a total of ferrite and bainitic ferrite of 25-80%, and 3-20% martensite, and having, in terms of volume fraction, at least 10% retained austenite; the average crystalline particle diameter of the retained austenite being not more than 2[mu]m; the average Mn volume (mass%) in the retained austenite being at least 1.2 times the Mn volume (mass%) in the steel; and retained austenite aggregates, in which seven or more retained austenite crystal particles having the same orientation are grouped together, accounting for, in terms of area ratio, at least 60% of the total retained austenite.
Owner:JFE STEEL CORP
Preparation method of compact silicon carbide ceramic
The invention discloses a preparation method of compact silicon carbide ceramic, which is characterized in that the compact accumulation among silicon carbide crystalline particles is realized so as to obtain a polycrystalline block ceramic with high compactness by using decomposition and chemical combination reactions generated by pure silicon carbide or a material source of silicon carbide at the temperature of 2,250-2,500DEG C, wherein the material source of silicon carbide is generated by a chemical combination reaction, adopting a high-temperature physical gaseous phase transmission technology and controlling the gaseous phase recrystallizing and arraying accumulation process. For the compact silicon carbide ceramic prepared by the method, crystals are directly bonded by pure silicon carbide interfaces, silicon carbide crystalline particles are directionally arrayed and compactly accumulated according to the preferred orientation of crystals and the volume density of the ceramic can approach to the theoretical density.
Owner:咸阳瞪羚谷新材料科技有限公司
High efficiency solid state directional lighting including luminescent nanocrystal particles
InactiveUS8508126B1Reduce heat transferPoint-like light sourceDischarge tube main electrodesBeam divergencePhysics
A solid state directional lighting device includes a semiconductor light source emitting a primary short wavelength light and a light collimation component disposed in light-reflecting relation to the light source to generate a collimated light with beam divergence angle less than forty degrees (40°). A luminescent nanocrystal conversion layer is disposed in the path of the collimated light and includes a luminescent nanocrystal conversion layer including luminescent nanocrystal particles with nano-particles sizes less than fifteen nanometers (15 nm). The luminescent nanocrystal particles absorb at least a portion of the collimated short wavelength light and convert the absorbed light into at least one long wavelength spectral light. A mixture of leakage primary short wavelength light and long wavelength light converted by the luminescent nano-particles produces a directional white light with luminous efficacy of at least one hundred lumens per Watt (100 lm / W). Light scattering is reduced due to the nano-particle size.
Owner:LEDNOVATION
apparatus and process for producing crystals
InactiveUS20110288060A1Organic active ingredientsNervous disorderUltrasonic radiationUltrasound Radiation
This invention provides an oscillating baffled reactor apparatus for preparing crystalline particles of at least one substance comprising: a reactor vessel; means for supplying a first flowing stream; means for oscillating fluid within the reactor vessel; a plurality of baffles; source of ultrasonic radiation; and means for collecting said particles.
Owner:PROSONIX
Water supply device, water supply method, and washing machine having water supply device
InactiveUS20060186222A1Easily dryEffect is exertedBiocideWater/sewage treatment by electrochemical methodsInjectorAntimicrobial effect
A water feeding apparatus (300) has an ion eluter (100) and a shower emitter (200). The shower emitter (200) receives water via a coupling pipe (250) from the ion eluter (100), and sprays the water, in the form of a shower, onto laundry. Liquid droplets in the form of a shower are small and easy to dry, and thus produce crystals having smaller particles (with large surface areas), having more lattice defects, and easier to dissolve. With these crystals attached to the laundry, when the crystals make contact with moisture next time, the silver ion in the liquid droplets easily dissolves. Even when the laundry is made of water-repellent or hydrophobic cloth, the solution dries up on the surface of the cloth before water is repelled. Thus, even this type of laundry can benefit from the antimicrobial effect of the silver ion.
Owner:SHARP KK
Stabilized individually coated ramipril particles, compositions and methods
InactiveUS20060159742A1Improve stabilityMaintain potencyBiocidePowder deliveryDecompositionOral therapy
The present invention relates to novel ramipril crystalline particles with improved stability and bioavailability. More particularly, the present invention is directed to individually coated, single ramipril crystalline particles for pharmaceutical and biopharmaceutical applications in oral therapies that are stabilized against decomposition into degradation products, namely, ramipril-DKP and ramipril-diacid, during formulation and storage conditions. The present invention also relates to stabilized ramipril pharmaceutical compositions, novel anhydrous pharmaceutical grade ramipril powders, methods for improving ramipril bioavailability, and methods of manufacture and stabilization of ramipril formulations. The novel, anhydrous pharmaceutical grade ramipril powders and ramipril compositions and dosage forms formed therewith are useful in the treatment of cardiovascular disorders and have the advantage that they provide greater stability against decomposition into ramipril-DKPs and ramipril-diacids under formulation and storage conditions. In addition, they maintain consistent label ramipril potency over extended shelf-life and provide reduced in vivo variability in the bioavailability of ramipril among subjects when administered orally.
Owner:KING PHARMA RES & DEV
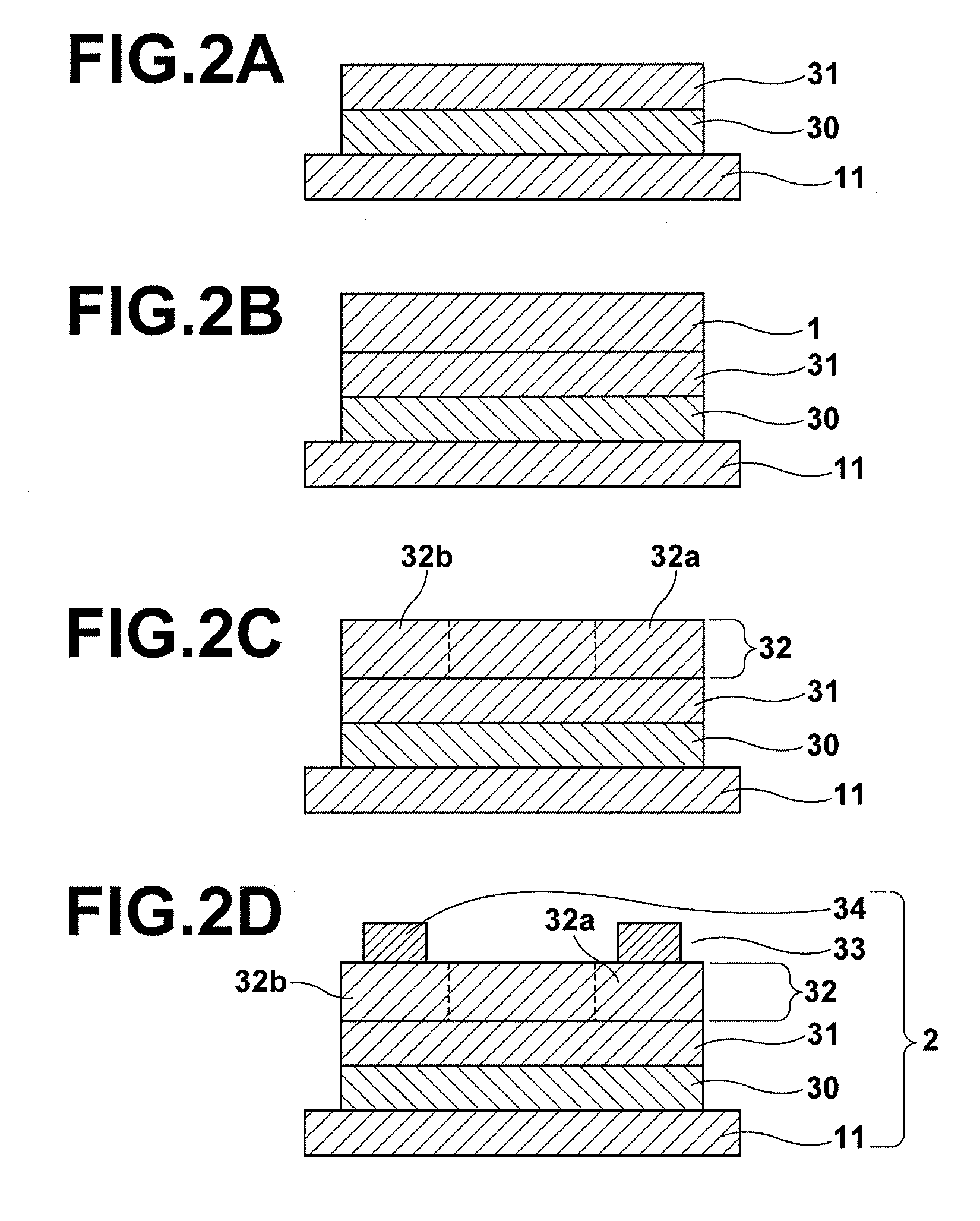
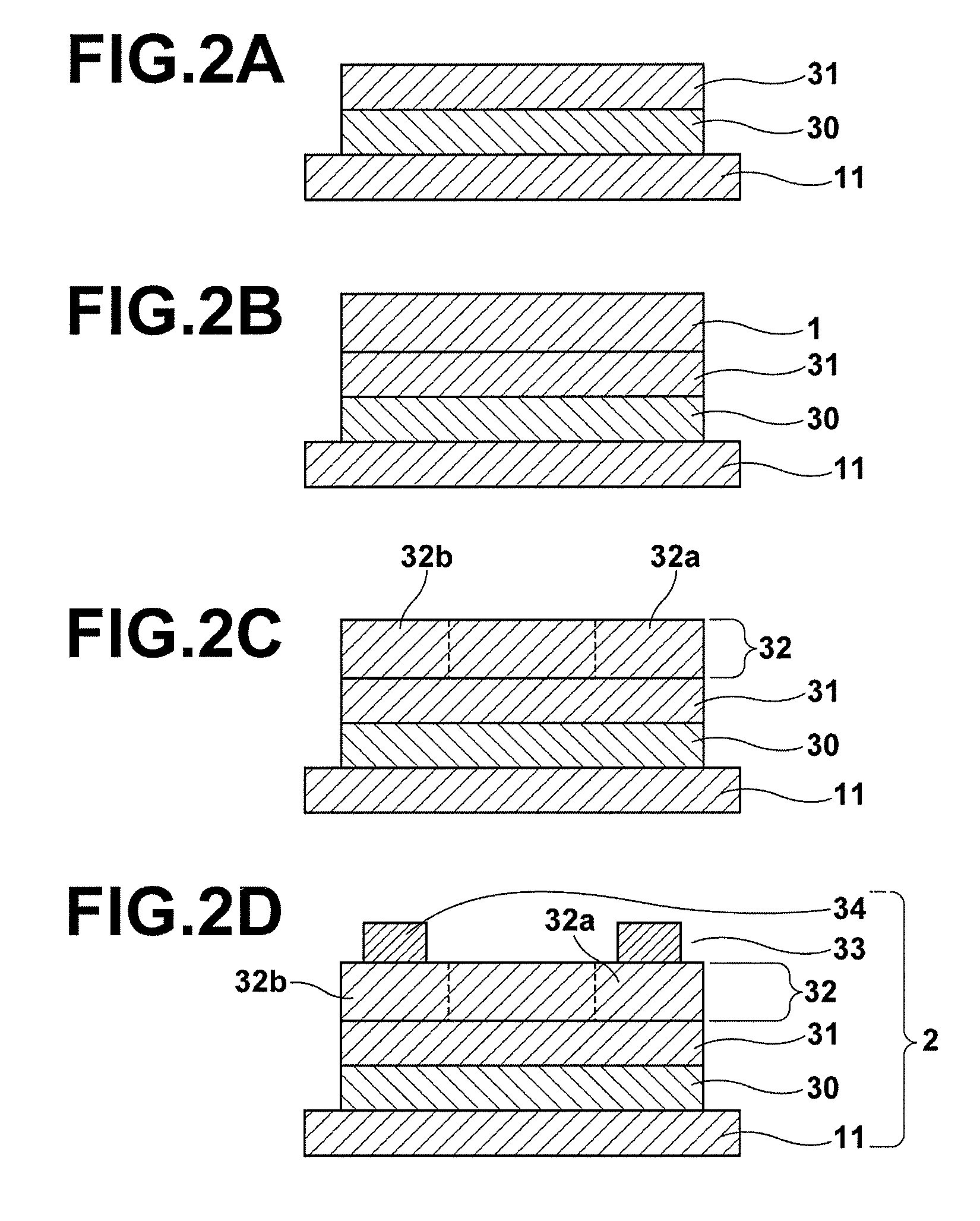
Piezoelectric/electrostrictive film type device and method of manufacturing the same
InactiveUS20060066176A1Superior piezoelectric/electrostrictive characteristicLarge displacementPiezoelectric/electrostriction/magnetostriction machinesPiezoelectric/electrostrictive device material selectionLithiumNiobium
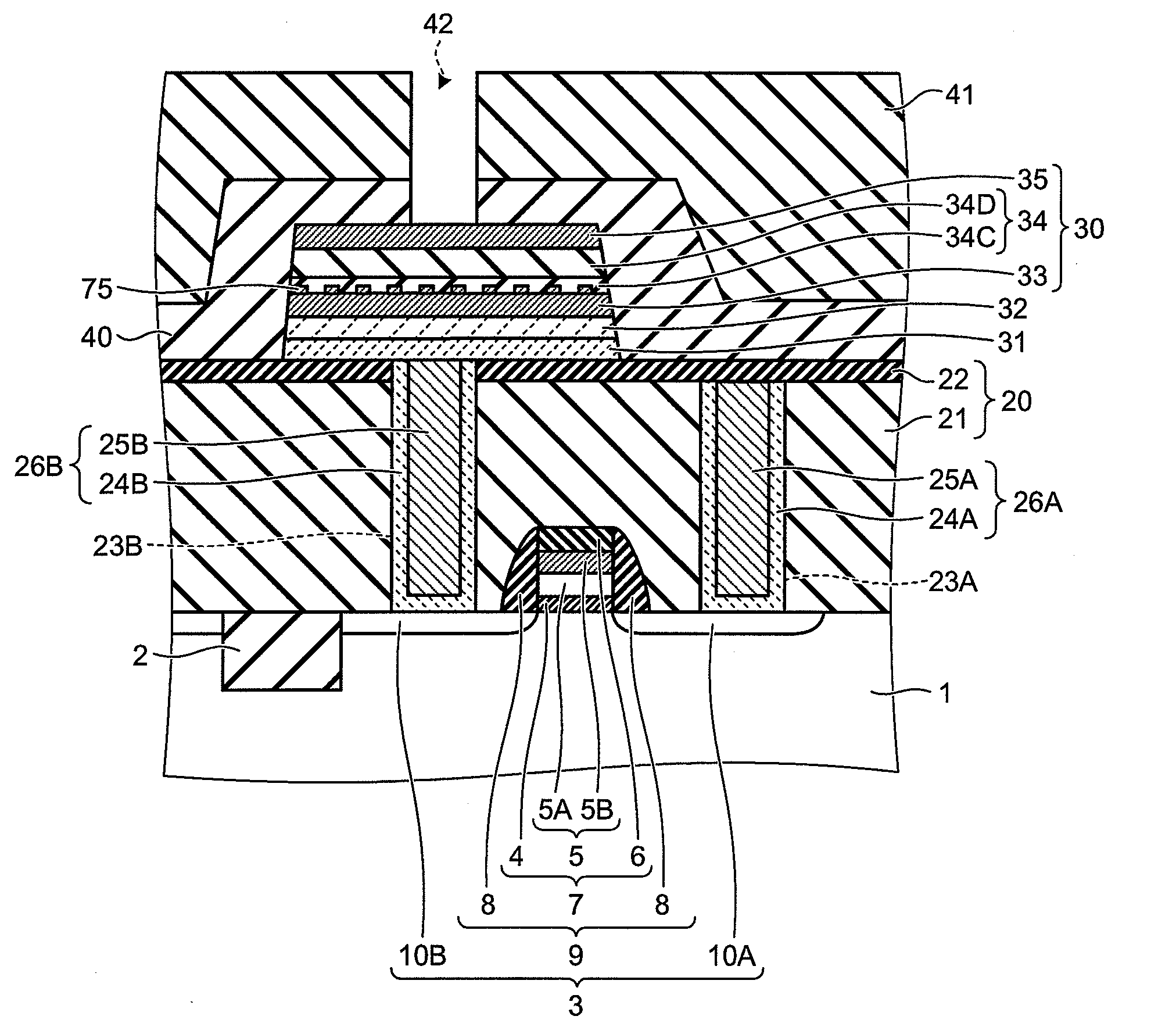
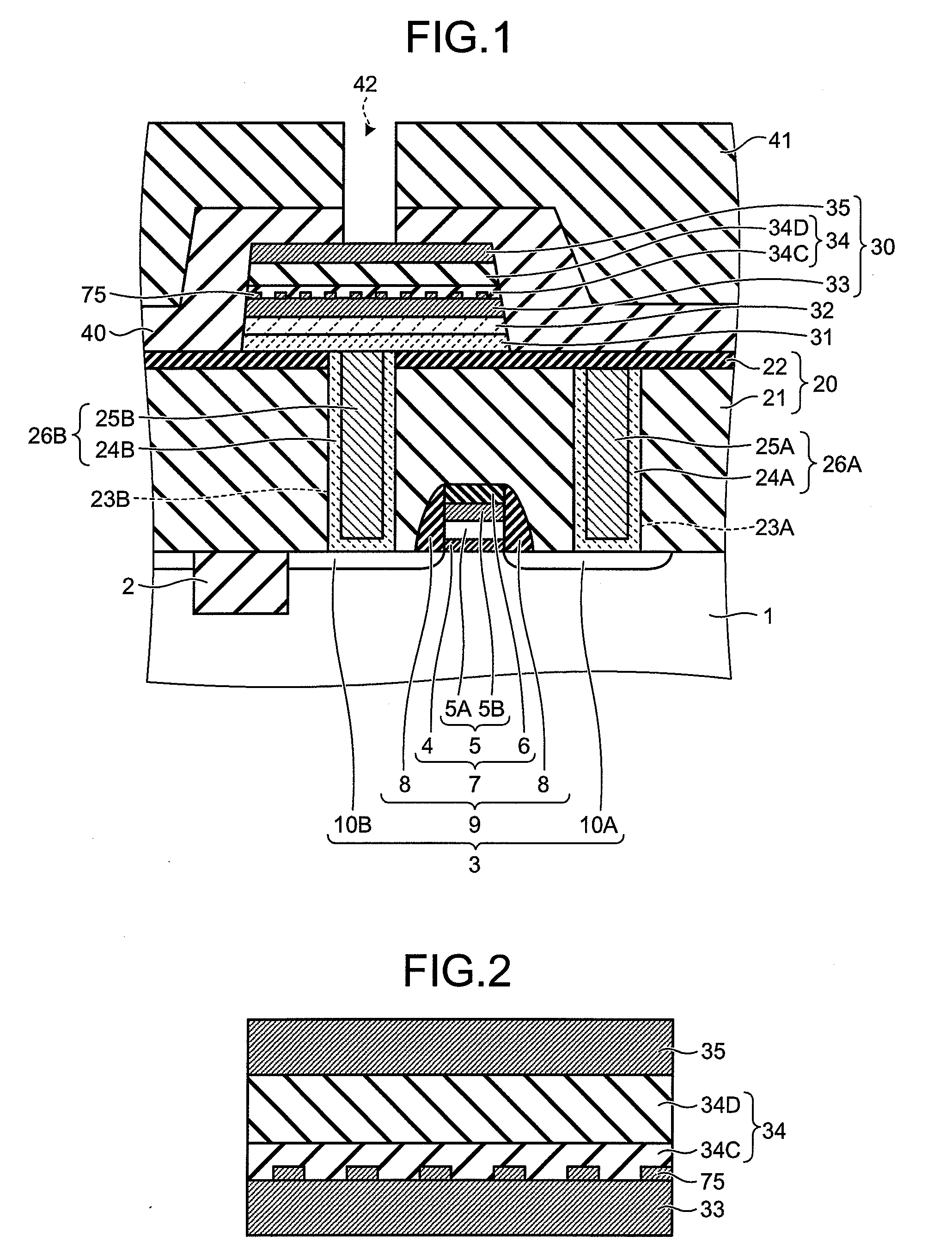
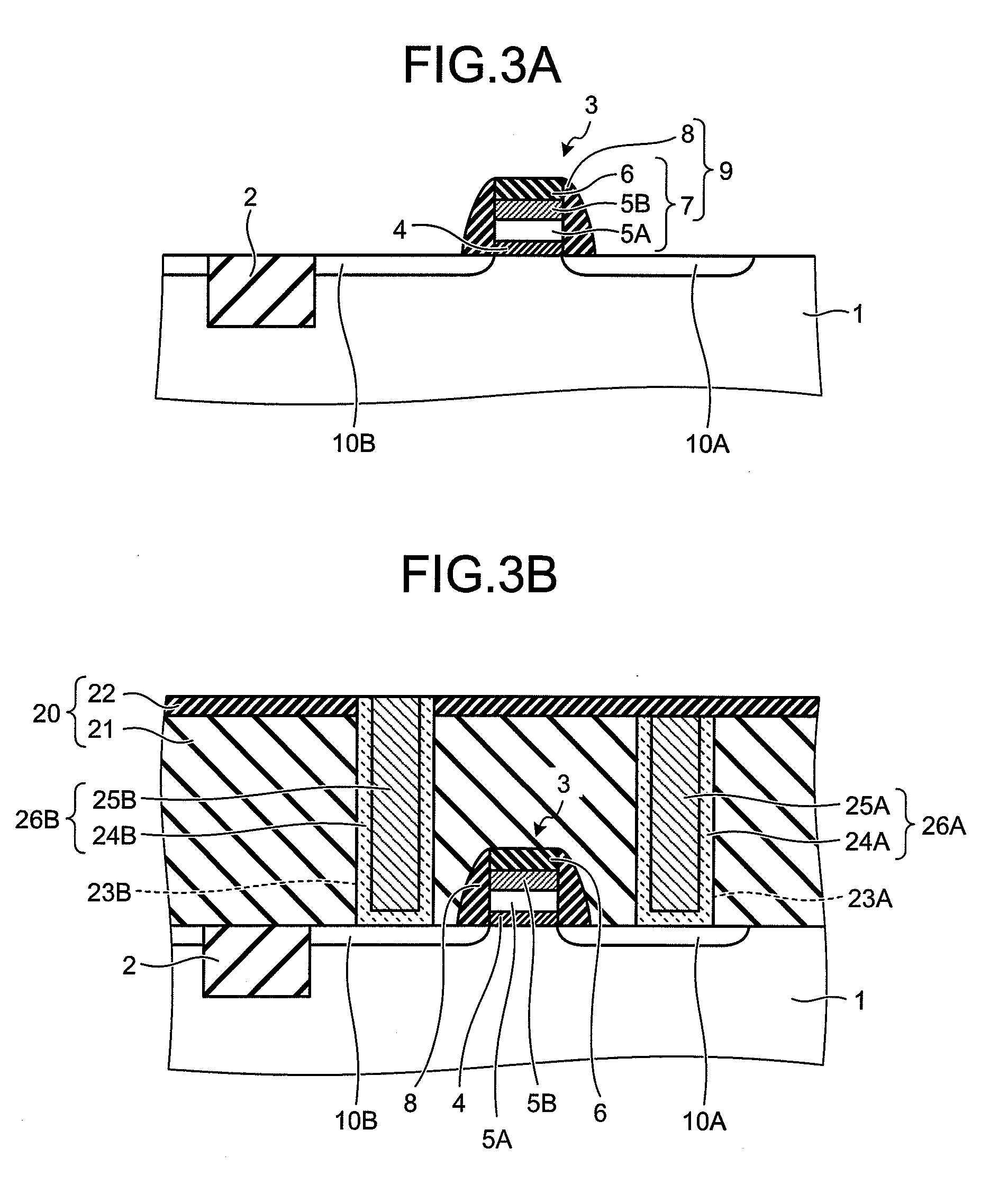
There is disclosed a piezoelectric / electrostrictive film type device 1 provided with: a thin substrate 2 made of a ceramic; and a piezoelectric / electrostrictive operating portion 5 disposed on the substrate 2 and constituted by successively laminating a lower electrode film 3a, a piezoelectric / electrostrictive film 4 containing a large number of crystal particles constituted of a piezoelectric / electrostrictive composition, and an upper electrode film 3b. The piezoelectric / electrostrictive composition contains one or more alkali metal elements selected from the group consisting of lithium (Li), potassium (K), and sodium (Na), and one or more metal elements selected from the group consisting of niobium (Nb), tantalum (Ta), antimony (Sb), and silver (Ag) (however, surely containing niobium (Nb), and circle equivalent diameters of 90% or more of the large number of crystal particles are in a range of 0.3 to 50 μm. It is possible to exhibit a superior piezoelectric / electrostrictive characteristic without containing lead (Pb) and obtain an especially large displacement.
Owner:NGK INSULATORS LTD
Surface-modified zirconia nanocrystal particle and method for producing same
InactiveUS20120071680A1Improve stabilityEasily substitutedMaterial nanotechnologyIndividual molecule manipulationNanoparticleSolvent
The invention provides a surface-modified zirconia nanocrystal particle, wherein the surface of the zirconia nanoparticle is modified by organic sulfonyloxy groups, and a method of producing a zirconia nanocrystal particle whose surface is modified by carbonyloxy groups, organic phosphoryloxy groups or aryloxy groups. This makes it possible a highly stable surface-modified zirconia nanocrystal particle having a solvent dispersibility by a simple method. Further, it is possible to the surface-modified zirconia nanocrystal particle of the invention is equipped with a surface modifier having a structure that can be easily substituted with a desired functional group according to use. Furthermore, it is possible to the method of producing the surface-modified zirconia nanocrystal particle which is capable of easily producing that.
Owner:HOYA CORP
Method for using blast furnace slag to produce micro-crystalline light brick
The invention discloses a method for using blast furnace slag to produce a micro-crystalline light brick. The light brick uses the blast furnace slag as main raw material. The method for using the blast furnace slag to produce the micro-crystalline light brick includes that tempering and clearing the blast furnace slag to obtain slag with qualified ingredients, feeding to a water quenching pool to form micro-crystalline particles through water quenching, grinding and sieving the micro-crystalline particles to obtain micro-crystalline particles with proper granularity, uniformly mixing with foaming agent, putting the mixture in a casting mould, and sintering to form the micro-crystalline light brick with uniform air bubbles and stable ingredients. Compared with the traditional clay brick, the micro-crystalline light brick has lower density and higher intensity, so that the micro-crystalline light brick has better bearing ability, and moreover, the micro-crystalline light brick has good heat preservation effect and sound insulation effect due to the uniform air hole structure, so that the micro-crystalline light brick can replace the clay brick or ordinary light brick in a certain degree to serve as novel building material.
Owner:江西璞晶新材料股份有限公司
Magnetic core for saturable reactor, magnetic amplifier type multi-output switching regulator and computer having magnetic amplifier type multi-output switching regulator
InactiveUS6270592B1Stable outputImprove reliabilityVariable inductancesInorganic material magnetismMagnetic amplifierMagnetic flux
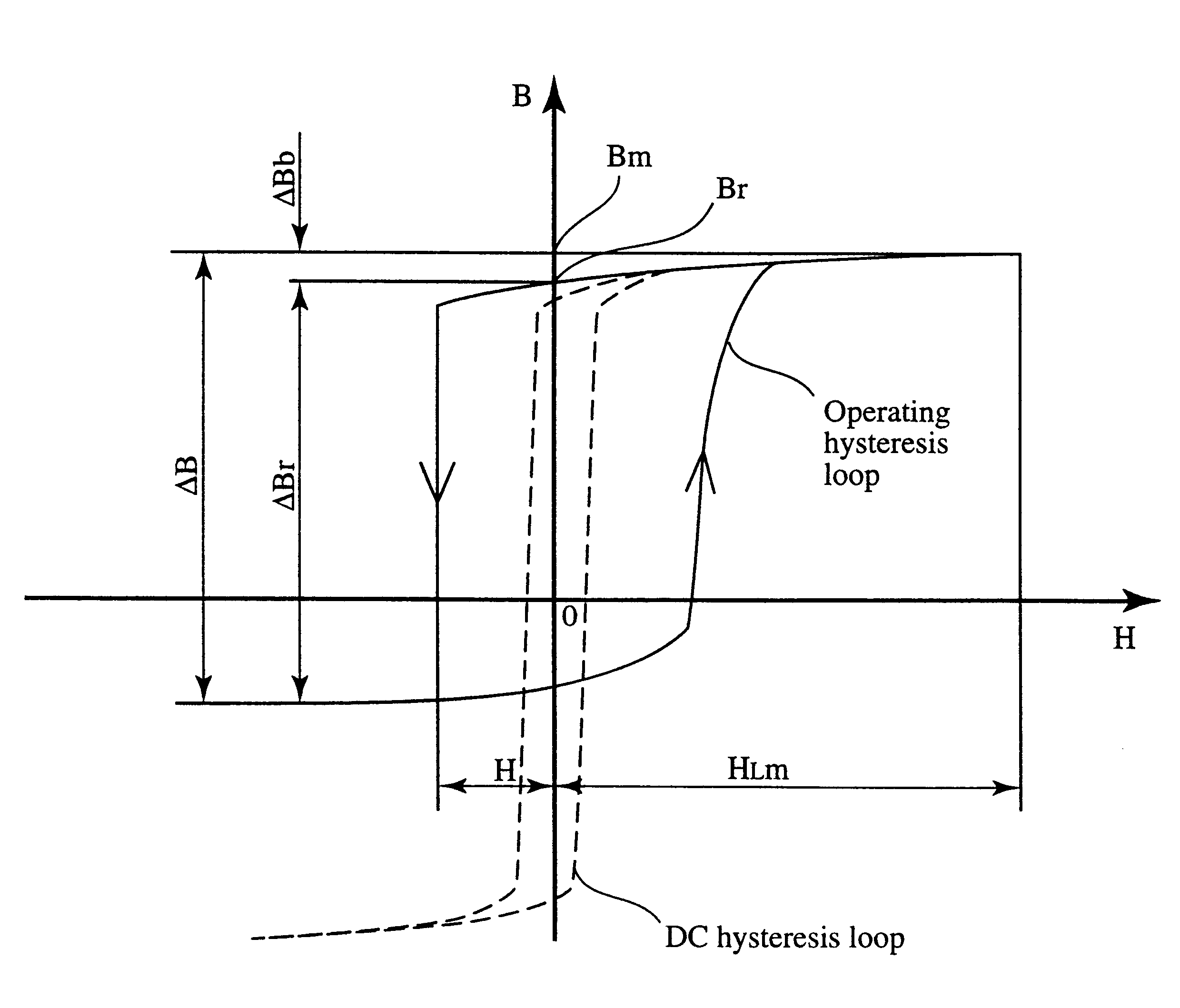
A magnetic core for use in a saturable reactor made of an Fe-based soft-magnetic alloy comprising as essential alloying elements Fe, Cu and M, wherein M is at least one element selected from the group consisting of Nb, W, Ta, Zr, Hf, Ti and Mo, and having an alloy structure at least 50% in area ratio of which being fine crystalline particles having an average particle size of 100 nm or less. The magnetic core has control magnetizing properties of a residual operating magnetic flux density DELTABb of 0.12 T or less, a total control operating magnetic flux density DELTABr of 2.0 T or more, and a total control gain Gr of 0.10-0.20 T / (A / m) calculated by the equation: Gr=0.8x(DELTABr-DELTABb) / Hr, wherein Hr is a total control magnetizing force defined as a control magnetizing force corresponding to 0.8x(DELTABr-DELTABb)+DELTABb.
Owner:HITACHI METALS LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com