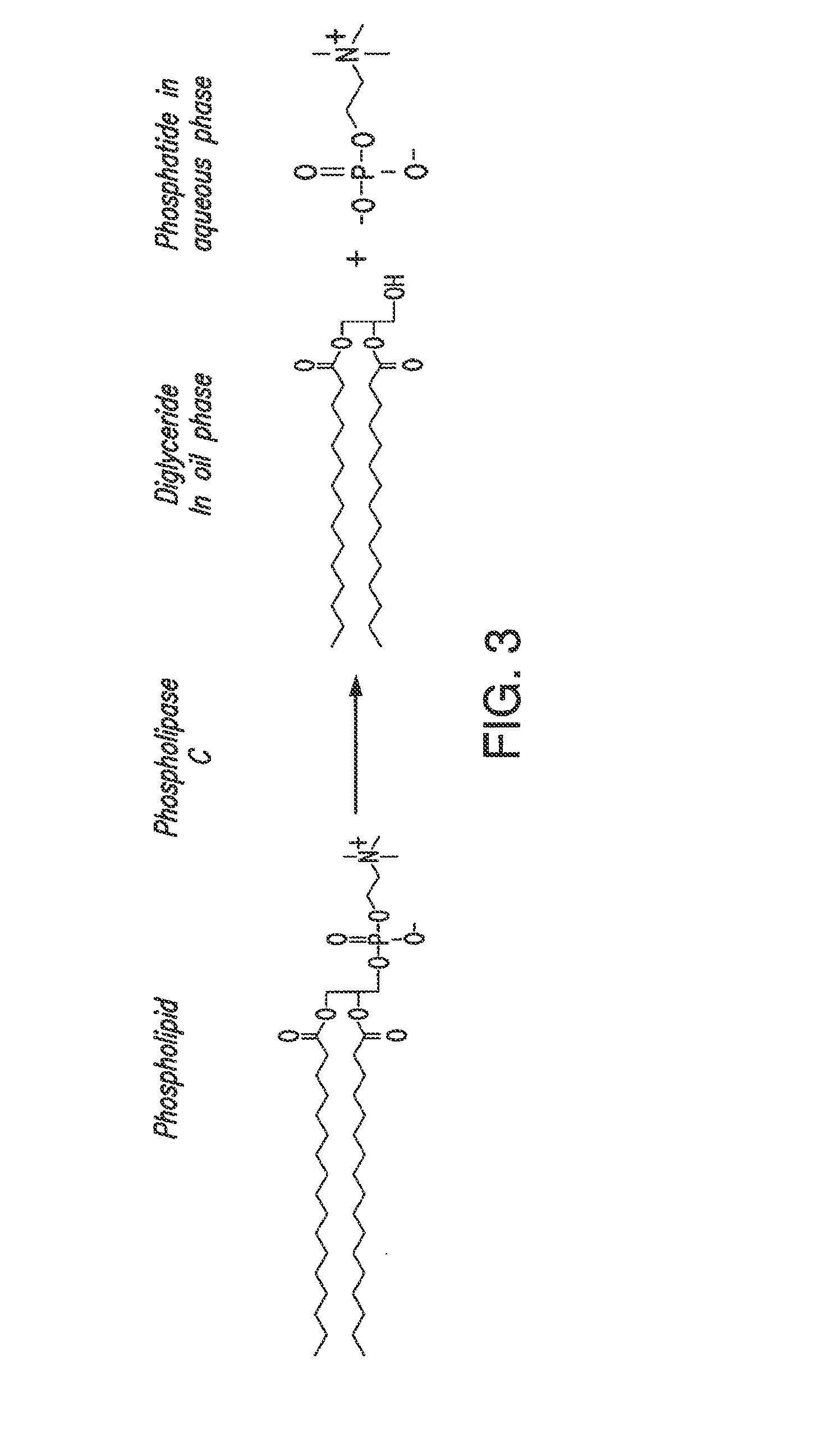
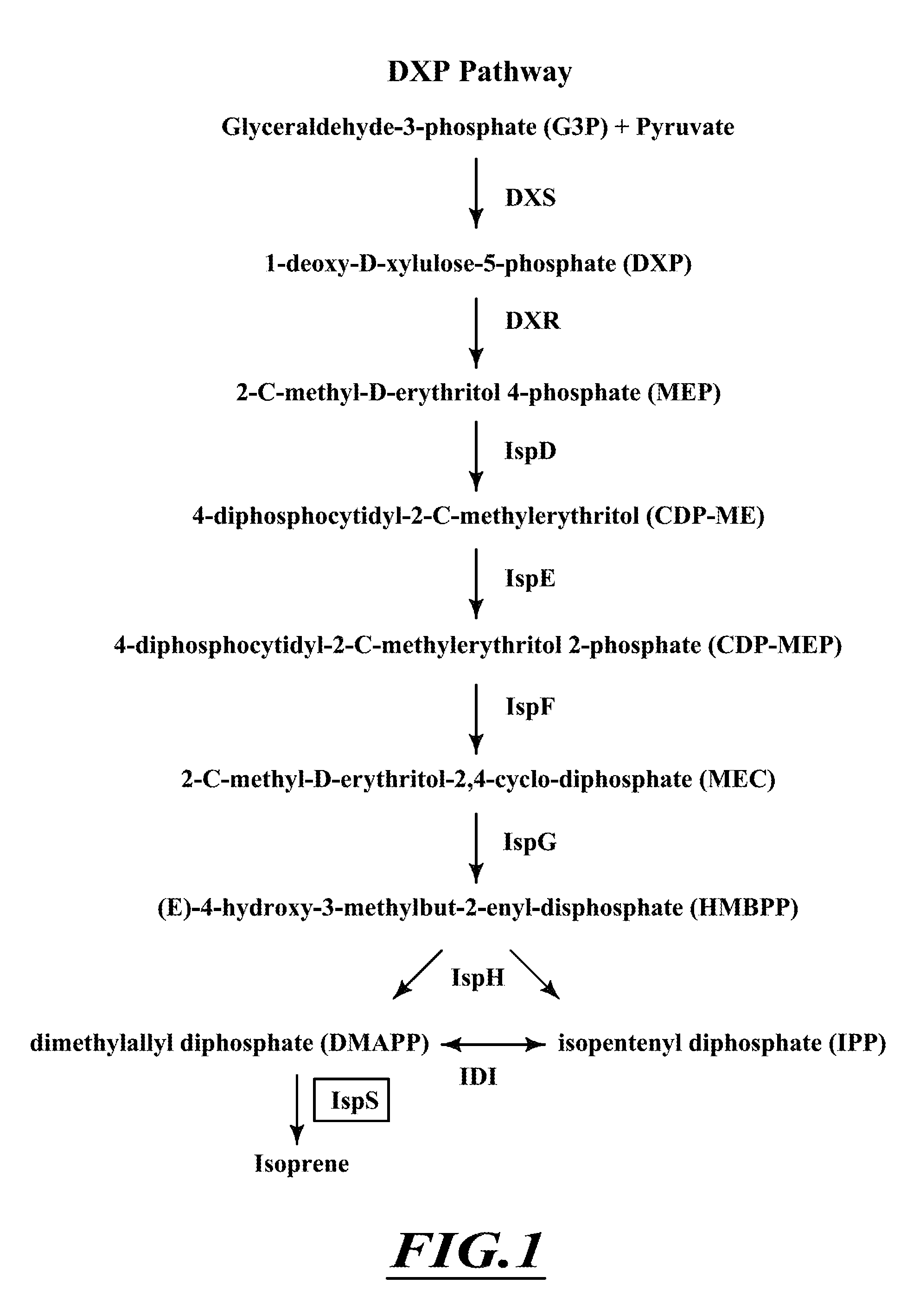
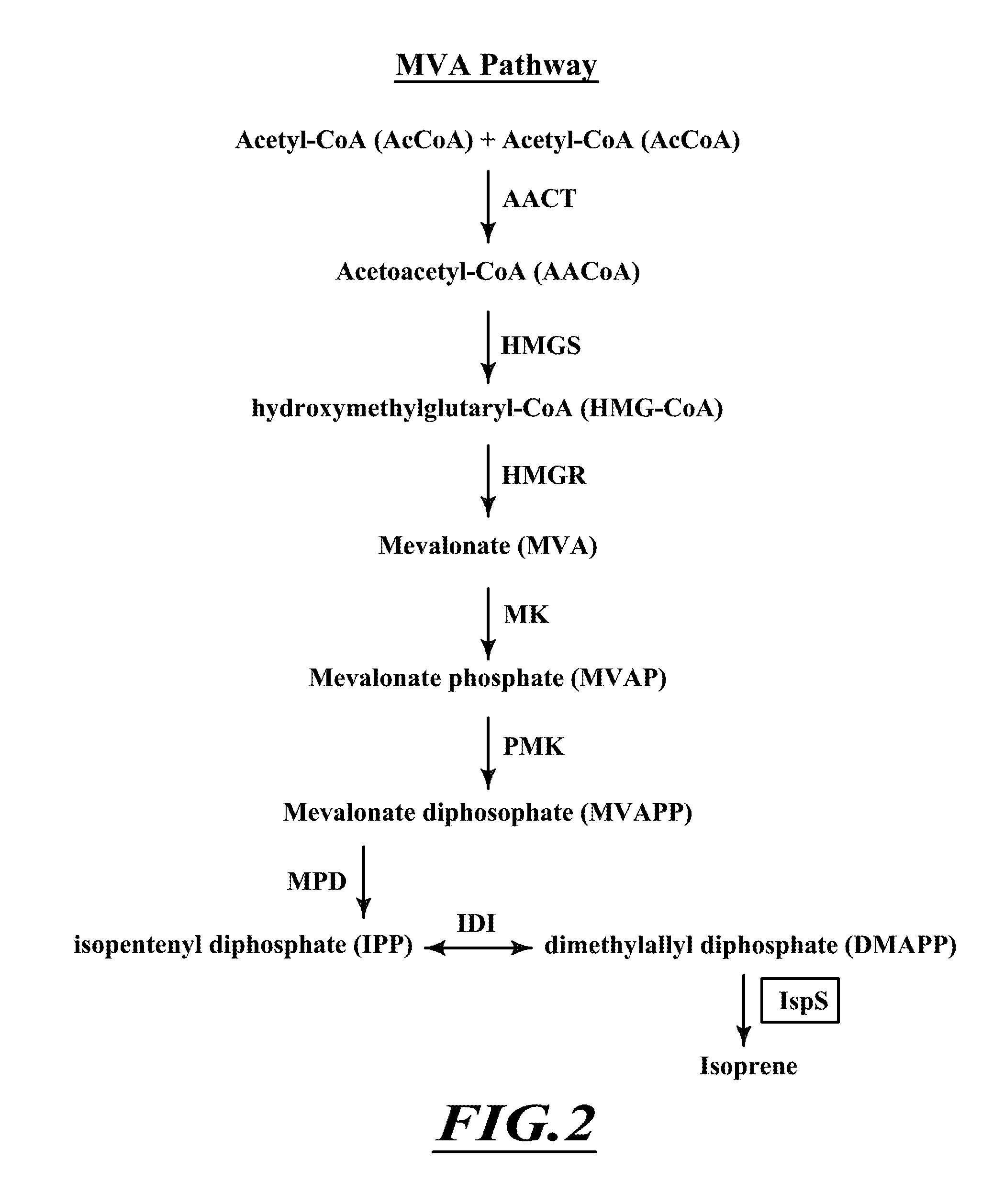
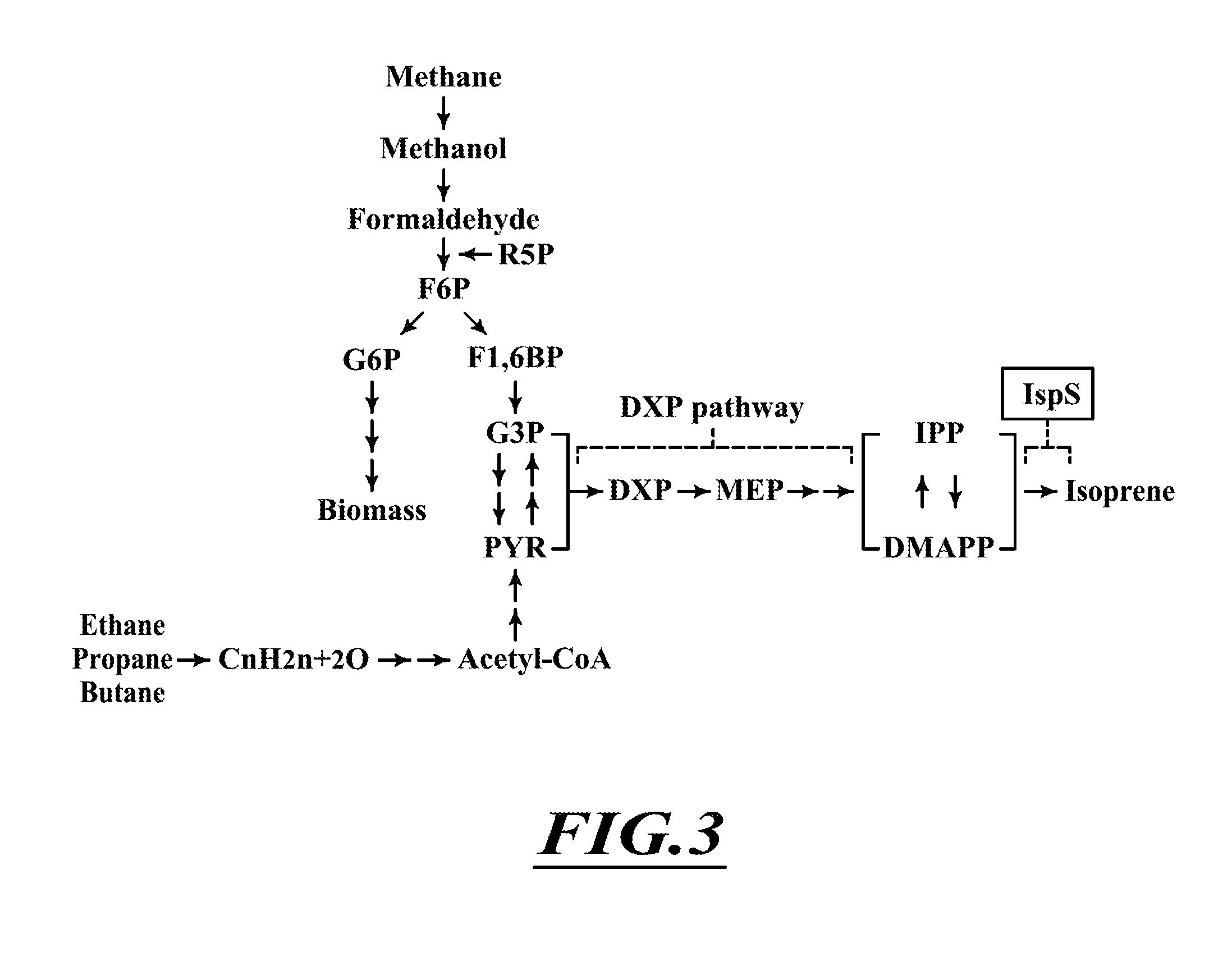
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
96results about "Phosphorus-oxygen lyases" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
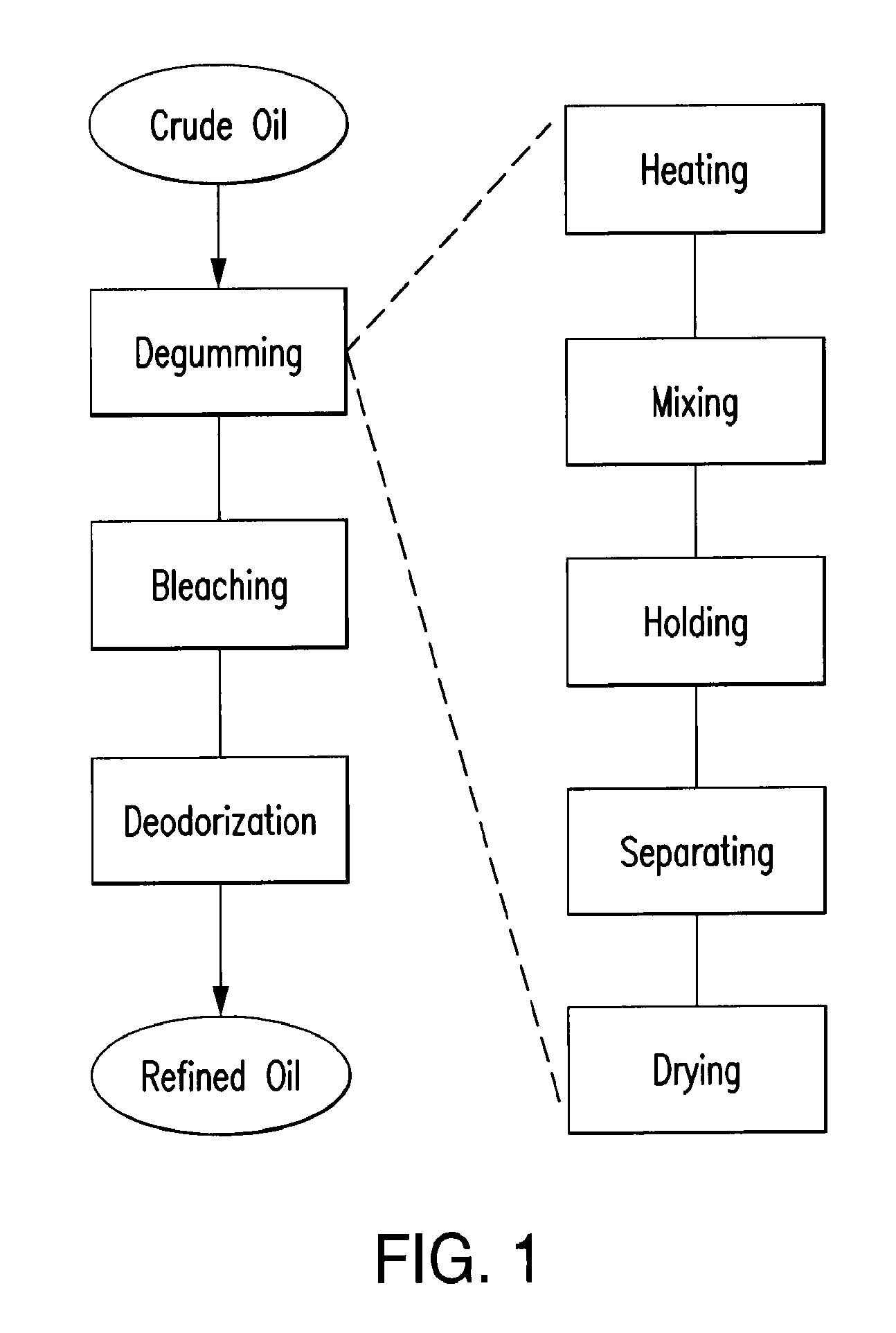
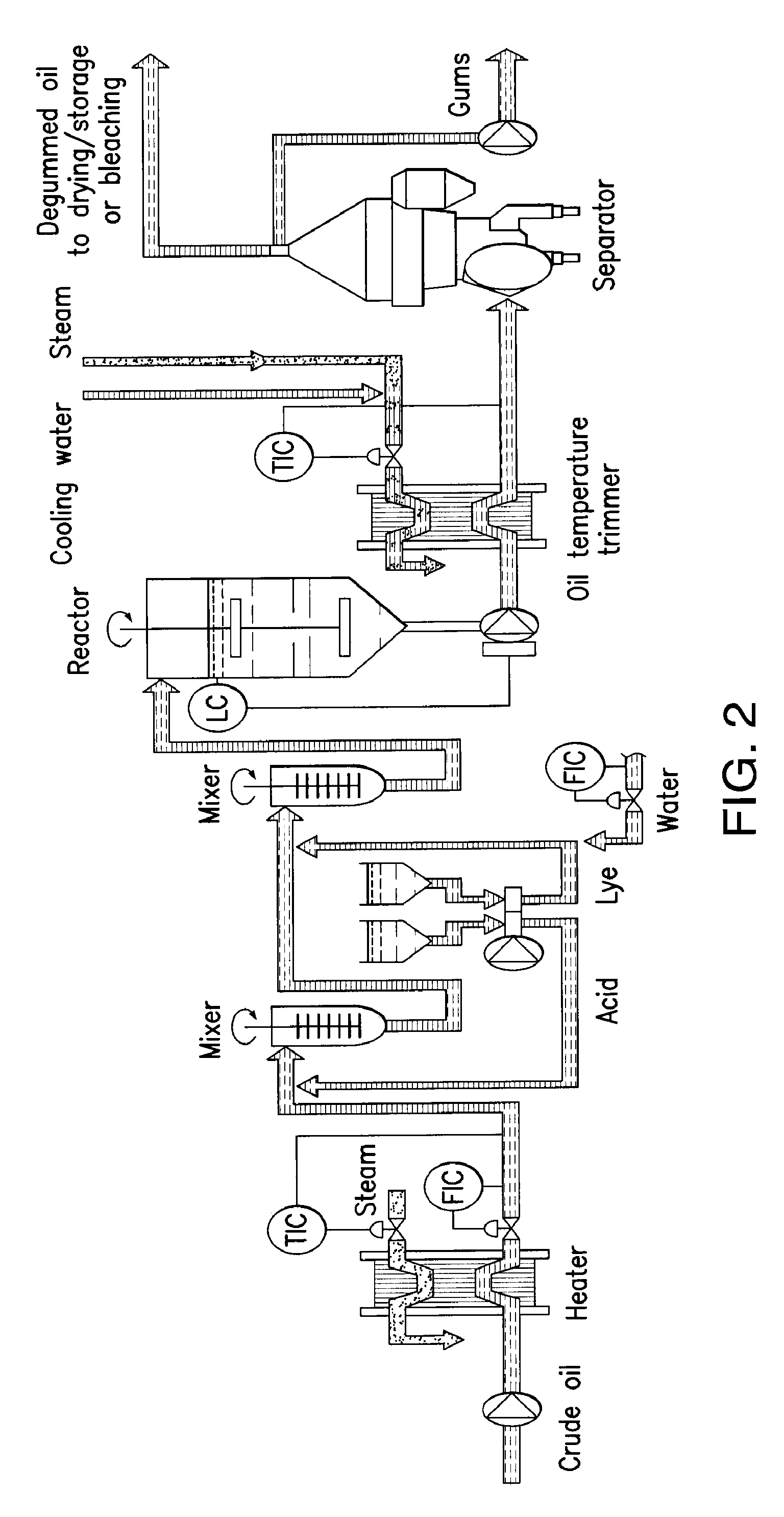
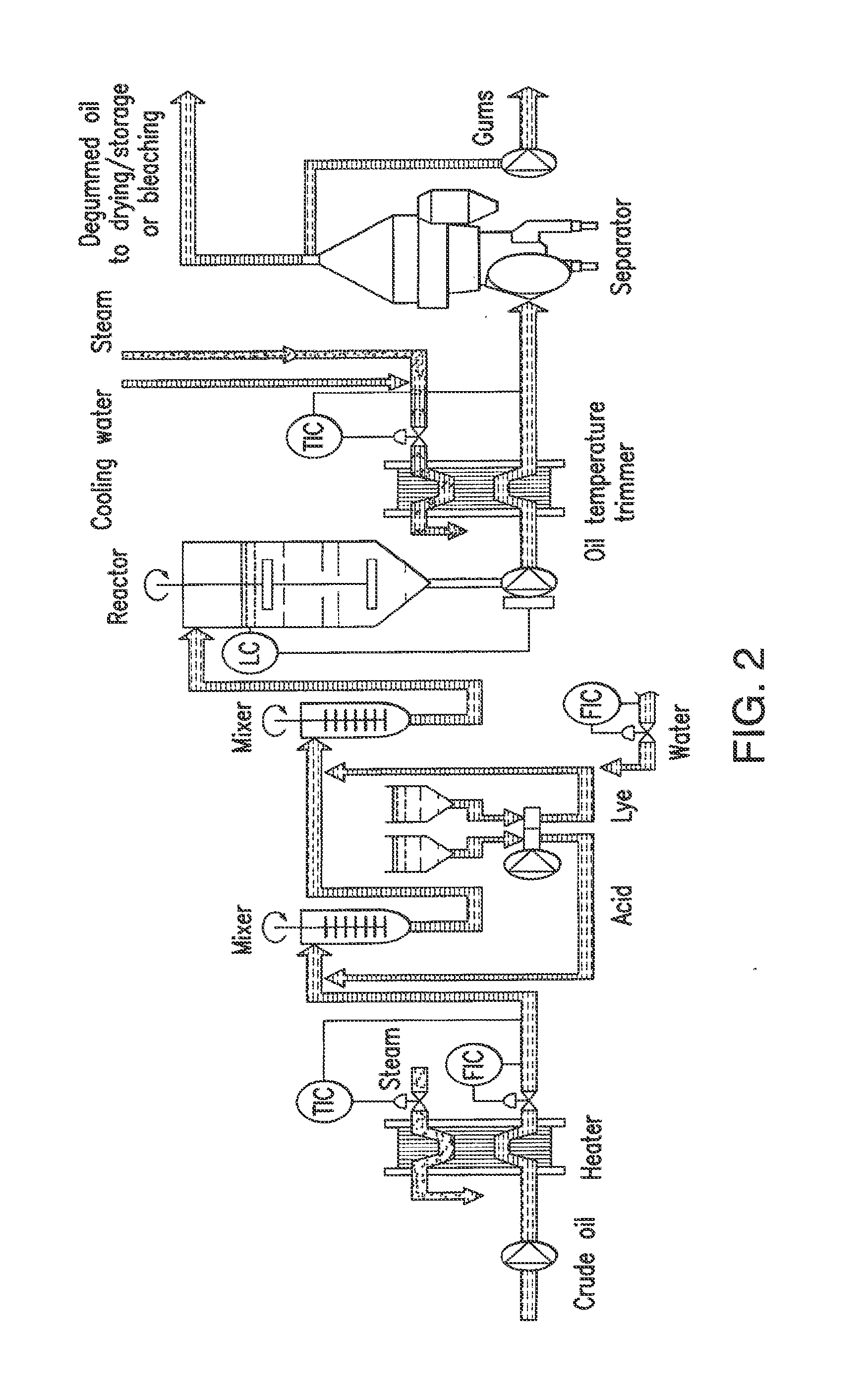
Oil degumming methods
ActiveUS20130011887A1Improve reaction speedImprove heat resistanceFatty acids production/refiningOther chemical processesVegetable oilPhospholipase
In alternative embodiments, the invention provides phosphatidylinositol-specific phospholipase C (PI-PLC) enzymes, nucleic acids encoding them, antibodies that bind specifically to them, and methods for making and using them. Industrial methods and products comprising use of these phospholipases are also provided. In certain embodiments, provided herein are methods for hydration of non hydratable phospholipids (NHPs) within a lipid matrix. The methods enable migration of NHPs to an oil-water interface thereby allowing the NHPs to be reacted and / or removed from the lipids. In certain embodiments, provided is a method for removing NHPs, hydratable phospholipids, and lecithins from vegetable oils to produce a degummed oil or fat product that can be used for food production and / or non-food applications. In certain embodiments, provided herein are methods for hydration of NHPs followed by enzymatic treatment and removal of various phospholipids and lecithins. The methods provided herein can be practiced on either crude or water-degummed oils.
Owner:DSM IP ASSETS BV +1
Host cells with artificial endosymbionts
The present invention is directed generally to eukaryotic host cells comprising artificial endosymbionts and methods of introducing artificial endosymbionts into eukaryotic host cells. The invention provides artificial endosymbionts that introduce a phenotype to host cells that is maintained in daughter cells. The invention additionally provides eukaryotic host cells containing magnetotactic bacteria.
Owner:BELL BIOSYST
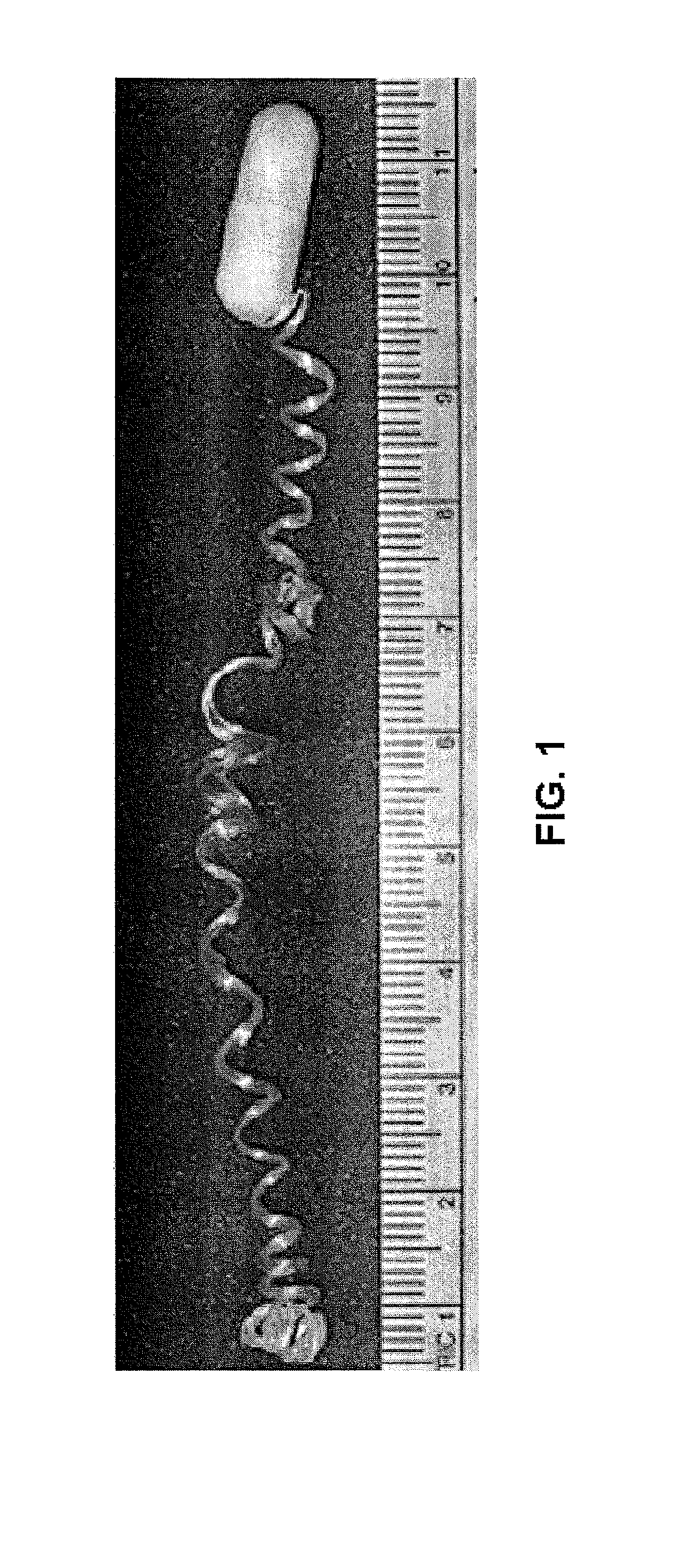
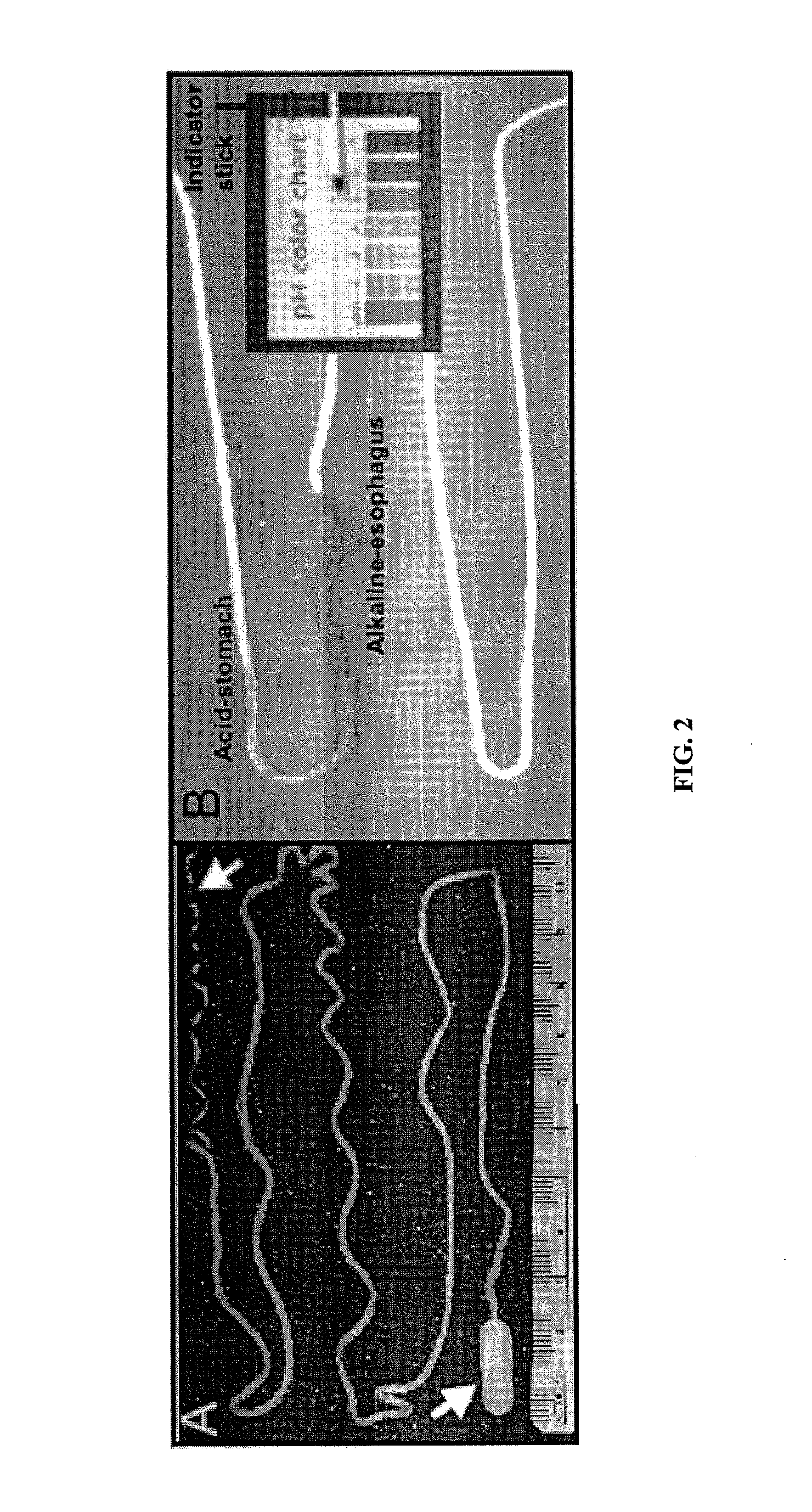
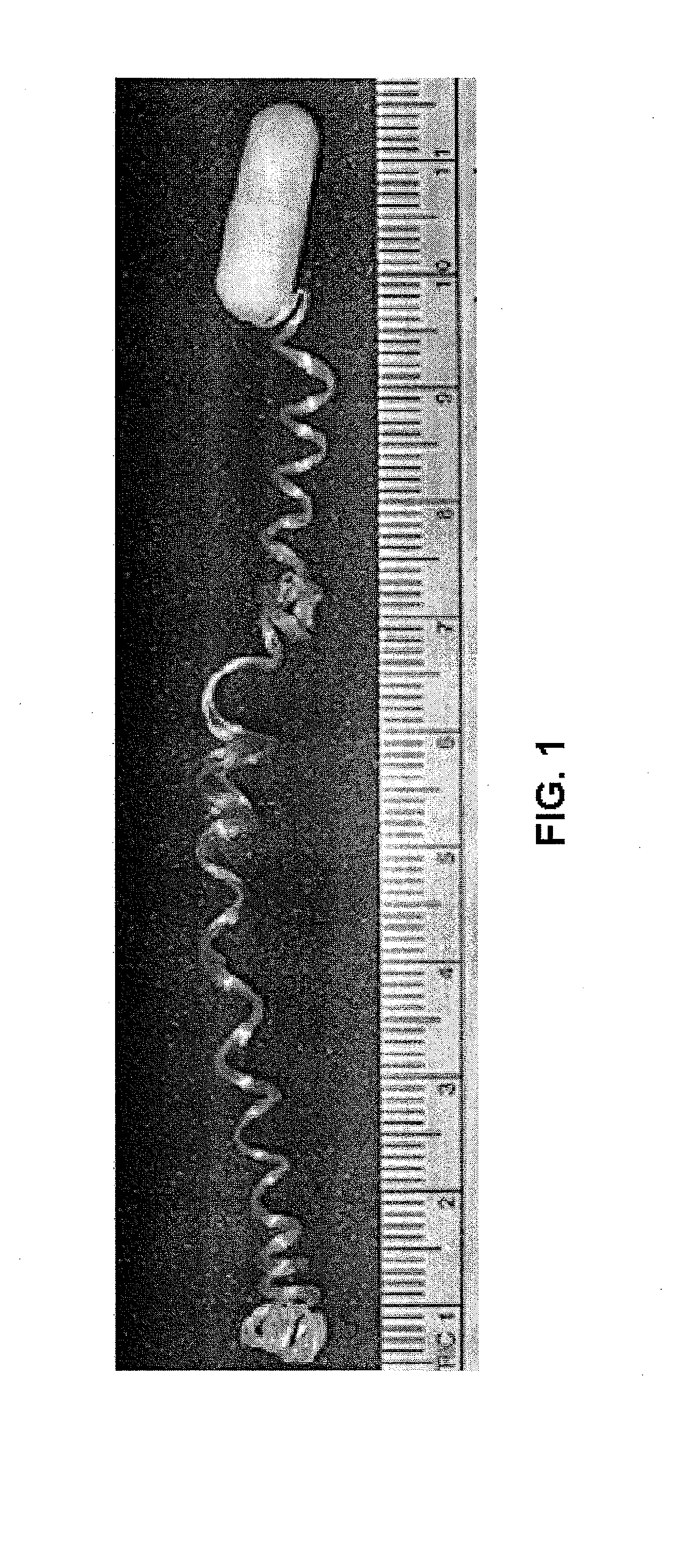
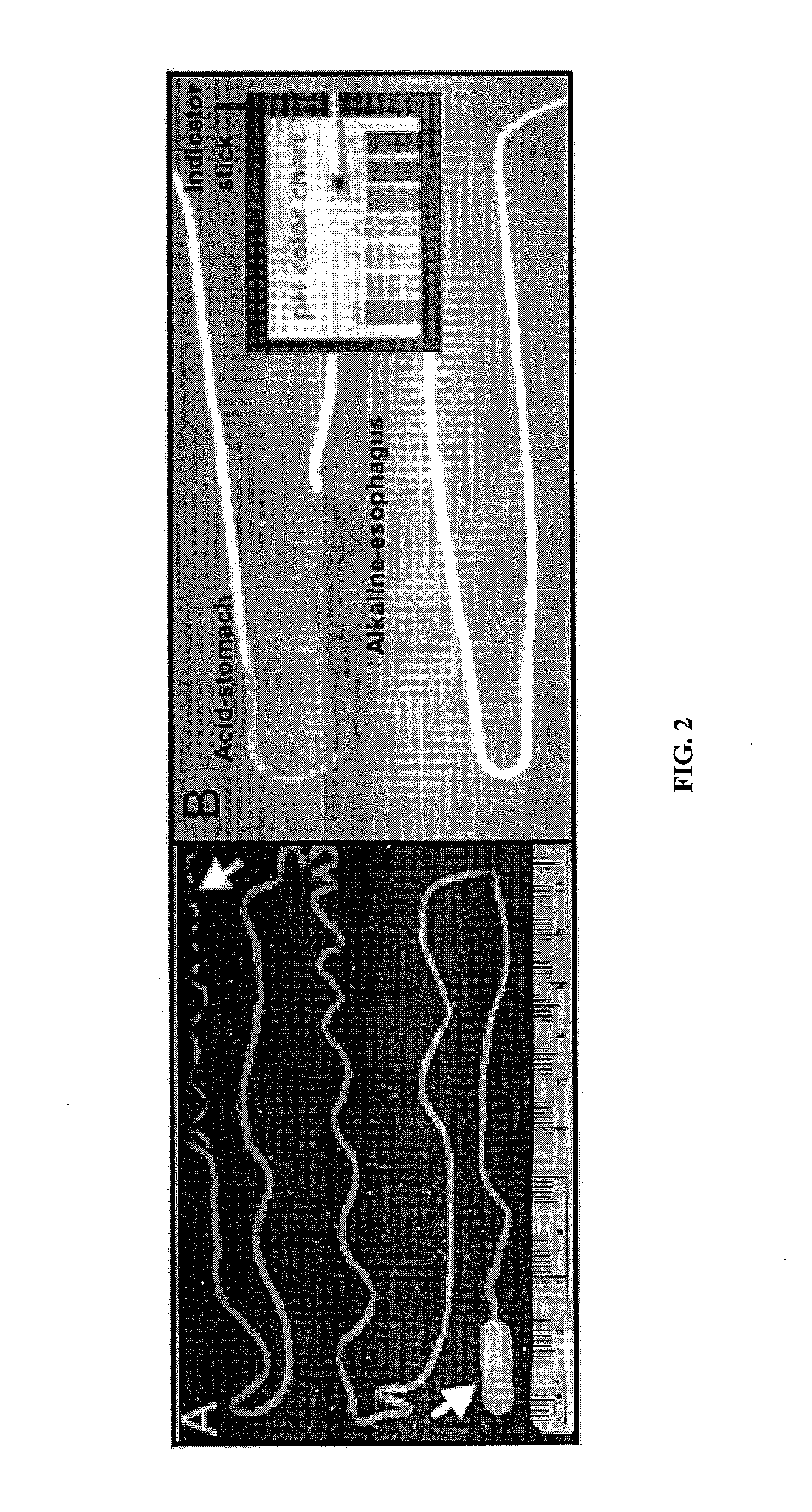
Minimally-invasive measurement of esophageal inflammation
Owner:UNIV OF COLORADO THE REGENTS OF +1
Host cells with artificial endosymbionts
Owner:BELL BIOSYST
Recombinant microorganisms and uses therefor
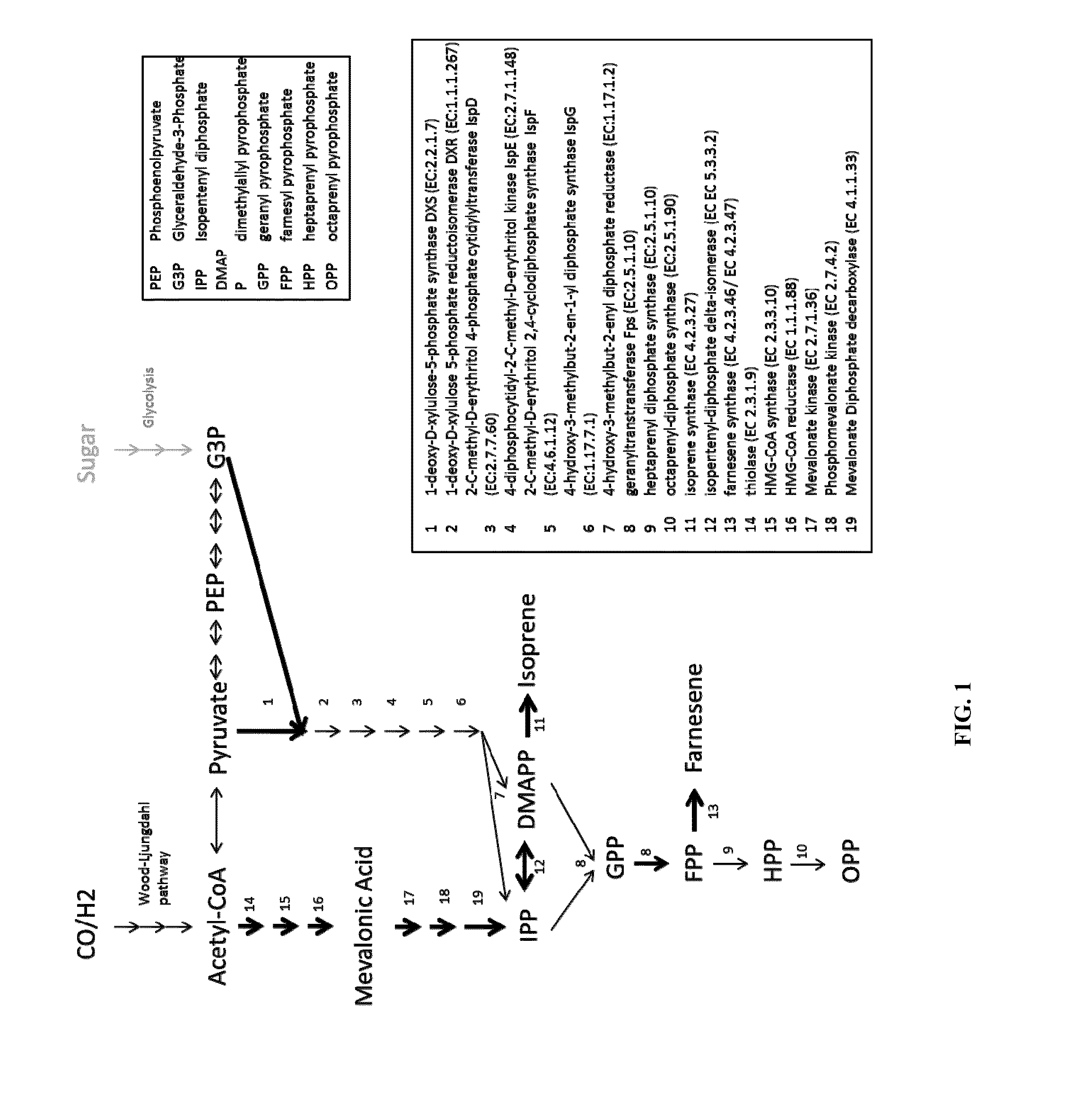
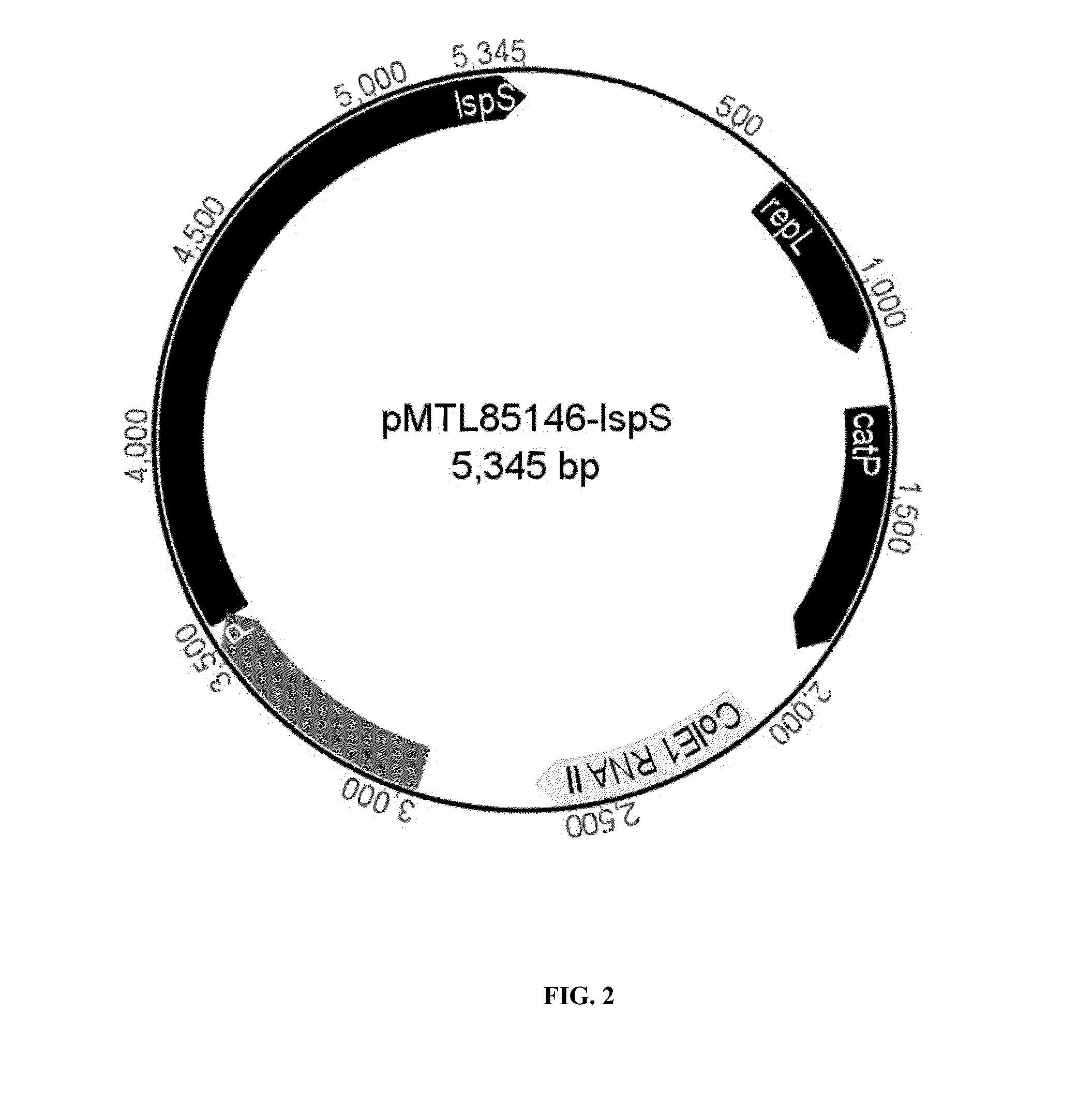
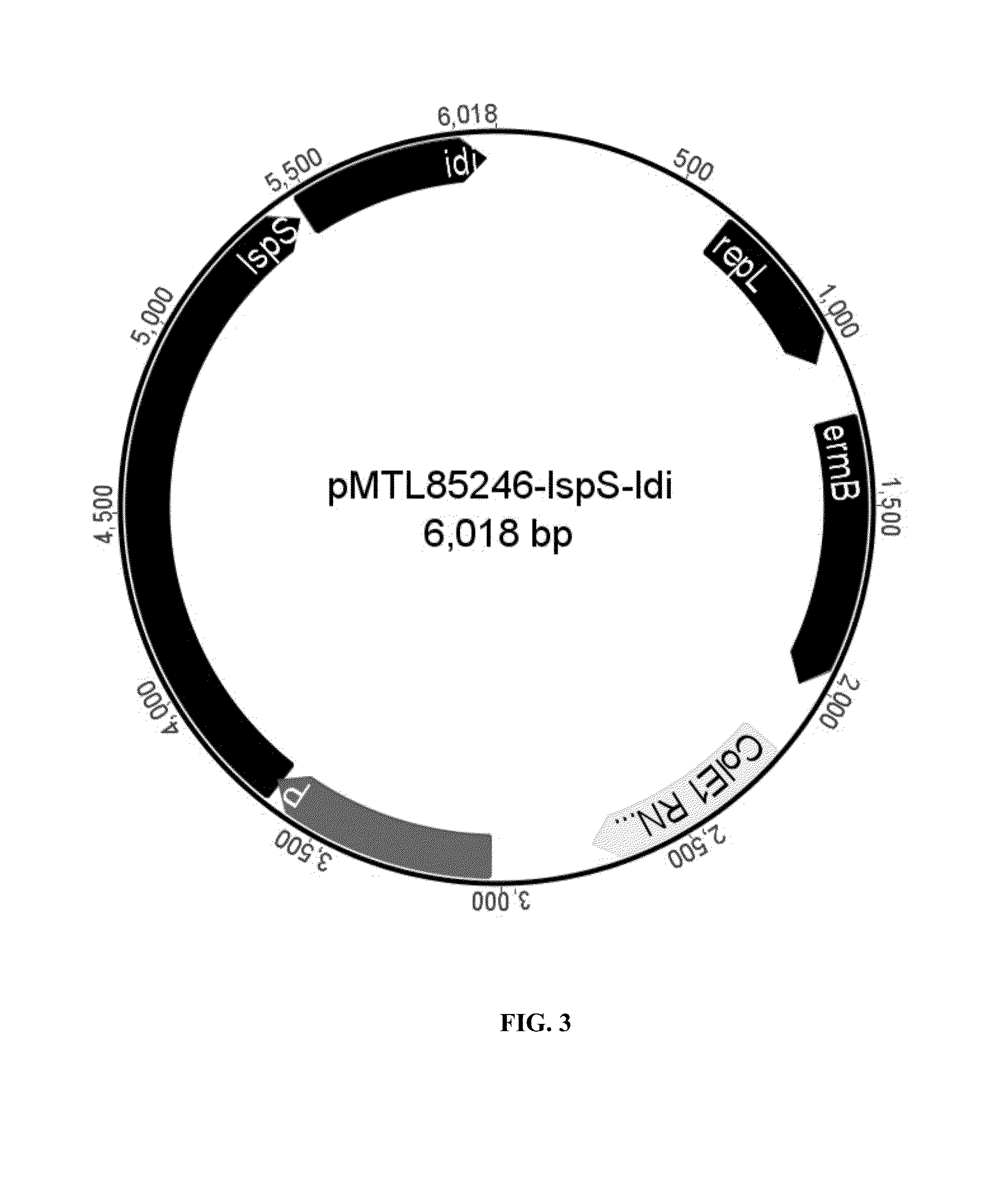
Terpenes are valuable commercial products used in a diverse number of industries. Terpenes may be produced from petrochemical sources and from terpene feed-stocks, such as turpentine. However, these production methods are expensive, unsustainable and often cause environmental problems including contributing to climate change. Microbial fermentation provides an alternative option for the production of terpenes. One or more terpenes and / or precursors can be produced by microbial fermentation of a substrate comprising CO. Recombinant microorganisms may be used in such methods. Carboxydotrophic, acetogenic, recombinant microorganisms can be used in such methods. The recombinant microorgnsims may contain exogenous mevalonate (MVA) pathway enzymes and / or DXS pathway enzymes, for example.
Owner:LANZATECH NZ INC
Minimally-invasive measurement of esophageal inflammation
The methods and apparatus of the present invention allow the evaluation of inflammation of the esophagus. Measurements may be utilized, for example, to diagnose a disease of the esophagus, to monitor inflammation of the esophagus, or to access the treatment of a disease of the esophagus. In one embodiment, the invention comprises a method for measuring esophageal inflammation comprising deploying a device into the esophagus of a subject, removing the device after a predetermined period of time, analyzing the device for a diagnostic indicator of esophageal inflammation and evaluating the diagnostic indicator to diagnose esophageal inflammation.
Owner:UNIV OF COLORADO THE REGENTS OF +1
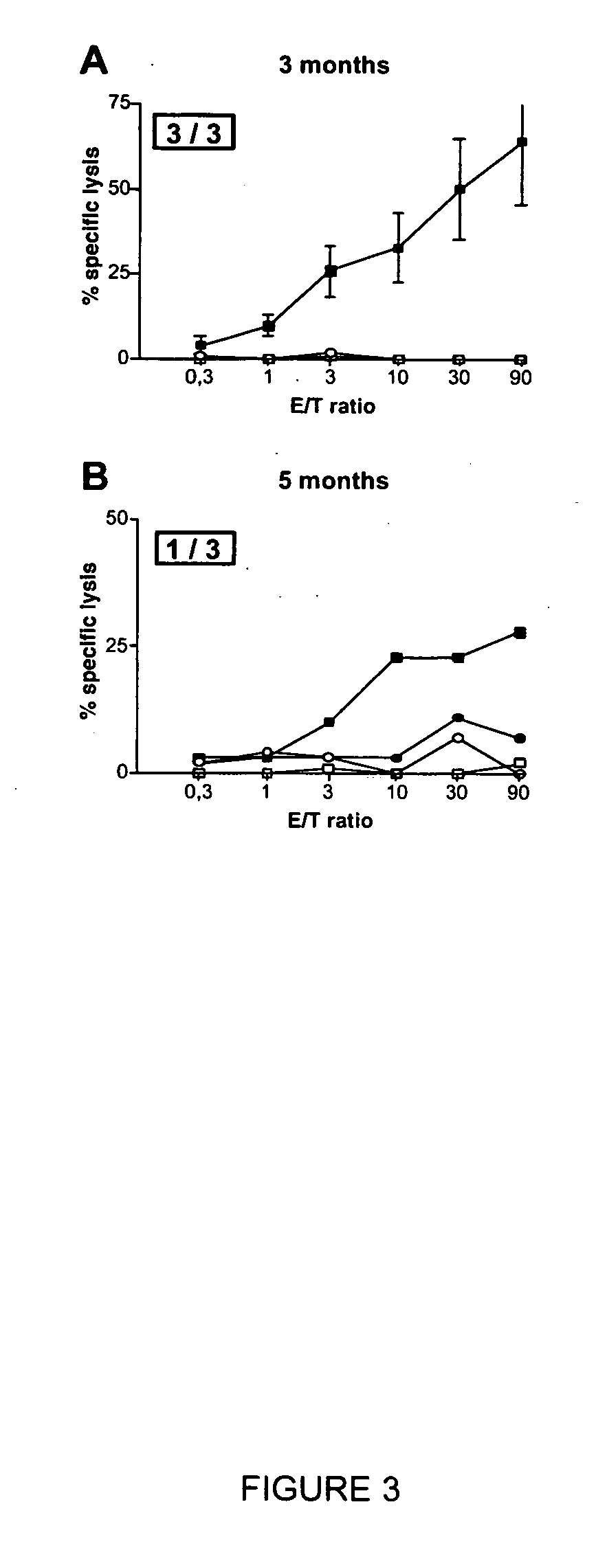
Recombinant adenylate cyclase toxin of Bordetella induces T cell responses against tumoral antigens
An immunogenic composition comprising a recombinant protein comprising a Bordetella CyaA, or a fragment thereof, and a peptide that corresponds to a tumor antigen is provided as a cancer treatment. Methods of treatment with this immunogenic composition are also provided. In an embodiment, the therapeutic composition is a treatment for melanoma, and comprises epitopes from the HLA*0201 epitope. These epitopes include Tyr or GnT-V, and are present in the recombinant proteins CyaA-E5-Tyr and CyaA-E5-GnT-V.
Owner:INST PASTEUR +3
Oil degumming methods
ActiveUS20140371476A1Improve heat resistanceImprove thermal stabilityOrganic chemistryPhosphorus-oxygen lyasesPhospholipaseVegetable oil
In alternative embodiments, the invention provides phosphatidylinositol-specific phospholipase C (PI-PLC) enzymes, nucleic acids encoding them, antibodies that bind specifically to them, and methods for making and using them. Industrial methods and products comprising use of these phospholipases are also provided. In certain embodiments, provided herein are methods for hydration of non hydratable phospholipids (NHPs) within a lipid matrix. The methods enable migration of NHPs to an oil-water interface thereby allowing the NHPs to be reacted and / or removed from the lipids. In certain embodiments, provided is a method for removing NHPs, hydratable phospholipids, and lecithins from vegetable oils to produce a degummed oil or fat product that can be used for food production and / or non-food applications. In certain embodiments, provided herein are methods for hydration of NHPs followed by enzymatic treatment and removal of various phospholipids and lecithins. The methods provided herein can be practiced on either crude or water-degummed oils.
Owner:BUNGE GLOBAL INNOVATION LLC
Polyamine-polyphenol modified graphene oxide carrier, and preparation method and application thereof
ActiveCN111961660AShorten the time of self-assemblyImprove stabilityCarbon compoundsPhosphorus-oxygen lyasesPolyphenolHigh activity
The invention discloses a crosslinking enzyme polymer based on polyamine-polyphenol codeposition, and a preparation method and application thereof, and belongs to the technical field of immobilized enzyme application. Graphene oxide is dispersed in a buffer solution, and then, polyphenol solution and multi-amido polymer solution are added into the buffer solution so as to react to obtain the polyamine-polyphenol modified graphene oxide. The polyamine-polyphenol modified graphene oxide carrier obtained by the method is used for the immobilized enzyme so as to obtain the crosslinking enzyme polymer. The polyamine-polyphenol modified graphene oxide carrier has the advantages of universality, high activity, simpleness in operation and the like.
Owner:NANJING TECH UNIV
Compositions and methods for biological production of isoprene
InactiveUS20160017374A1Increase in red pigmentationIncreased red pigmentationOrganic chemistryBacteriaEngineeringMethanotrophic bacterium
The present disclosure provides compositions and methods for biologically producing isoprene using methanotrophic bacteria that utilize carbon feedstock, such as methane or natural gas.
Owner:CALYSTA
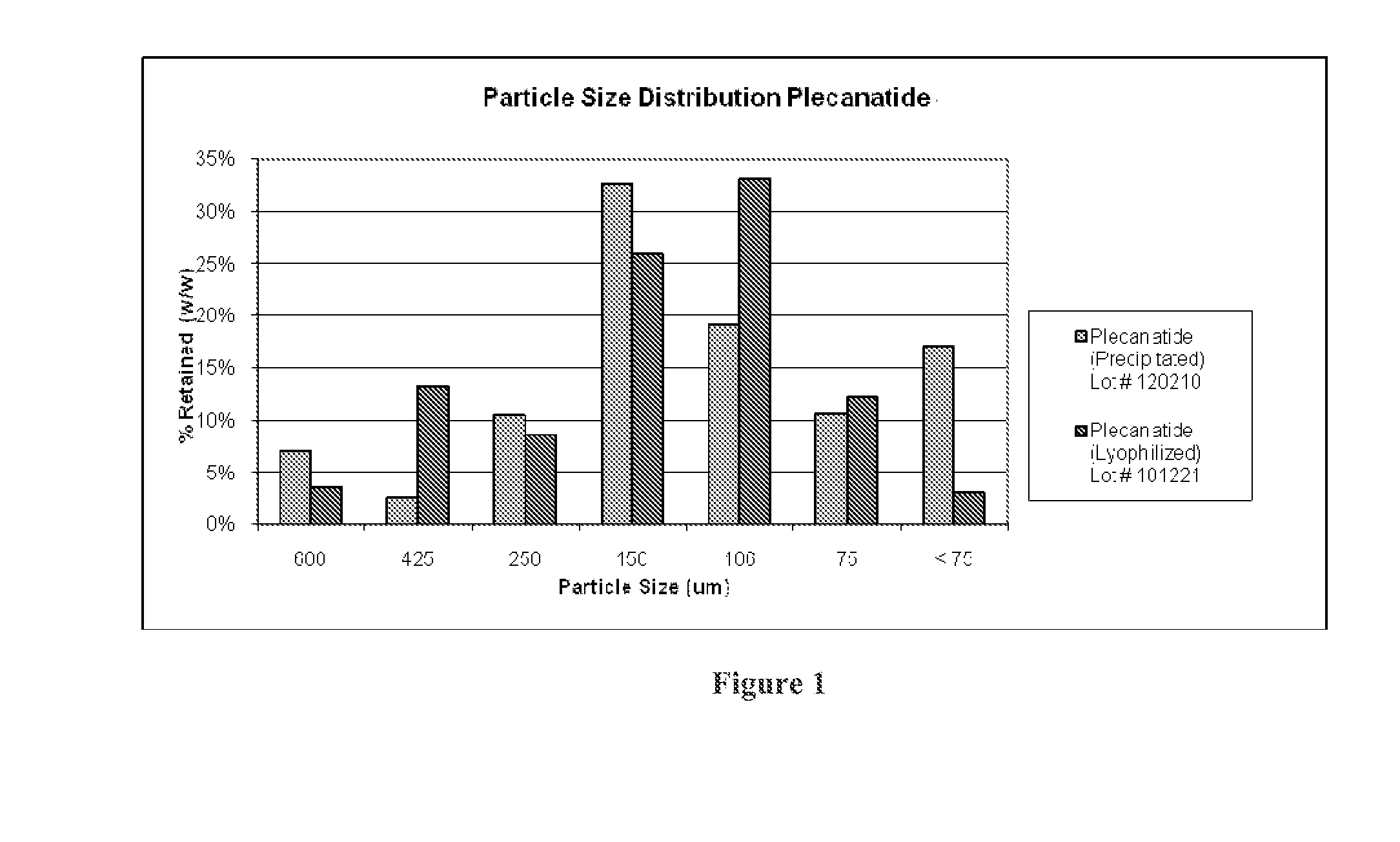
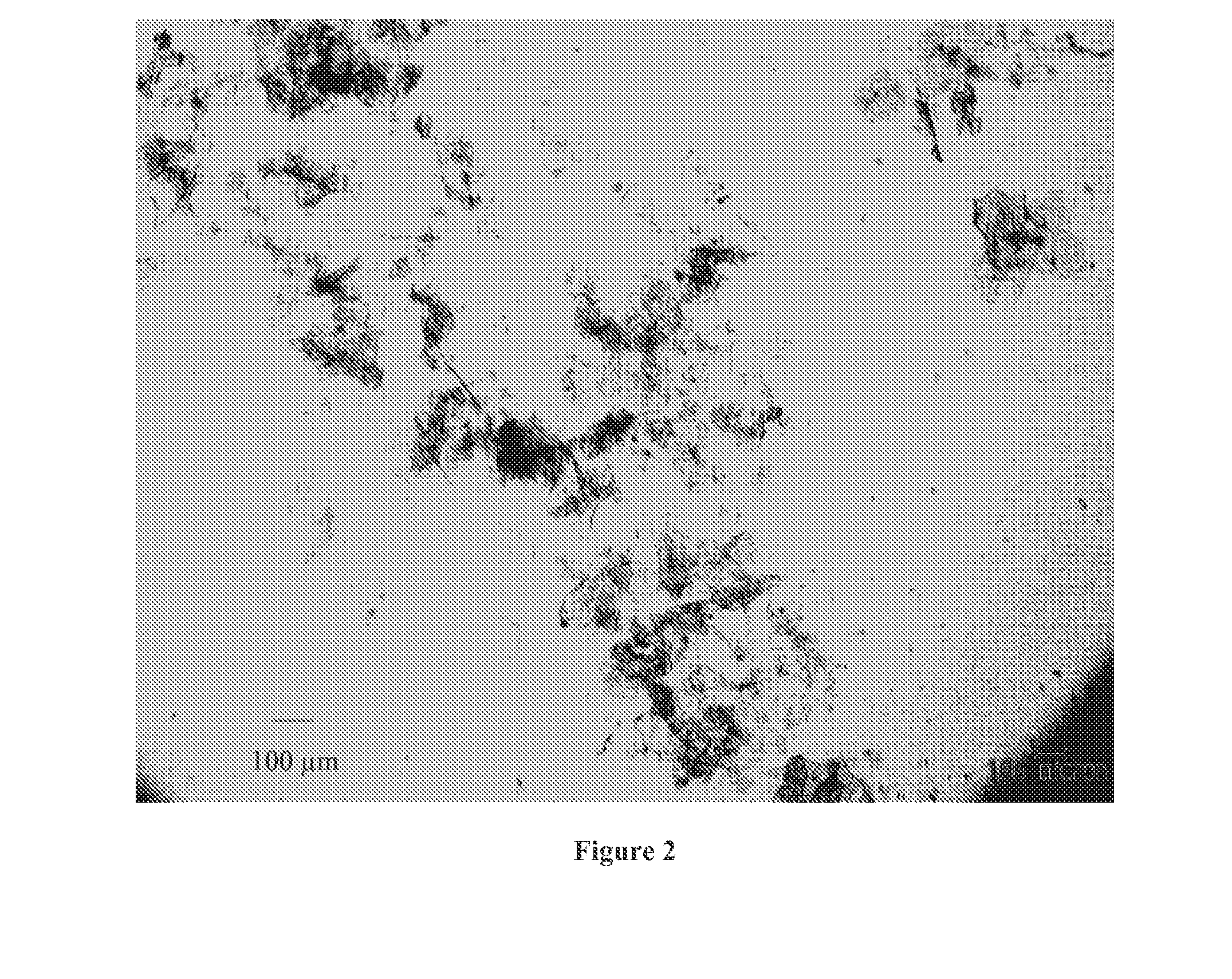
Ultra-pure agonists of guanylate cyclase C, method of making and using same
ActiveUS10011637B2Reduce the amount requiredPeptide/protein ingredientsPhosphorus-oxygen lyasesFreeze-dryingSolvent
Owner:BAUSCH HEALTH IRELAND LTD
Formulations of guanylate cyclase c agonists and methods of use
ActiveUS20150366935A1Unexpected efficacyMaintain good propertiesAntibacterial agentsPeptidesMedicineAgonist peptide
The invention provides low-dose formulations of guanylate cyclase-C (“GCC”) agonist peptides and methods for their use. The formulations of the invention can be administered either alone or in combination with one or more additional therapeutic agents, preferably an inhibitor of cGMP-dependent phosphodiesterase or a laxative.
Owner:BAUSCH HEALTH IRELAND LTD
Microbial fermentation for the production of terpenes
The invention provides a method for producing a terpene or a precursor thereof by microbial fermentation. Typically, the method involves culturing a recombinant bacterium in the presence of a gaseous substrate whereby the bacterium produces a terpene or a precursor thereof, such as mevalonic acid, isopentenyl pyrophosphate, dimethylallyl pyrophosphate, isoprene, geranyl pyrophosphate, farnesyl pyrophosphate, and / or farnesene. The bacterium may comprise one or more exogenous enzymes, such as enzymes in mevalonate, DXS, or terpene biosynthesis pathways.
Owner:LANZATECH NZ INC
Recombinant escherichia coli for producing cyclic adenosine monophosphate with high yield and application thereof in synthetization of cyclic adenosine monophosphate
PendingCN110157653AMeet the requirements of large-scale industrial productionEasy to separateBacteriaPhosphorus-oxygen lyasesEscherichia coliRecombinant escherichia coli
The invention discloses recombinant escherichia coli for producing cyclic adenosine monophosphate with high yield and application of the recombinant escherichia coli in synthetization of cyclic adenosine monophosphate. An adenylate cyclase gene is cloned from a cAMP production strain, a crude enzyme solution obtained after recombinant bacteria are broken has good catalytic activity and stability,adenosine triphosphate (ATP) and Zn2 + are added, a reaction system is simple, the conditions are mild, a period is short, few byproducts are produced, the method is clean and pollution-free and is asimple, rapid and efficient production way, and the conversion rate of substrate ATP reaches 90% or above.
Owner:NANJING UNIV OF TECH
Asx-specific protein ligase
ActiveUS10590407B2Highly versatilePhosphorus-oxygen lyasesPeptide preparation methodsCyclase activityNucleic acid
Owner:NANYANG TECH UNIV
Method for preparing cyclic adenosine monophosphate by adenylate cyclase
PendingCN112063670AImprove stabilityExtended half-lifePhosphorus-oxygen lyasesFermentationCyclaseAdenosine
The invention discloses a method for preparing cyclic adenosine monophosphate by adenylate cyclase. The method comprises the following steps that the adenylate cyclase serves as a catalyst, adenosinetriphosphate serves as a substrate, and in the presence of Mg <2+>, a reaction solution containing the cyclic adenosine monophosphate is obtained. According to the adenylate cyclase, an amino acid sequence as shown in SEQ ID NO.14 is provided, the stability is good, the half-life period is long at medium and low temperatures, ATP can be efficiently catalyzed to generate cAMP in one step at mediumtemperature, a substrate conversion rate reaches 90 % or above, the advantages of simple industry, mild conditions, short period, few by-products and the like are achieved, the clean and pollution-free effects are achieved, and the energy conservation, consumption reduction and emission reduction in the cAMP production process can be realized.
Owner:杭州美亚药业股份有限公司
Polypeptide with immune cell targeted recognition function and application thereof
ActiveCN110885805ASpecific phenotypic response is not obviousWeak stainingPhosphorus-oxygen lyasesGeneral/multifunctional contrast agentsCyclaseNucleic acid sequencing
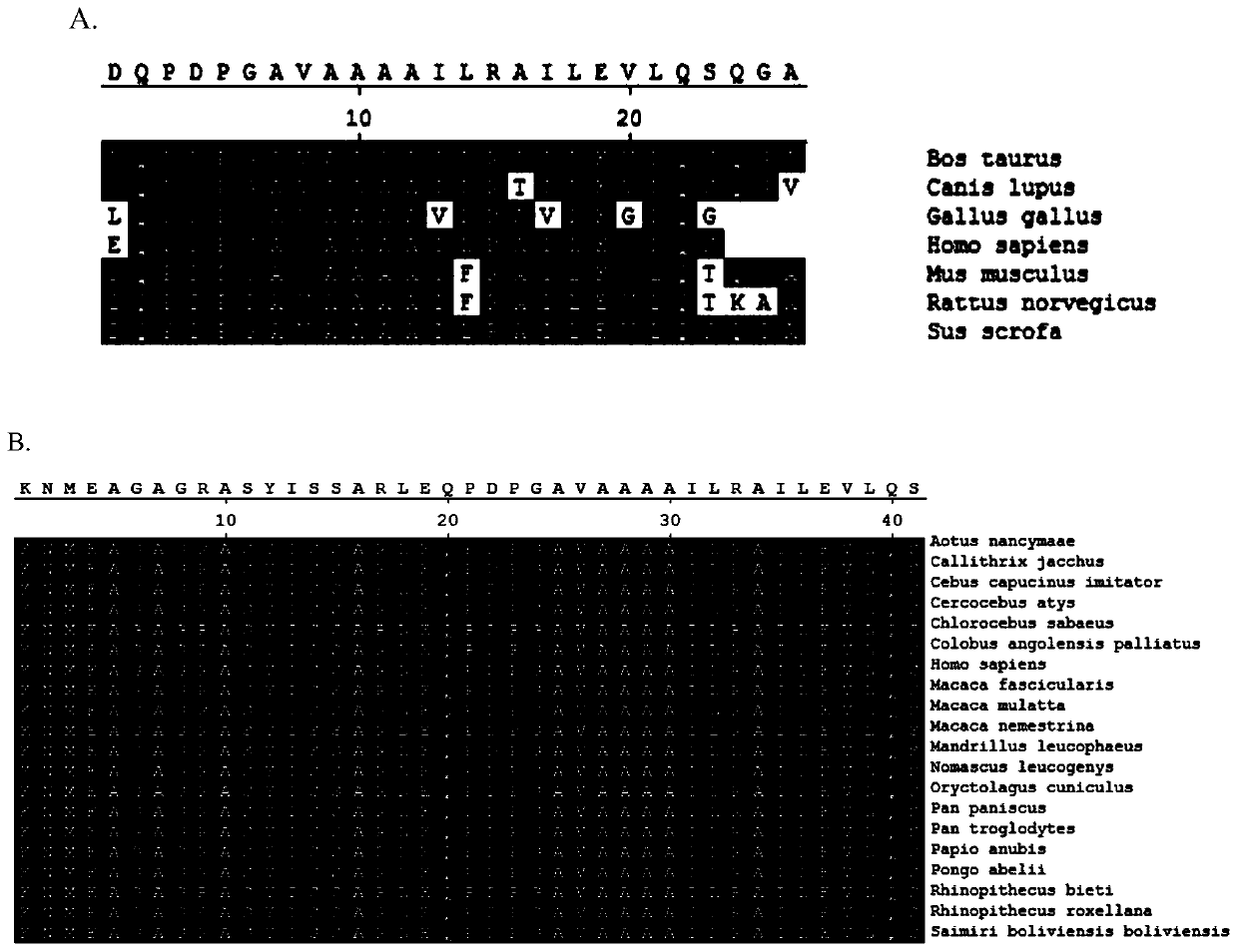
The invention discloses a polypeptide for recognizing immune cells. The polypeptide comprises the following amino acid sequences: (a) an amino acid sequence of a C-terminal fragment sequence EQPDPGAVAAAAILRAILE containing human Triokinase / FMN cyclase; or (b) an amino acid sequence which is basically the same as the amino acid sequence in (a), and the basically the same means that the amino acid sequence in (a) has more than 80% of identity with that in (b). The invention also discloses a nucleic acid sequence for encoding the polypeptide; the polypeptide for targeted recognition of immune cells comprising the polypeptide and a polypeptide probe with a reporter group; a kit comprising the probe; and an application of the polypeptide or the probe in preparation of the kit for imaging livingcells of mammals.
Owner:KEYANGLE LIFE TECH CO LTD
Raav-guanylate cyclase compositions and methods for treating leber's congenital amaurosis-1 (LCA1)
Disclosed are viral vector compositions comprising polynucleotide sequences that express one or more biologically-active mammalian guanylate cyclase proteins. Also disclosed are methods for their use in preventing, treating, and / or ameliorating at least one or more symptoms of a disease, disorder, abnormal condition, or dysfunction resulting at least in part from a guanylate cyclase deficiency in vivo. In particular embodiments, the use of recombinant adeno-associated viral (rAAV) vectors to treat or ameliorate symptoms of Leber's congenital amaurosis, as well as other conditions caused by an absence or reduction in the expression of a functional retinal-specific guanylate cyclase 1 (retGC1).
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Crystals and structures of 2C-methyl-D-erythritol 2,4-cyclodiphosphate synthase MECPS
InactiveUS20030073134A1Convenient bindingEnhanced interactionPhosphorus-oxygen lyasesPeptidesErythritolMutant
The present invention provides machine readable media embedded with the three-dimensional molecular structure coordinates of 2C-methyl-D-erythritol 2,4-cyclodiphosphate synthase, and subsets thereof, including binding pockets, methods of using the structure to identify and design affecters, including inhibitors and activator, mutants of MECPS, and compounds and compositions that affect MECPS activity.
Owner:STRUCTURAL GENOMIX
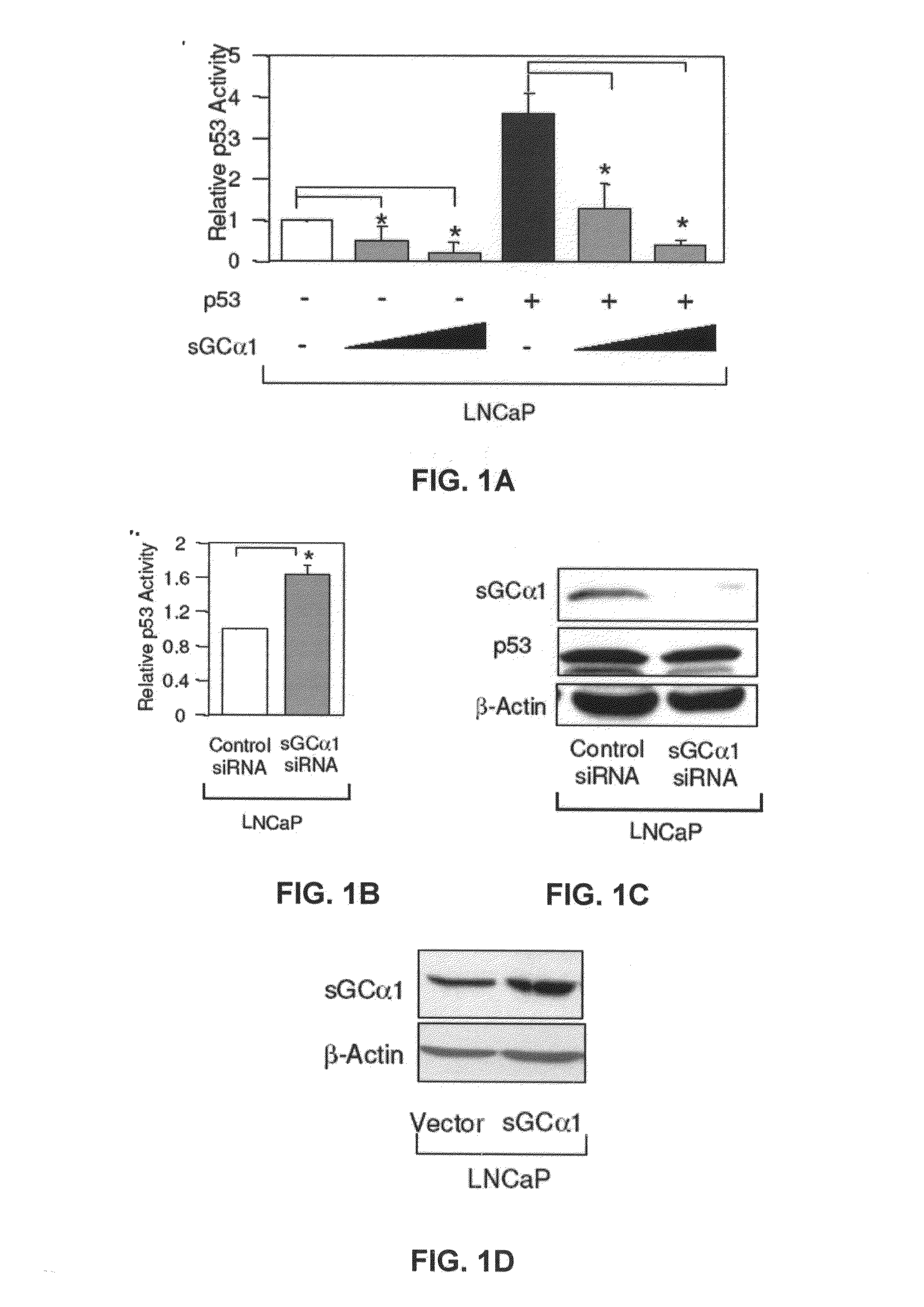
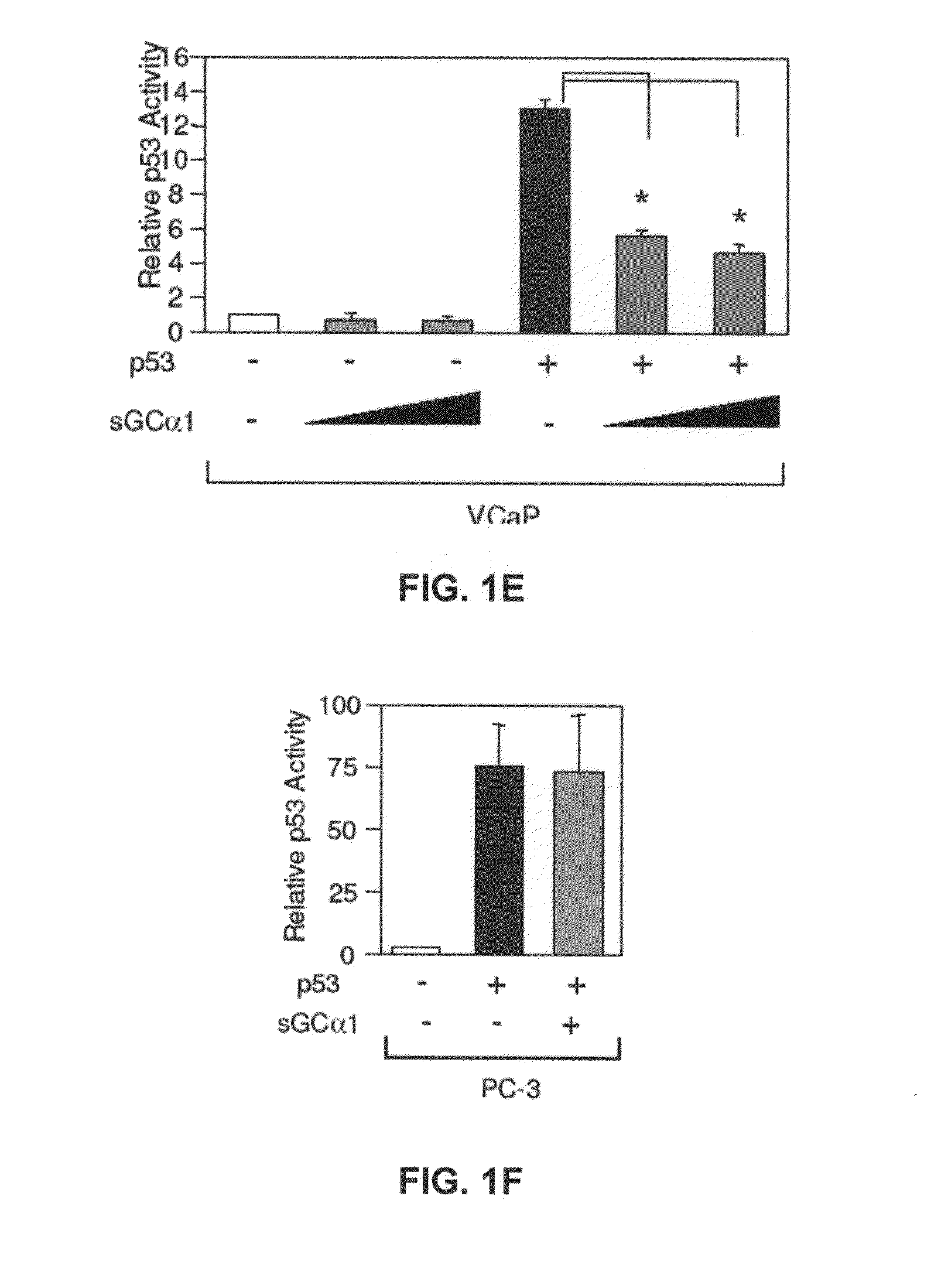
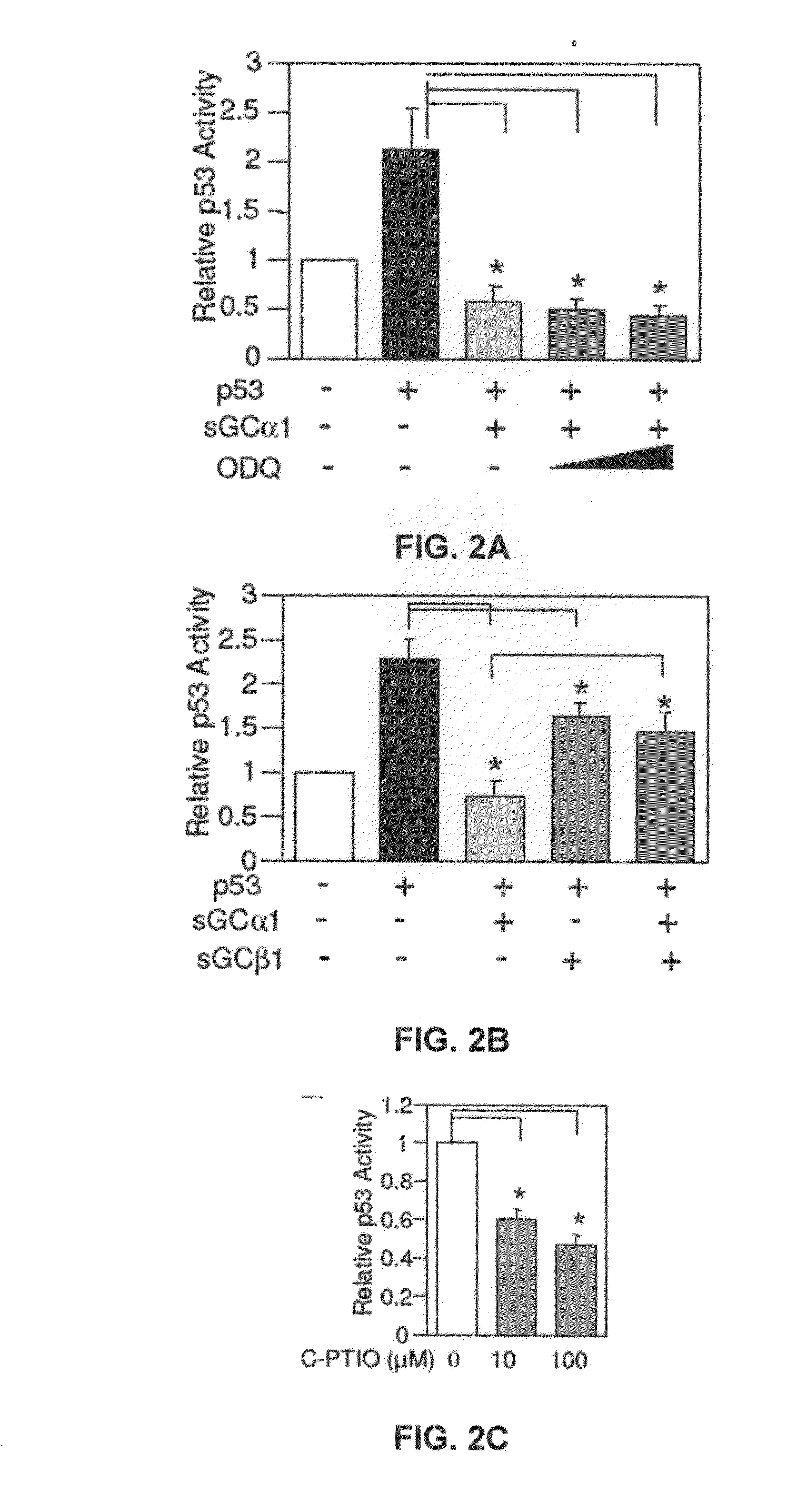
sGCalpha1 Inhibiting Compositions, and Methods of Treatment of Cancers Therewith
The present invention provides materials and methods useful to treat various sGCα1-expressing cancers. Materials include peptides which interfere with sGCα1's pro-survival functions, thereby resulting in apoptosis of sGCα1-expressing cells. In addition, the present invention provides screening assays, diagnostic assays, methods to prognose, methods to treat, and kits.
Owner:UNIVERSITY OF TOLEDO
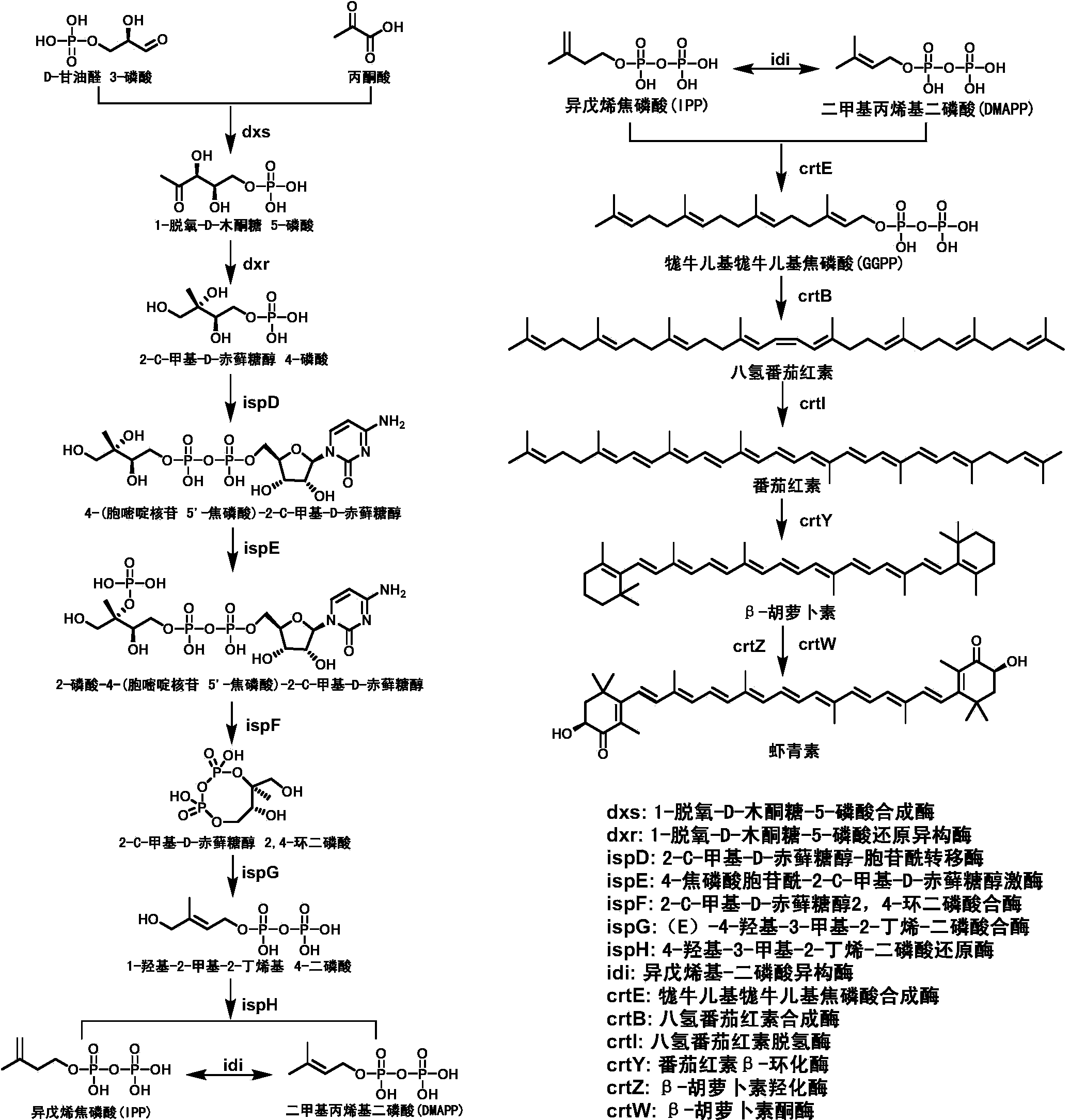
Astaxanthin synthetase of sphingomonas, encoding gene of astaxanthin synthetase and method for genetic manipulation of sphingomonas
The invention discloses astaxanthin synthetase of sphingomonas, an encoding gene of astaxanthin synthetase and a method for genetic manipulation of sphingomonas. The invention verifies that the sphingomonas can be used for producing astaxanthin, a biological synthesis way capable of producing the astaxanthin is contained, an MEP way and a carotenoid synthesis way are used for producing the astaxanthin in the sphingomonas, and the fact that in the sphingomonas, crtE and crtZ having relatively low homology with the known genes have the function of synthesizing the astaxanthin, can be further proven. Both the enzyme and the encoding gene thereof required by the astaxanthin biosynthetic way of the sphingomonas are not reported in the prior art, so that more resources are provided to the biosynthetic metabolism and transformation of the carotenoid. Genetic manipulation can be carried out on the sphingomonas by taking pBBR1MCS2 as a carrier and taking EGFP as a reporter gene, so that a foundation is laid for the genetic modification of the environmental microorganism of the type and the finding of the degradation mechanisms.
Owner:WUHAN INST OF BIOTECH +1
Cycle adenosine monophosphate-incompetent adenylyl cyclase and compositions and methods for treating heart failure and increasing cardiac function
The invention provides methods for treating, ameliorating or protecting (preventing) an individual or a patient having or at risk of having heart disease or heart failure, or decreased cardiac function, comprising: providing a cyclic adenosine monophosphate-incompetent (cAMP-incompetent) adenylyl cyclase type 6 (AC6) protein or polypeptide (also called "an AC6mut"), or an AC6mut -encoding nucleic acid or a gene operatively linked to a transcriptional regulatory sequence.
Owner:RGT UNIV OF CALIFORNIA
Polypeptide having immune cell target recognition function and use thereof
ActiveCN111051504AGood choiceStrong specificityGeneral/multifunctional contrast agentsPhosphorus-oxygen lyasesCyclaseSomatic cell
Disclosed is a polypeptide that recognizes immune cells, the polypeptide comprising the following amino acid sequence: (a) an amino acid sequence containing C terminal fragment sequence EQPDPGAVAAAAILRAILE of human Triokinase / FMN cyclase and the homologous sequence thereof; or (b) an amino acid sequence in which the amino acid sequence is substantially the same as described in (a), the substantially the same referred to amino acid sequence having at least 80% sequence identity to the amino acid sequence as described above in (a). Also disclosed in the present invention are a nucleotide sequence encoding the polypeptide; a polypeptide probe that targets recognized immune cells, comprises the polypeptide as described above and has a reporter group; a kit comprising the above-mentioned probe;and the use of the polypeptide or probe as described above in the preparation of the kit for mammalian living cell imaging.
Owner:KEYANGLE LIFE TECH CO LTD
Formulations of guanylate cyclase C agonists and methods of use
Owner:BAUSCH HEALTH IRELAND LTD
Methods for the expression of peptides and proteins
ActiveUS20160138066A1Inhibitory activityInefficient transportAntibody mimetics/scaffoldsPhosphorus-oxygen lyasesRecombinant peptideADAMTS Proteins
The present invention lies in the field of molecular biology, recombinant peptide and protein expression and relates to methods comprising nucleic acid sequences comprising allocrites of T1SSs or fragments thereof for the efficient production of recombinant Pe OIs and Pr OI. The allocrites or fragments thereof improve the expression of PeOI and Pr OI as IB and function as IB-tags.
Owner:NUMAFERM GMBH
Recombinant adenylate cyclase toxin of Bordetella induces T cell responses against tumoral antigens
An immunogenic composition comprising a recombinant protein comprising a Bordetella CyaA, or a fragment thereof, and a peptide that corresponds to a tumor antigen is provided as a cancer treatment. Methods of treatment with this immunogenic composition are also provided. In an embodiment, the therapeutic composition is a treatment for melanoma, and comprises epitopes from the HLA*0201 epitope. These epitopes include Tyr or GnT-V, and are present in the recombinant proteins CyaA-E5-Tyr and CyaA-E5-GnT-V.
Owner:INST PASTEUR +3
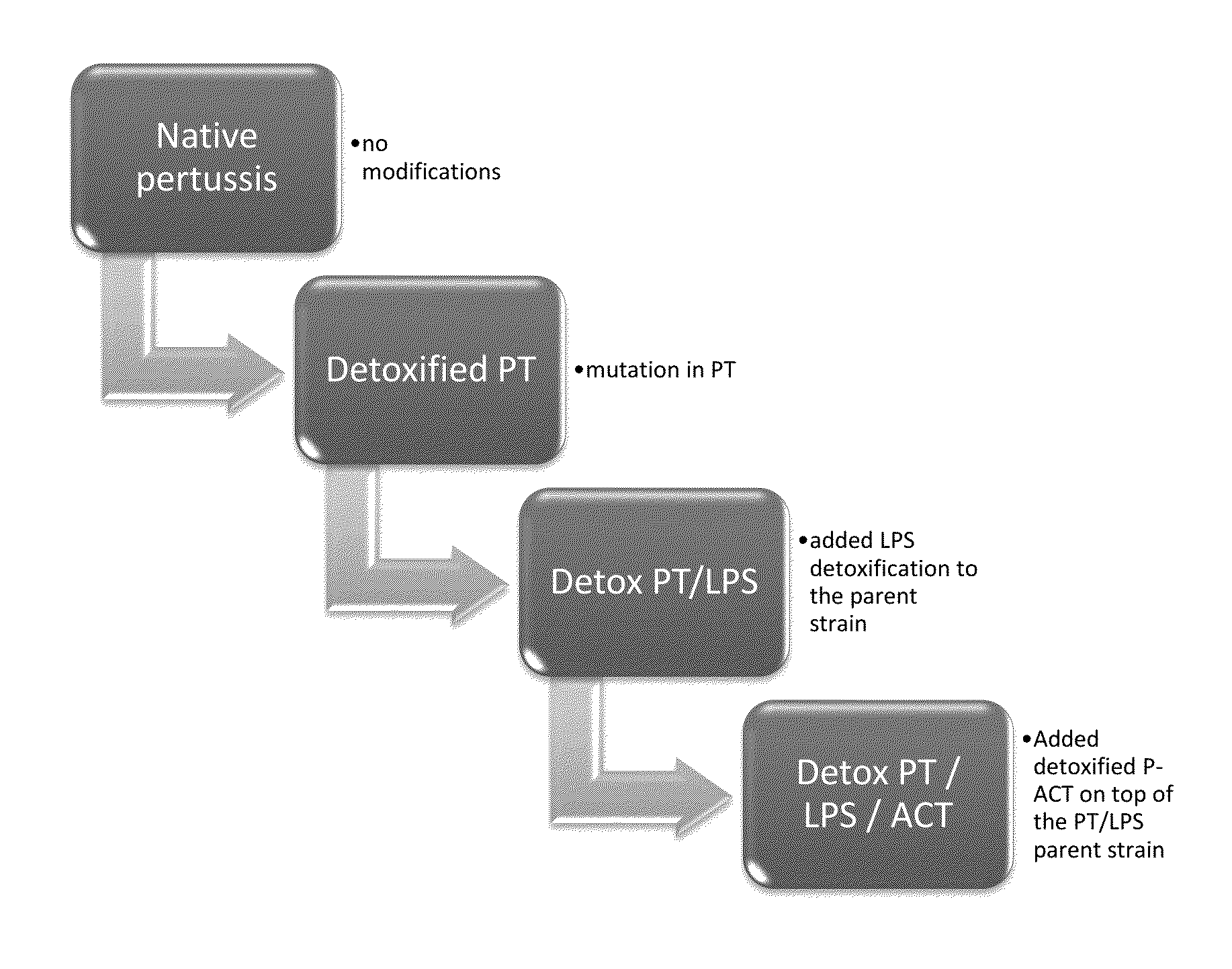
Genetically detoxified pertussis vaccine that maintains intrinsic adjuvant activity
The invention is directed to a method for the production of a pertussis vaccine and, in particular, a detoxified pertussis vaccine comprising detoxified pertussis toxin (PT), detoxified pertussis lipopolysaccharide (P-LPS), and detoxified pertussis adenylate cyclase toxin (P-ACT). The invention is also directed to the manufacture of a vaccine or the invention and methods for the administration of a detoxified vaccine to patients.
Owner:INVENTPRISE INC
Improved preparation method of adenylate cyclase
InactiveCN107058271APhosphorus-oxygen lyasesNucleic acid vectorCell-free protein synthesisGenetic engineering
The invention discloses an improved preparation method of adenylate cyclase. The preparation method comprises the following steps: (1) optimizing a codon of a to-be-expressed gene; (2) carrying out PCR reaction on a target fragment; (3) purifying and recycling a PCR product; (4) constructing a recombinant expression carrier; and (5) carrying out cell-free protein synthesis reaction on a target gene. The preparation method disclosed by the invention has the advantages that molecular biology and genetic engineering methods are adopted, the recombinant expression carrier of an adenylate cyclase gene is constructed, and expression is induced, so that a recombinant protein is obtained.
Owner:WUHAN GENECREATE BIOLOGICAL ENG CO LTD
Ultra-pure agonists of guanylate cyclase c, method of making and using same
ActiveUS20160145307A1Reduce the amount requiredPeptide/protein ingredientsPhosphorus-oxygen lyasesCyclaseFreeze-drying
Owner:BAUSCH HEALTH IRELAND LTD
Genetically Detoxified Pertussis Vaccine that Maintains Intrinsic Adjuvant Activity
The invention is directed to a method for the production of a pertussis vaccine and, in particular, a detoxified pertussis vaccine comprising detoxified pertussis toxin (PT), detoxified pertussis lipopolysaccharide (P-LPS), and detoxified pertussis adenylate cyclase toxin (P-ACT). The invention is also directed to the manufacture of a vaccine or the invention and methods for the administration of a detoxified vaccine to patients.
Owner:INVENTPRISE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com