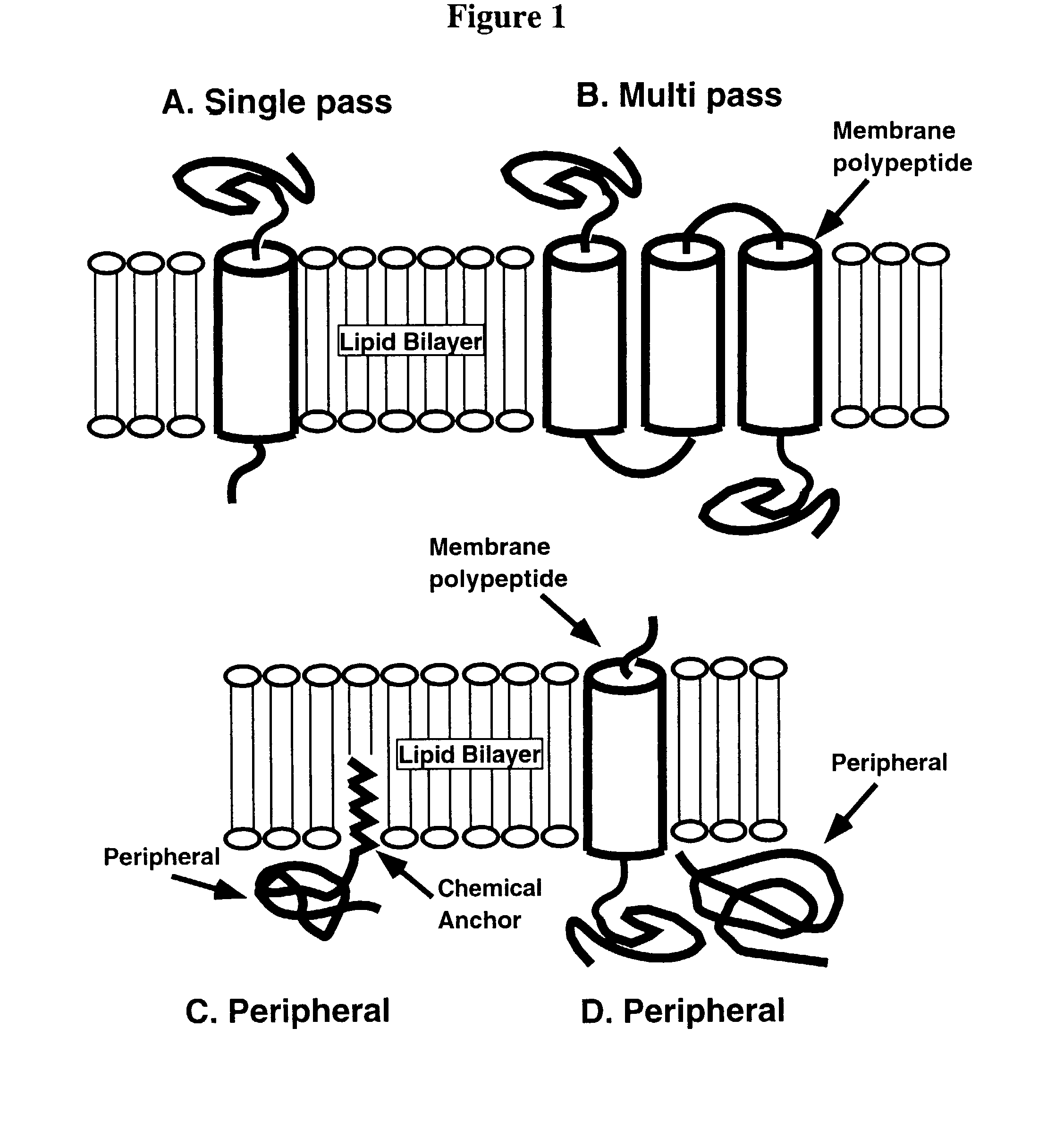
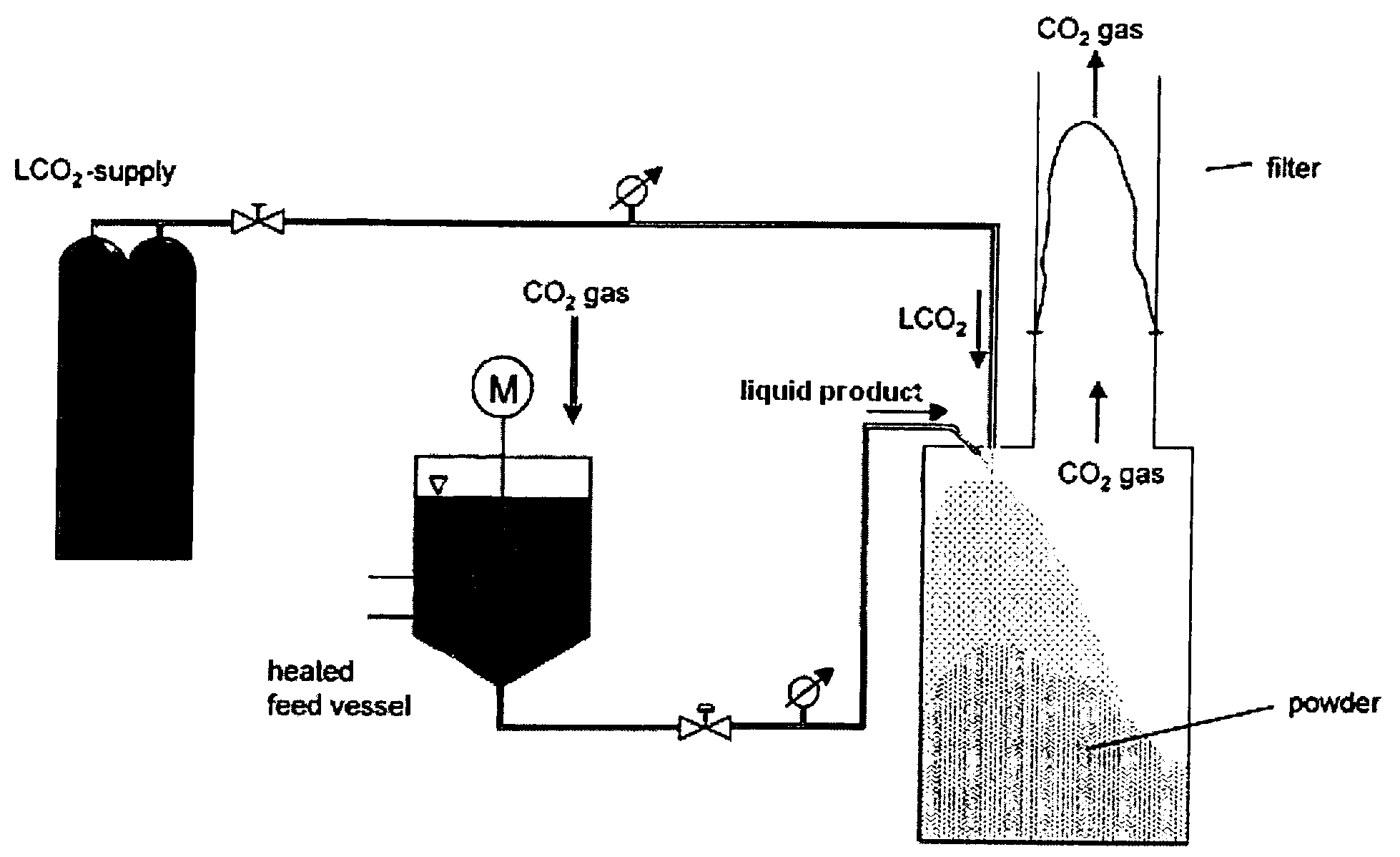
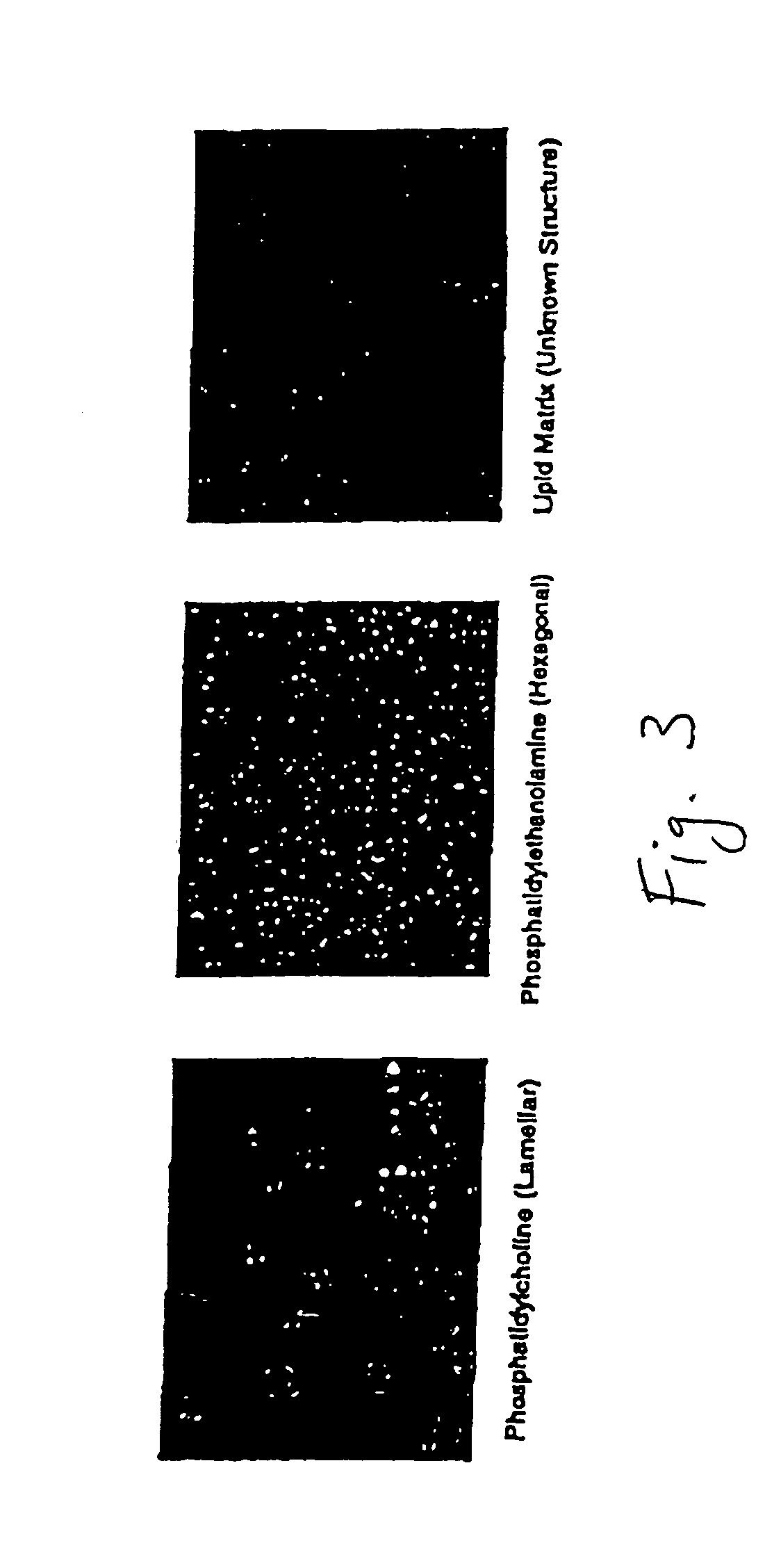
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "Lipid matrix" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The lipid matrix which fills the space between them. A disruption of the lipid layer, or the simple lack of. skin lipids, can create moisture deficiency and dry skin. The SLM acts as an excellent skin renovator by. providing new skin lipids.
Process for the manufacture of pharmaceutical composition with modified release of active principle comprising the matrix
The invention relates to a process for the manufacture of a pharmaceutical composition with modified release of active principle comprising at least one active principle, a lipid matrix agent composed of ester of alcohol with at least one fatty acid and at least one adjuvant.This process is characterized in that:a powder composed of at least one component selected in the group comprising the active principle and the adjuvant, is mixed, while heating and fluidizing, in order to obtain individual grains;the said lipid matrix agent is liquefied separately under warm conditions;the said powder is then coated under warm conditions by spraying the said lipid matrix agent over the individual grains;finally, the temperature of the combined product is lowered in order to allow the lipid matrix agent to solidify.
Owner:GATTEFOSSE HLDG
Solid lipid particles, particles of bioactive agents and methods for the manufacture and use thereof
InactiveUS6207178B1Suppresses decrease in specific surface areaImprove bioavailabilityBiocideCosmetic preparationsLipid formationLipid particle
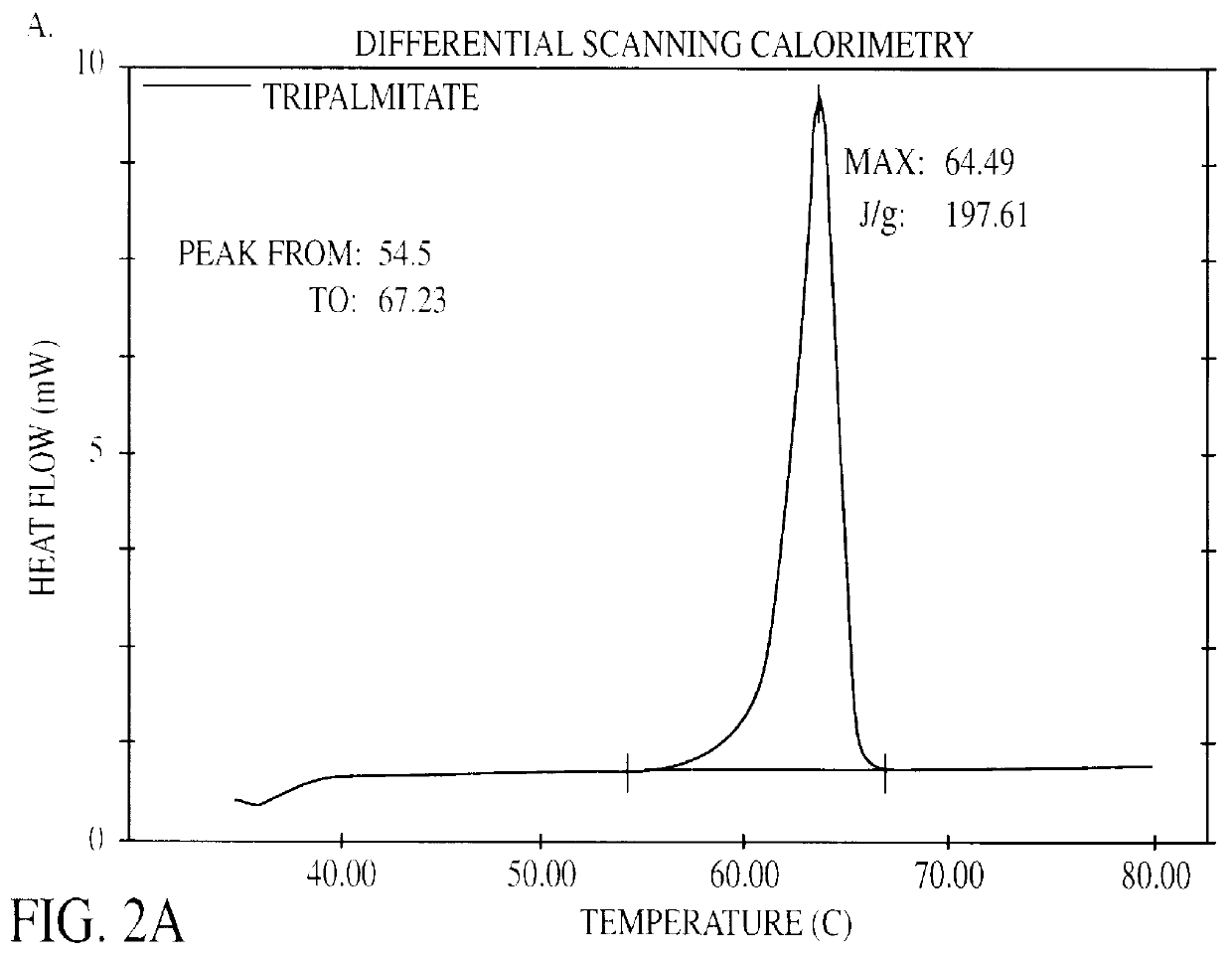
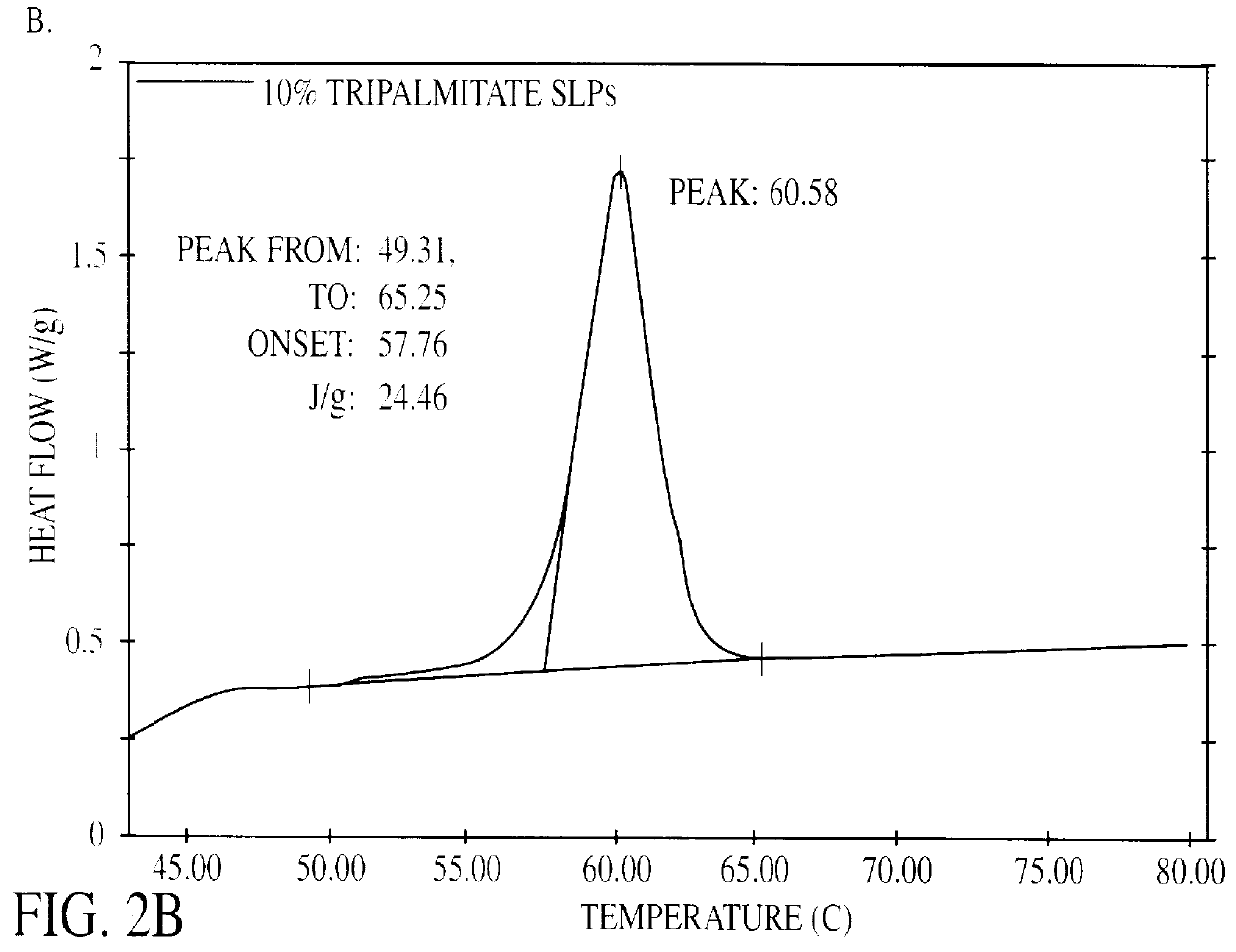
The present invention is in the area of administration forms and delivery systems for drugs, vaccines and other biologically active agents. More specifically the invention is related to the preparation of suspensions of colloidal solid lipid particles (SLPs) of predominantly anisometrical shape with the lipid matrix being in a stable polymorphic modification and of suspensions of micron and submicron particles of bioactive agents (PBAs); as well as to the use of such suspensions or the lyophilizates thereof as delivery systems primarily for the parenteral administration of preferably poorly water-soluble bioactive substances, particularly drugs, and to their use in cosmetic, food and agricultural products. SLPs and PBAs are prepared by the following emulsification process: (1) A solid lipid or bioactive agent or a mixture of solid lipids or bioactive agents is melted. (2) Stabilizers are added either to the lipid or bioactive agent and to the aqueous phase or to the aqueous phase only depending on their physicochemical characteristics. Stabilizers may also be added or exchanged after homogenization. (3) Drugs or other bioactive substances to be incorporated into the SLPs may be melted together with the lipids if the physicochemical characteristics of the substance permit or may be dissolved, solubilized or dispersed in the lipid melt before homogenization. (4) The aqueous phase is heated to the temperature of the melt before mixing and may contain for example stabilizers, isotonicity agents, buffering substances, cryoprotectants and / or preservatives. (5) The molten lipid compounds and the bioactive agents are emulsified in an aqueous phase preferably by high-pressure homogenization.
Owner:PHARMACIA AB
Lipid formulations comprising a thiolated antioxidant
ActiveUS20110230569A1Reduce degradationImprove stabilityBiocideAnimal repellantsAntioxidantChemistry
The present invention provides a formulation comprising:i) a lipid matrix;ii) at least one thiolated antioxidant;iii) optionally at least one bioactive agent; andiv) optionally at least one chelating agent.
Owner:CAMURUS AB
Enhancement of oral bioavailability of non-emulsified formulations of prodrug esters with lecithin
InactiveUS20050113337A1Prevent degradationImprove efficiencyBiocideDispersion deliveryAntibiotic YLiposome
A method for enhancing the oral bioavailability of a prodrug ester by formulating the ester as a non-emulsified formulation with lecithin; as well as a pharmaceutical composition of at least one antibiotic and lecithin in a non-emulsified formulation; a method of treating infections with the non-emulsified formulation, and a method for preparing tablets by direct compression of blends of drugs with lecithin are disclosed. Non-emulsified formulations include solids, tablets, capsules, lozenges, suspensions, elixirs and solutions, and exclude emulsions, liposomes, lipid matrix systems and micro-emulsions. A suitable prodrug ester is a cephalosporin β-lactam antibiotic such as cefditoren pivoxil, and a suitable non-emulsified formulation is a solid formulation.
Owner:TAP PHARM PROD INC
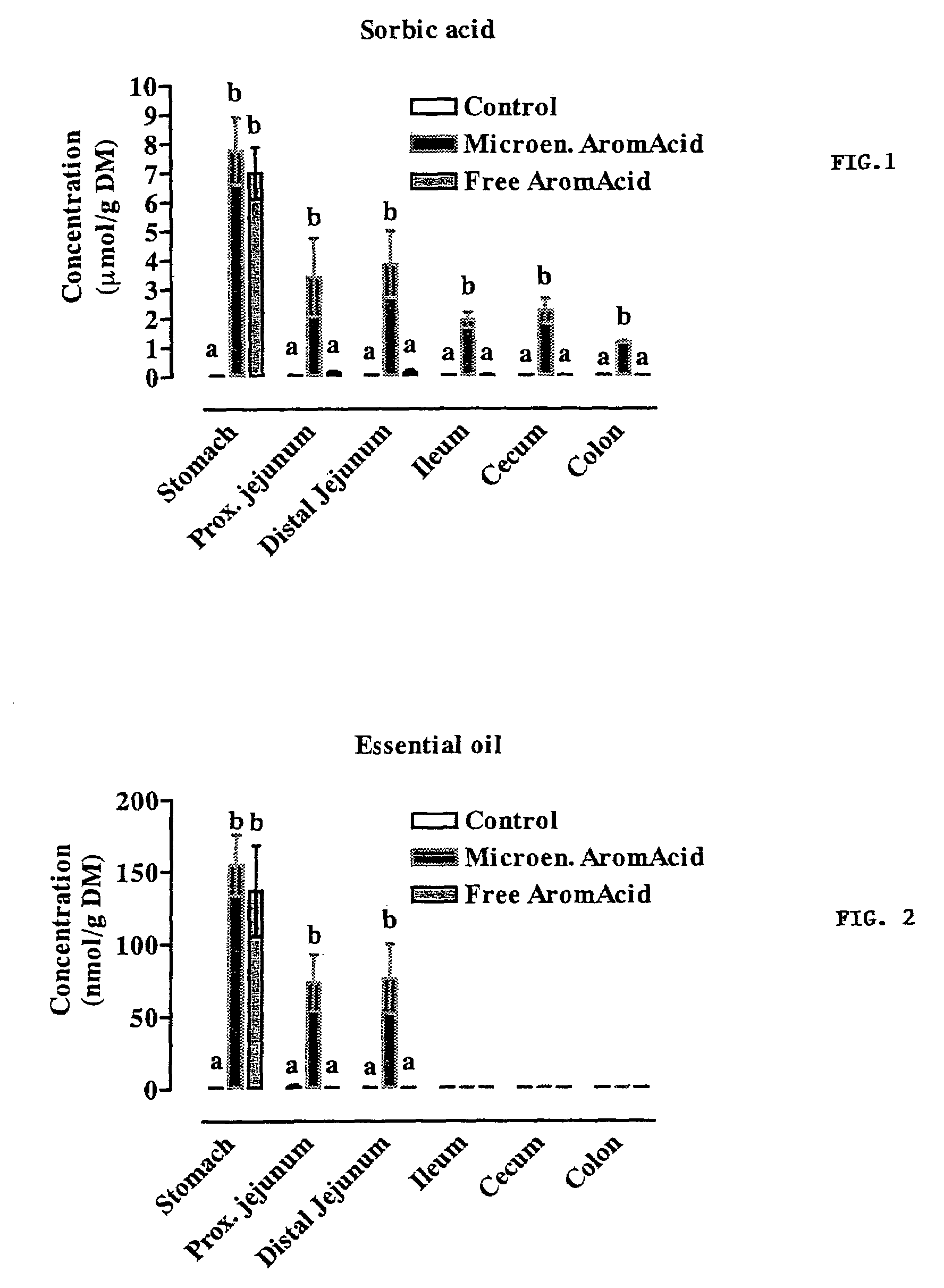
Composition for use in animal nutrition comprising a controlled release lipid matrix, method for preparing the composition and method for the treatment of monogastric animals
ActiveUS20040009206A1Simple compositionDigestive systemAnimal feeding stuffBiotechnologyOrganic acid
The present invention relates to a composition for use in animal nutrition comprising a controlled release matrix and to a method for preparing said composition. Moreover, the present invention relates to a method for the treatment of monogastric animals in which said composition is used as addition of active substances such as for instance organic acids and / or inorganic acids for preserving and acidifying food for monogastric animals, including swine, sheep, rabbits, birds, horses, pets and humans.
Owner:VETAGRO
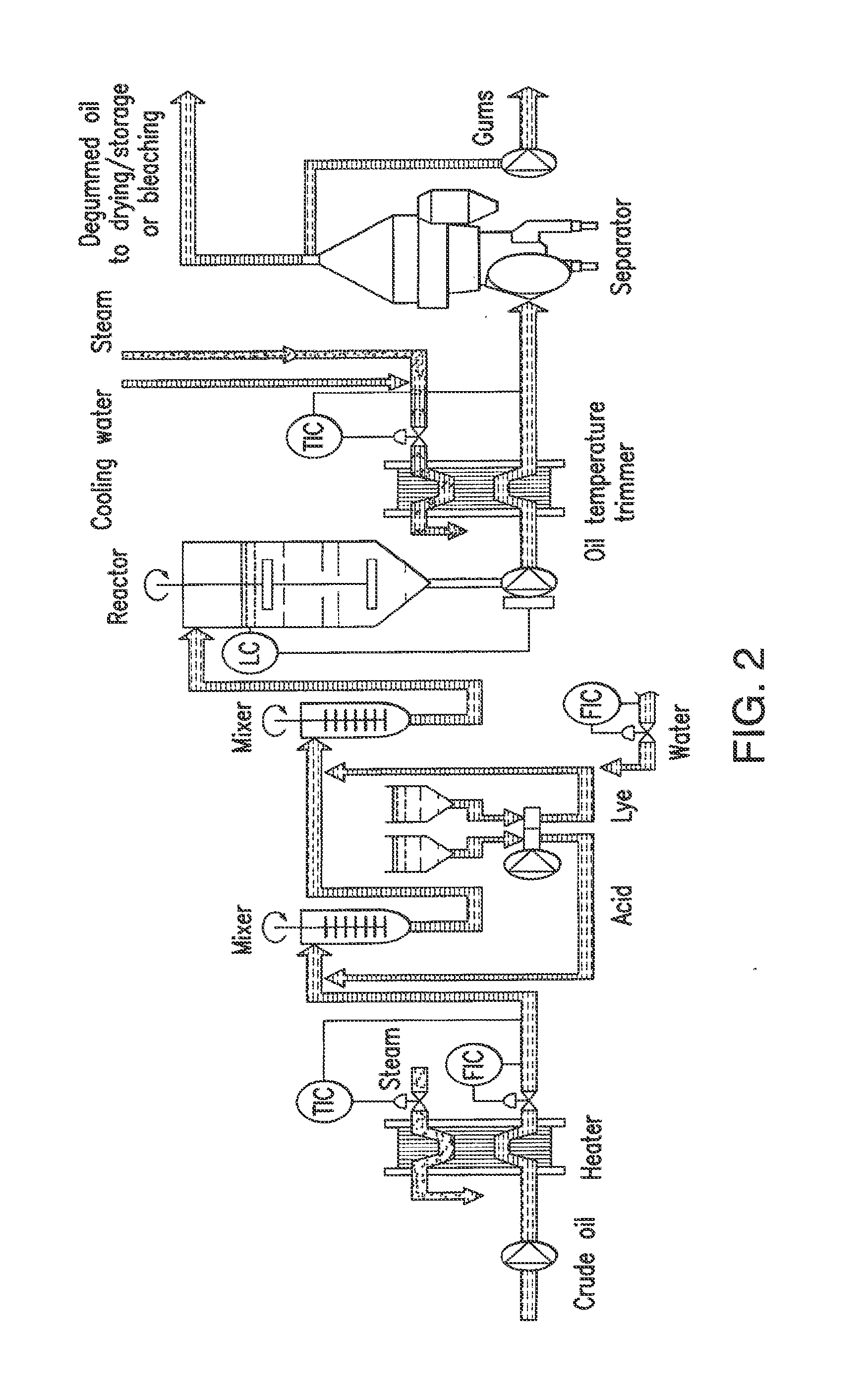
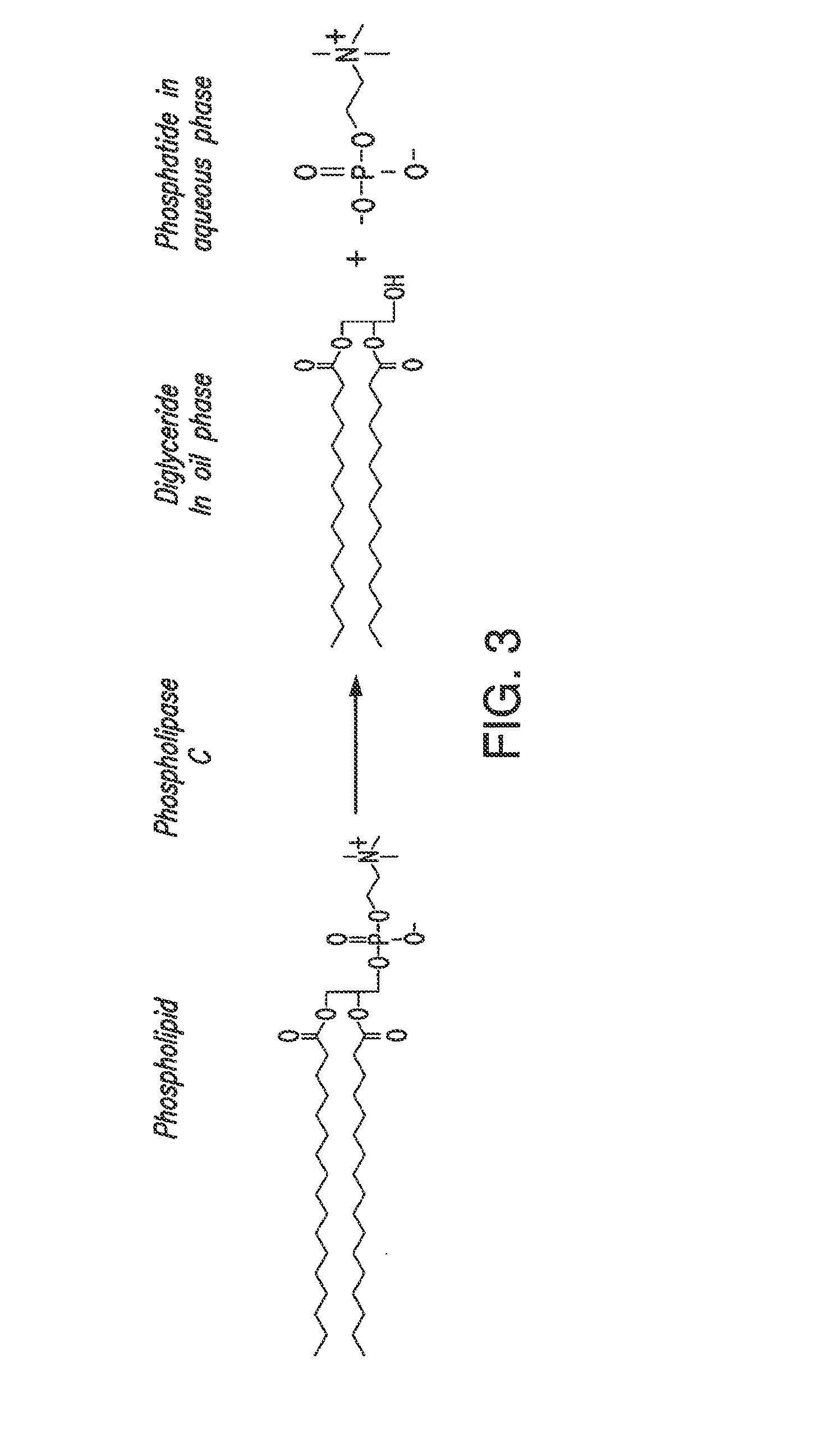
Oil degumming methods
ActiveUS20130011887A1Improve reaction speedImprove heat resistanceFatty acids production/refiningOther chemical processesVegetable oilPhospholipase
In alternative embodiments, the invention provides phosphatidylinositol-specific phospholipase C (PI-PLC) enzymes, nucleic acids encoding them, antibodies that bind specifically to them, and methods for making and using them. Industrial methods and products comprising use of these phospholipases are also provided. In certain embodiments, provided herein are methods for hydration of non hydratable phospholipids (NHPs) within a lipid matrix. The methods enable migration of NHPs to an oil-water interface thereby allowing the NHPs to be reacted and / or removed from the lipids. In certain embodiments, provided is a method for removing NHPs, hydratable phospholipids, and lecithins from vegetable oils to produce a degummed oil or fat product that can be used for food production and / or non-food applications. In certain embodiments, provided herein are methods for hydration of NHPs followed by enzymatic treatment and removal of various phospholipids and lecithins. The methods provided herein can be practiced on either crude or water-degummed oils.
Owner:DSM IP ASSETS BV +1
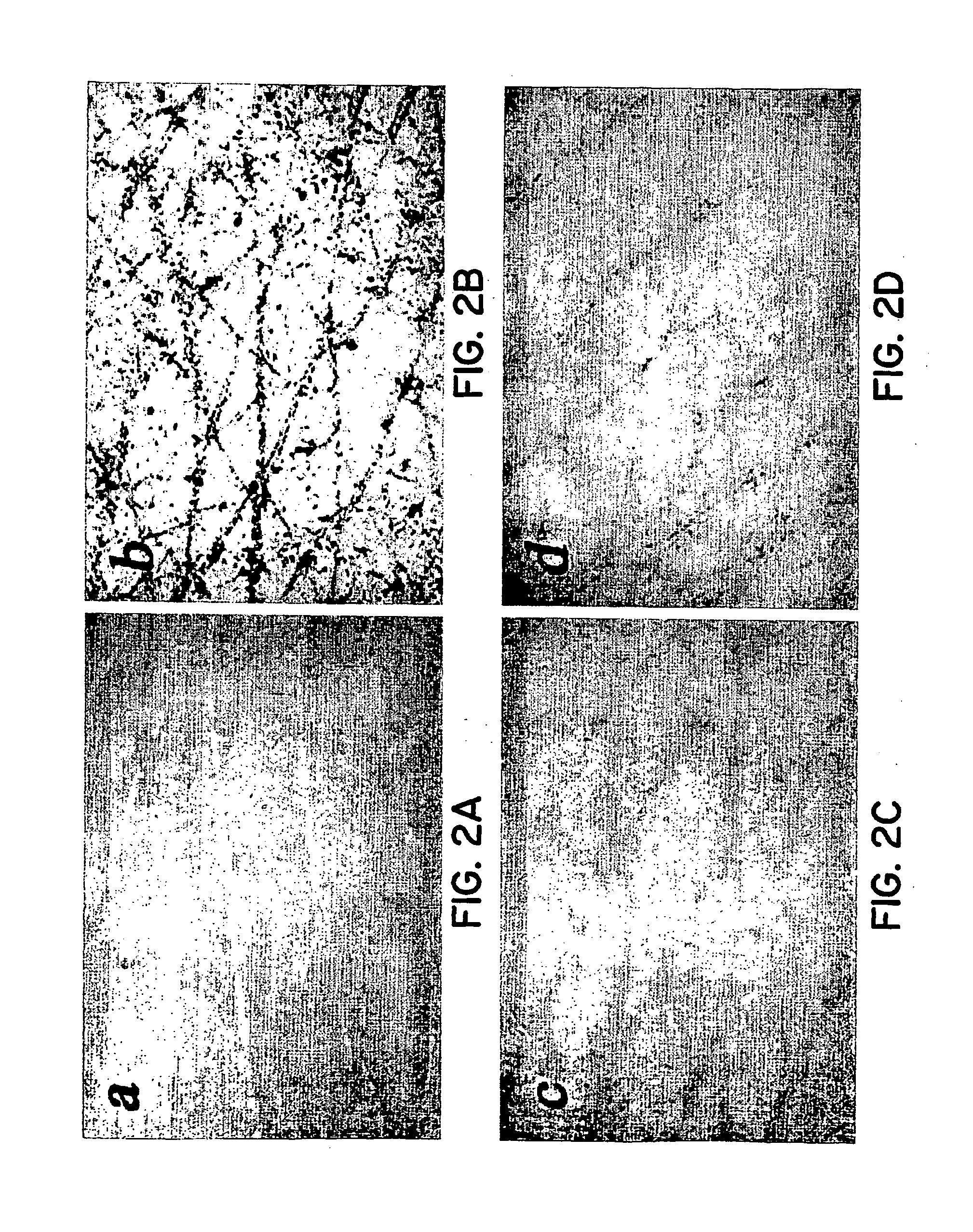
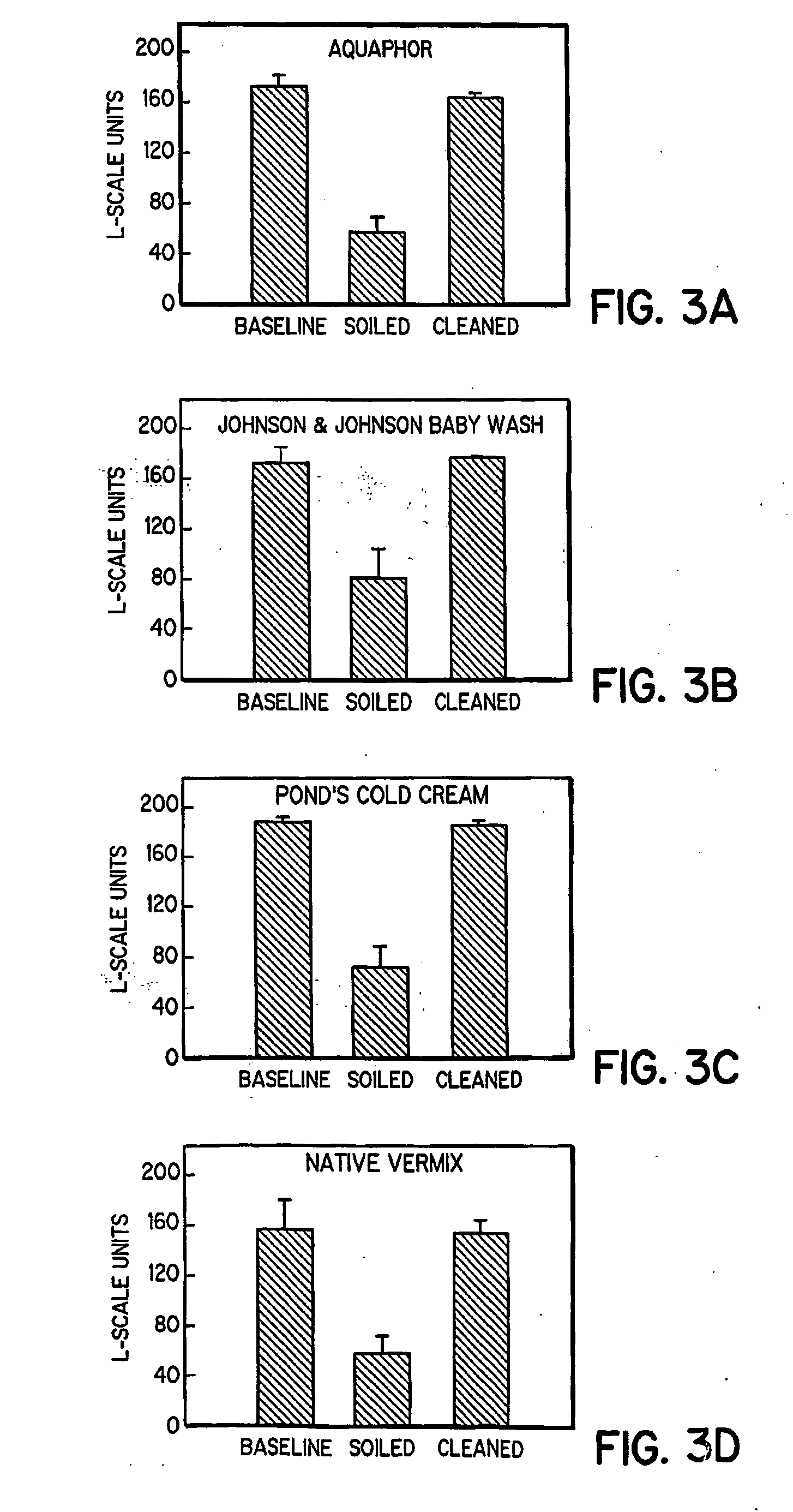
Simulated vernix compositions for skin cleansing and other applications
InactiveUS20050163812A1Effective treatmentCosmetic preparationsToilet preparationsSkin surfaceWater vapor
A composition and a method of producing a composition which simulates hydration, cleansing and other properties of native vernix. The composition contains, in one embodiment, hydrated synthetic cells in a lipid matrix to provide properties which are substantially similar to those of native vernix, and may also contain proteins. In one embodiment, the composition contains water-in-oil emulsified particles providing water vapor transport and evaporative water loss properties simulating native vernix. In one embodiment, the composition contains cubosomes / water with up to 30% protein and about 5% lipid to about 30% lipid. The composition may be used to cleanse newborn skin, compromised skin surfaces, as well as normal skin, to provide hydration / barrier function, and other applications.
Owner:CINCINNATI UNIV OF +1
Simulated vernix compositions for skin cleansing and other applications
InactiveUS20050232890A1Effective treatmentCosmetic preparationsToilet preparationsLipid formationSkin surface
A composition and a method of producing a composition which simulates hydration, cleansing and other properties of native vernix. The composition contains hydrated synthetic cells in a lipid matrix to provide rheological properties which are substantially similar to those of native vernix, and may also contain proteins. In one embodiment, the composition contains cubosomes / water with up to 30% protein and about 5% lipid to about 30% lipid. The composition may be used to cleanse newborn skin, compromised skin surfaces, as well as normal skin, to provide hydration / barrier function, and other applications.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
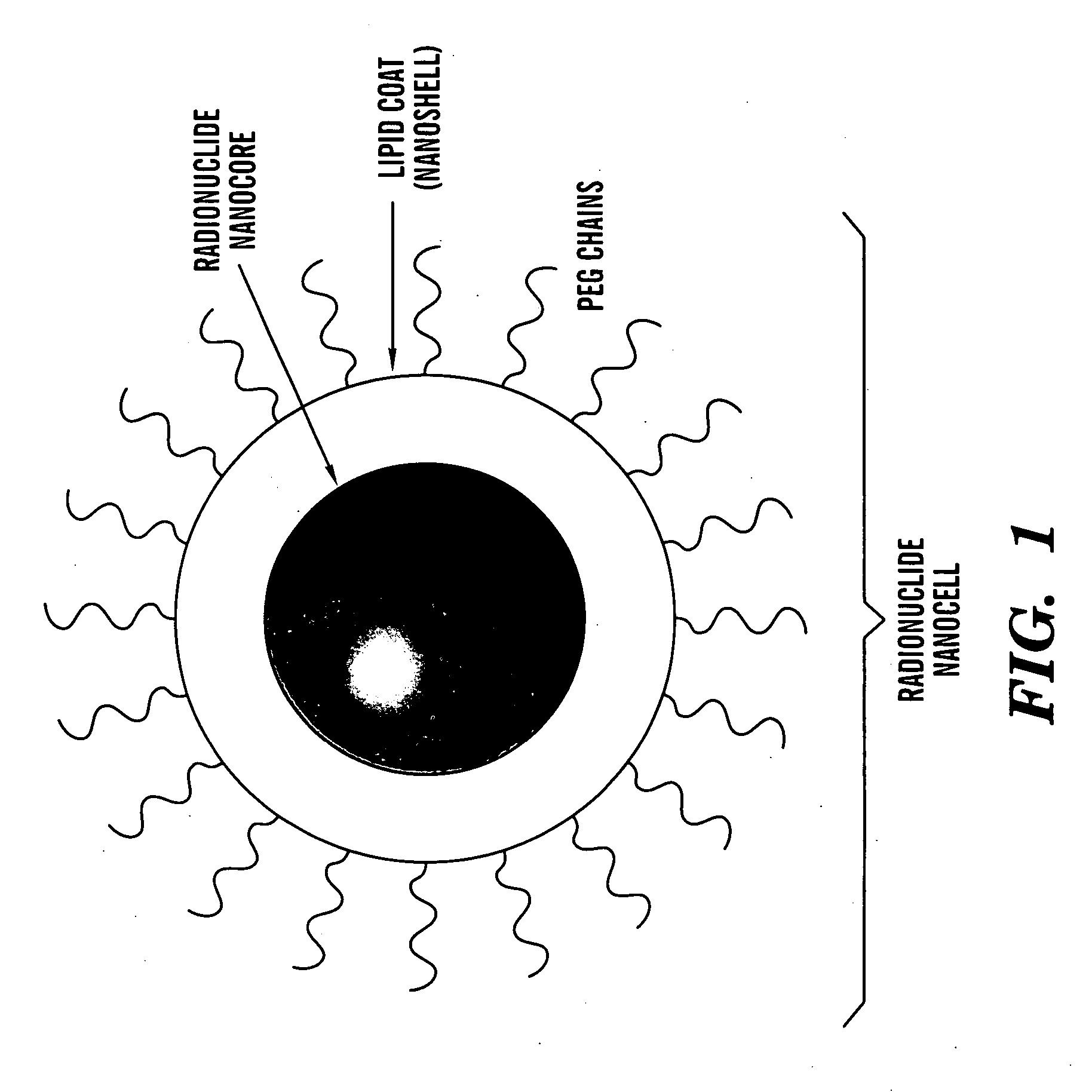
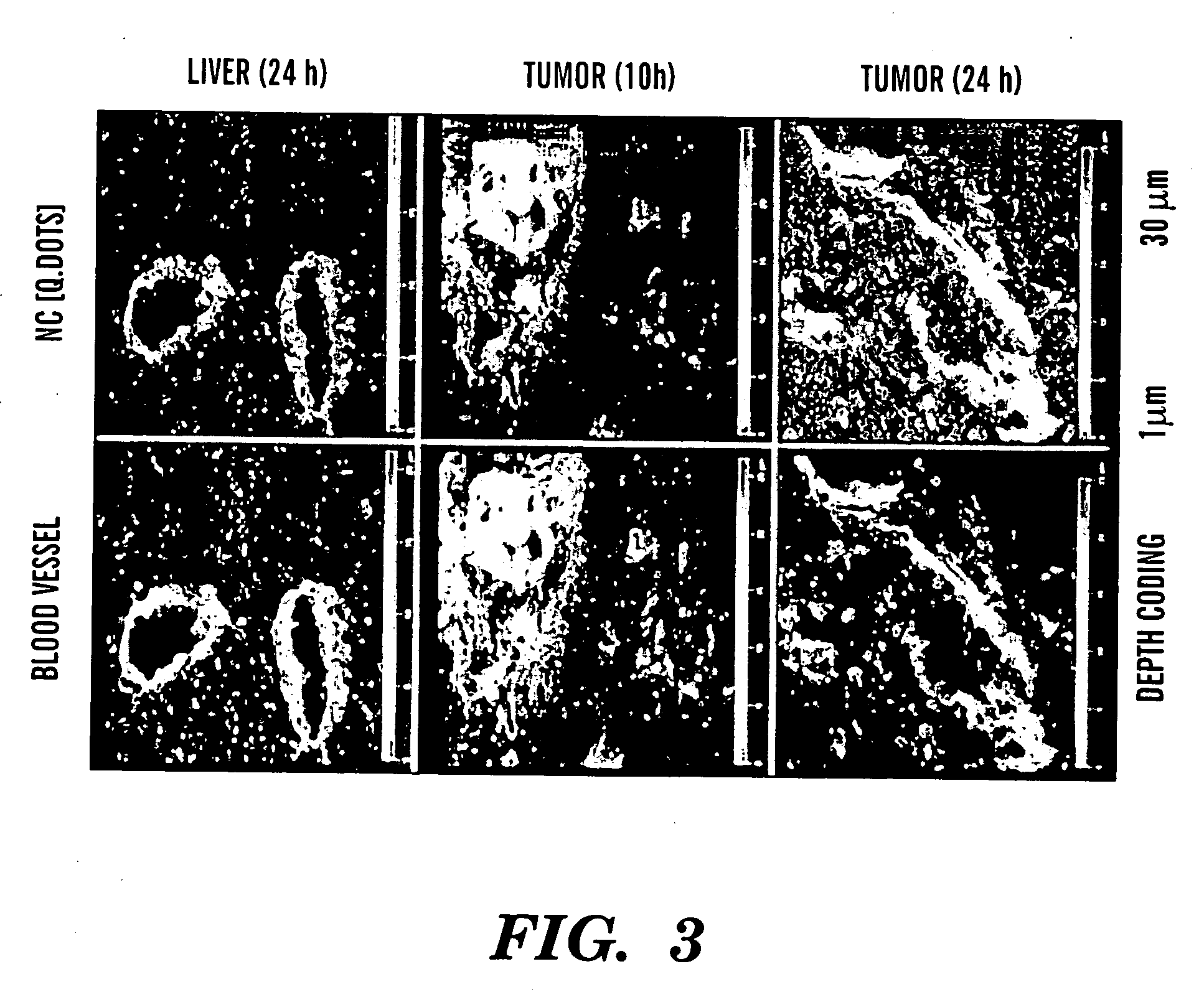
Nanocells for diagnosis and treatment of diseases and disorders
InactiveUS20070065359A1Fine tune deliveryReduce deliveryPowder deliverySenses disorderAbnormal tissue growthBrain cancers
The present invention relates to novel nanocell compositions and their use in imaging, diagnostic and treatment methods. In one embodiment, nanocells tailored for imaging methods comprise a nanocore surrounded by a lipid matrix, and are modified to contain a radionuclide core or a nanocore with an emission spectra. The nanocells may be size restricted such as being greater than about 60 nm so that they selectively extravasate at sites of angiogenesis (e.g. tumor) and do not pass through normal vasculature or enter non-tumor bearing tissue. In this way, angiogenic sites can be both detected and treated. In another embodiment, nanocells are tailored for various treatment methods, including the treatment of brain cancer, asthma, Grave's Disease, Cystic Fibrosis, and Pulmonary Fibrosis.
Owner:MASSACHUSETTS INST OF TECH
Composition for use in animal nutrition comprising a controlled release lipid matrix, method for preparing the composition and method for the treatment of monogastric animals
The present invention relates to a composition for use in animal nutrition comprising a controlled release matrix and to a method for preparing said composition. Moreover, the present invention relates to a method for the treatment of monogastric animals in which said composition is used as addition of active substances such as for instance organic acids and / or inorganic acids for preserving and acidifying food for monogastric animals, including swine, sheep, rabbits, birds, horses, pets and humans.
Owner:VETAGRO
Nanocells for Diagnosis and Treatment of Diseases and Disorders
InactiveUS20090110633A1Reduce deliveryFine tune deliveryPowder deliverySenses disorderAbnormal tissue growthDisease
Owner:MASSACHUSETTS INST OF TECH
Simulated vernix compositions for skin cleansing and other applications
InactiveUS7807188B2Effective treatmentCosmetic preparationsToilet preparationsLipid formationSkin surface
A composition and a method of producing a composition which simulates hydration, cleansing and other properties of native vernix. The composition contains hydrated synthetic cells in a lipid matrix to provide rheological properties which are substantially similar to those of native vernix, and may also contain proteins. In one embodiment, the composition contains cubosomes / water with up to 30% protein and about 5% lipid to about 30% lipid. The composition may be used to cleanse newborn skin, compromised skin surfaces, as well as normal skin, to provide hydration / barrier function, and other applications.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Use of a novel phytonutrient rich bioactive concentrate (Ri-ActiveTM) for the prevention and treatment of cardiovascular disease, diabetes and other health disorders
InactiveUS20060083700A1Potent and great hypolipidemicPotent and great and hypocholesterolemic effectCosmetic preparationsBiocideVegetable oilAdditive ingredient
The present invention is generally related to the discovery of the use of a novel composition (Ri-Active™), containing a concentrate of the bioactives, micronutrients and antioxidants present in the unsaponifiable fraction of rice bran oil or rice germ oil, in the prevention and treatment of health disorders. The novel use of the by-products of rice bran oil or rice germ (and other vegetable oils) industries directly after purification and stabilization for therapeutic purposes is a unique and innovative approach that has been proved in the pilot study conducted by the current inventors. The current inventors have pioneered a new approach to prevent and treat health disorders such as cardiovascular diseases, hypercholesterolemia and diabetes which is both effective and inexpensive. The proprietary technology developed by the current inventors to produce Ri-Active™ from rice bran oil by-products or rice germ is very different from others who use these by-products to either isolate or purify individual micronutrients of value for use in other nutraceutical formulations. Most of the by-products of the edible oil refining industry are either discarded or sold at a throw away cost to the soap industry for their free fatty acid content. The novel technology and approach developed by the current inventors to utilize these low cost by-products from the edible oil refining industry and produce a highly effective therapeutic product has yielded a new and valuable use for these materials. This invention extends to the uses (therapeutic usefulness) and cost effectivity of similar whole extract concentrates of unsaponifiable micronutrients prepared from other vegetable oils rich in unsaponifiable fractions. The current discovery specifically covers the uses of Ri-Active™ (derived either from rice bran oil or rice germ oil), in preventing or treating cardiovascular disease, diabetes, hyperlipidemia, hypercholesterolemia and arteriosclerosis. Ri-Active™ contains many unsaponifiable bioactives and antioxidants of rice bran and rice germ in very high concentrations in a natural lipid matrix. These constituents in their natural lipid matrix act synergistically in the treatment of cardiovascular disease and other disorders and therefore the whole extract concentrate containing these constituents is far more efficacious than administering a mixture of procured ingredients in similar concentrations. The efficacy of this novel composition (Ri-Active™) in the treatment of cardiovascular disease risk factors has been proved in the pilot animal study conducted by the current inventors. Ri-Active™ has been tested to be safe, and highly effective in small doses.
Owner:NATURRI
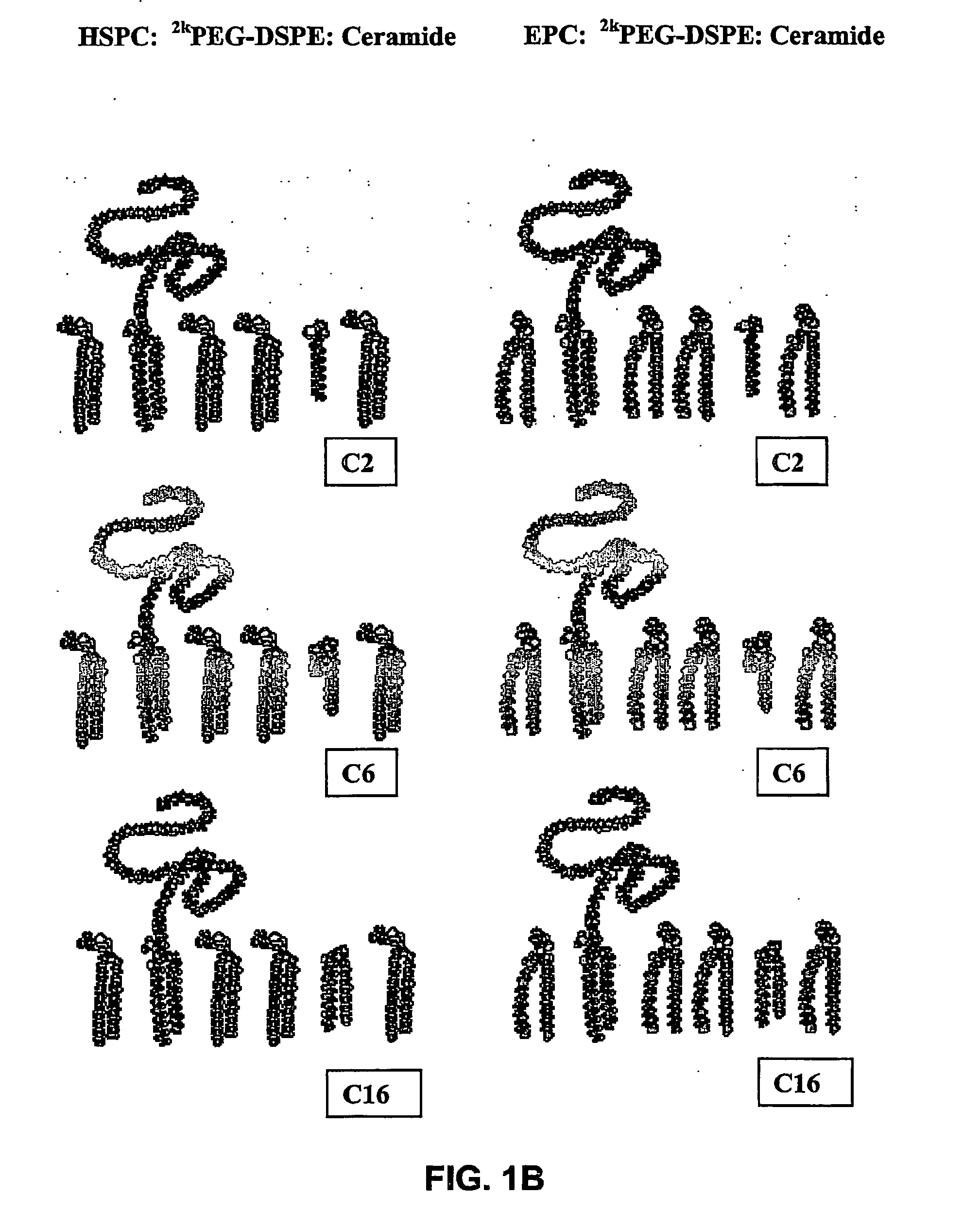
Stable liposomes or micelles comprising a sphinolipid and a peg-lipopolymer
InactiveUS20060198882A1Delay disease progressionUndesired symptomAntibody ingredientsAmide active ingredientsLipid formationPolyethylene glycol
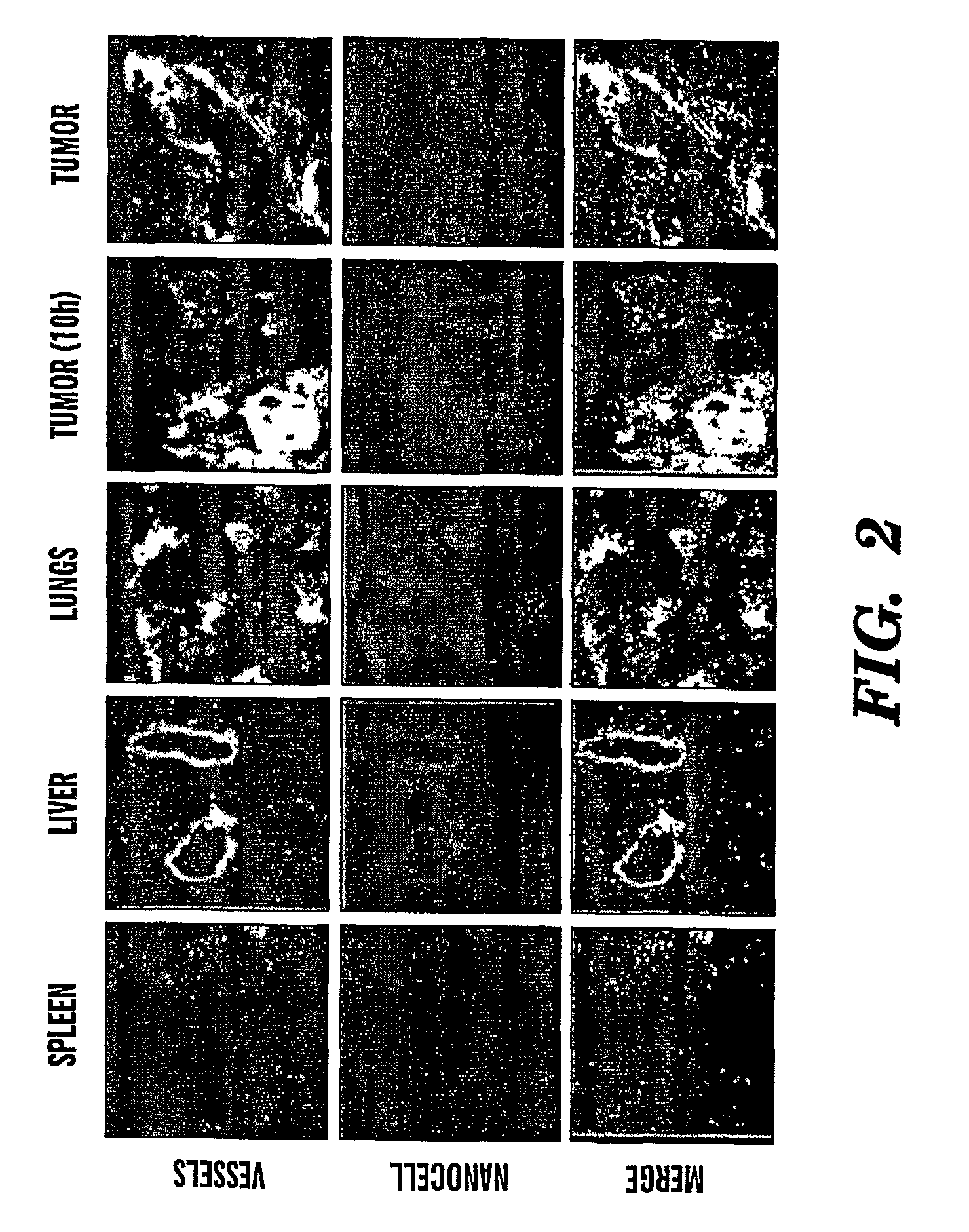
The present invention concerns a stable lipid assembly comprising a biologically active lipid having a hydrophobic region and a polar headgroup, wherein the atomic mass ratio between the headgroup and hydrophobic region is less than 0.3, and a lipopolymer having a hydrophobic lipid region and a polymer headgroup, wherein the atomic mass ratio between the headgroup and hydrophobic region is at least 1.5 and optionally a lipid matrix composed of liposome forming lipids. Specific lipid assemblies according to the invention comprise the biologically active lipid, ceramide, a lipid derivatized with polyethylene glycol (lipopolymer) and optionally in combination with a phospholipid (e.g. Egg phosphatidylcholine (EPC) and hydrogenated soybean phosphatidylcholine (HSPC)). The lipid assemblies of the invention exhibited a therapeutic effect in vitro in tumor cells as well as in vivo in animal models and they deliver the biologically active lipid to the disease site.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Anhydrous multiphase gel system
InactiveCN101511339AImprove permeabilityBig storageOrganic active ingredientsCosmetic preparationsDrug reservoirHigh concentration
The invention relates to an anhydrous multiphase gel system consisting of an outer lipid matrix and of an inner phase gelled by means of polymer, obtained by: a) melting the lipid phase while forming a liquid lipid phase; b) mixing and homogenizing polymers or polymer blends that are capable of swelling while forming a polymer phase to be dispersed; combining the polymer phase with the liquid lipid phase and homogenizing the phases, and; d) cold stirring the phase mixture until forming a solid gel-like mixed structure of the entire system. The anhydrous multiphase gel system is particularly suited for accommodating poorly soluble active substances in high concentrations and for topical and transdermal applications. The described system is characterized as an EDRS Entrapped Drug Reservoir System .
Owner:INTENDIS GMBH
Oil degumming methods
ActiveUS20140371476A1Improve heat resistanceImprove thermal stabilityOrganic chemistryPhosphorus-oxygen lyasesPhospholipaseVegetable oil
In alternative embodiments, the invention provides phosphatidylinositol-specific phospholipase C (PI-PLC) enzymes, nucleic acids encoding them, antibodies that bind specifically to them, and methods for making and using them. Industrial methods and products comprising use of these phospholipases are also provided. In certain embodiments, provided herein are methods for hydration of non hydratable phospholipids (NHPs) within a lipid matrix. The methods enable migration of NHPs to an oil-water interface thereby allowing the NHPs to be reacted and / or removed from the lipids. In certain embodiments, provided is a method for removing NHPs, hydratable phospholipids, and lecithins from vegetable oils to produce a degummed oil or fat product that can be used for food production and / or non-food applications. In certain embodiments, provided herein are methods for hydration of NHPs followed by enzymatic treatment and removal of various phospholipids and lecithins. The methods provided herein can be practiced on either crude or water-degummed oils.
Owner:BUNGE GLOBAL INNOVATION LLC
Veterinary enrofloxacin taste-masking sustained-release granule and preparation method thereof
InactiveCN109394727AImprove oral bioavailabilityImprove palatabilityAntibacterial agentsOrganic active ingredientsDiluentDissolution
The invention belongs to the field of preparation of veterinary medicine preparations, and particularly relates to a veterinary enrofloxacin taste-masking sustained-release granule and a preparation method thereof. The granule is prepared through the following step that lipid matrix nanocrystallization, wet granulation and film coating are combined; the granule is prepared by mixing, by mass, 2.5-15.0% of enrofloxacin, 5.0-35.0% of lipid matrix, 1.0-5.0% of a surfactant, 20.0-40.0% of a diluent; 10.0 %-20.0% of a flavoring agent and 5.0%-20.0% of a coating material. The invention provides an optimized formulation and a preparation method. The granule has the advantages that the appearance is white or light yellow, the particle size is 0.2-2 mm, and the loss on drying is less than 2%; the dissolution rate is less than 30% in simulated gastric juice when the pH value is equal to 2, and the dissolution rate in 30 min is less than 60% in the simulated gastric juice when the pH value is equal to 8.
Owner:HUAZHONG AGRI UNIV
Product based on conjugated linoleic acid and a method for the manufacture thereof
ActiveUS20100092569A1Correct formatEasy curingBiocidePowder deliveryProduct baseConjugated linoleic acid
A product based on conjugated linoleic acid (CLA) comprises an inner core in which the conjugated linoleic acid is substantially concentrated as well as a coating for covering and protecting the inner core; the coating in turn comprises a fraction greater than 80% by weight relative to the coating of a lipid matrix formed by glycerides of C16, C18, C20 and C22 saturated fatty acids.
Owner:SILA
Porcine Asian type-I foot-and-mouth disease virus inactivated vaccine adjuvant and preparation method thereof
The invention relates to a porcine Asian type-I foot-and-mouth disease virus inactivated vaccine adjuvant and a preparation method thereof. The adjuvant comprises recombined porcine interleukine-2 (pIL-2). The porcine Asian type-I foot-and-mouth disease virus inactivated vaccine adjuvant is a nanoparticle with the particle size of 50-1000nm, wherein the nanoparticle is formed by wrapping the porcine interleukine-2 with a lipid matrix and an emulsifier.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Compositions for lipid matrix-assisted chemical ligation
The present invention relates to methods and compositions for lipid matrix-assisted chemical ligation and synthesis of membrane polypeptides that are incorporated in a lipid matrix. The invention is exemplified in production of a prefolded membrane polypeptide embedded within a lipid matrix via stepwise chemoselective chemical ligation of unprotected peptide segments, where at least one peptide segment is embedded in a lipid matrix. Any chemoselective reaction chemistry amenable for ligation of unprotected peptide segments can be employed. Suitable lipid matrices include liposomes, micelles, cell membrane patches and optically isotropic cubic lipidic phase matrices. Prefolded synthetic and semi-synthetic membrane polypeptides synthesized according to the methods and compositions of the invention also permit site-specific incorporation of one or more detectable moieties, such as a chromophore, which can be conveniently introduced during synthesis. The methods and compositions of the invention have multiple uses. For example, they can be used to assay ligand binding to membrane polypeptides and domains comprising a receptor, and thus are extremely useful for structure / function studies, drug screening / selection / design, and diagnostics and the like, including high-throughput applications. The methods and compositions of the invention are particularly suited for FRET analyses of previously inaccessible membrane polypeptides.
Owner:AMYLIN PHARMA INC
Process and apparatus for cooling and atomizing liquid or pasty-like substances
InactiveUS20090211265A1Rapid cooling and atomizationGood reproducibilityLighting and heating apparatusIce productionPharmaceutical industryPaste substance
Owner:MERCK SERONO SA
Oral compositions having increased bioavailability and methods of using the same
The present invention provides an edible composition for oral delivery of an active agent such as paclitaxel or a retinide. The composition comprises, in the form of a dry flowable powder: (a) an active agent such as a retinide; (b) lipid matrix composition; (c) optionally sweetener; (d) flour. Compositions of the invention may be administered per se or mixed with a solid or liquid food carrier, for direct oral consumption by a subject or administration through a feeding tube.
Owner:MAURER BARRY J +5
Injectable long-acting local anesthetic semi-solid formulations and its compostions
ActiveCN105120839AOrganic active ingredientsPharmaceutical delivery mechanismControlled releaseSemi solid
A semi-solid controlled release composition containing biocompatible and bioerodible semi-solid lipid matrix incorporating local anesthetics agents to form a semi-solid solution arsd the methods of manufacturing are disclosed.
Owner:HUZHOU HUI ZHONG JI SHI BIOTECHNOLOGY CO LTD
Simulated vernix compositions for skin cleansing and other applications
InactiveUS7959935B2Effective treatmentCosmetic preparationsToilet preparationsSkin surfaceWater vapor
A composition and a method of producing a composition which simulates hydration, cleansing and other properties of native vernix. The composition contains, in one embodiment, hydrated synthetic cells in a lipid matrix to provide properties which are substantially similar to those of native vernix, and may also contain proteins. In one embodiment, the composition contains water-in-oil emulsified particles providing water vapor transport and evaporative water loss properties simulating native vernix. In one embodiment, the composition contains cubosomes / water with up to 30% protein and about 5% lipid to about 30% lipid. The composition may be used to cleanse newborn skin, compromised skin surfaces, as well as normal skin, to provide hydration / barrier function, and other applications.
Owner:CINCINNATI UNIV OF +1
Modification of solid 3-sn-phosphoglycerides
ActiveUS7407779B2Maximum rate of hydrolysisReduce latencyBiocideHydrolasesLipid formationMonoglyceride
Methods for hydrolyzing solid ungranulated lysophosphatidylcholine with phospholipase A2 are provided. Also disclosed are methods for making a lipid matrix of lysophosphatidylcholine, monoglyceride and fatty acid, and lipid matrices of particular structure.
Owner:BIOMOLECULAR PROD
Effective traditional Chinese medicine whitening and skin-smoothing cream
InactiveCN104688664APromote blood circulationPromote absorptionCosmetic preparationsToilet preparationsPolygonatum prattiiOfficinalis
The invention discloses an effective traditional Chinese medicine whitening and skin-smoothing cream, which is prepared from a traditional Chinese medicine prescription and a matrix, wherein the traditional Chinese medicine prescription comprises the following components according to the weight ratio: 15-25% of kaempferiae, 5-10% of white poria, 3-5% of mint, 7-12% of radix polygonati officinalis, 6-10% of saponin, 8-18% of silkworm larva and 5-12% of leonurus; the matrix comprises 0.2-0.5% of xanthan gum, 6-10% of lipid matrix and the balance of oxygen-enriched water. The whitening and skin-smoothing cream disclosed by the invention can effectively perfect skin blood circulation, whiten and smooth the skin, reduce the free radicals aging speed and recover the whiteness and tenderness of the skin.
Owner:SUZHOU INST OF TRADE & COMMERCE
ATRP (Atom Transfer Radical Polymerization) method for constructing high-transfection niosomes cation gene carrier
ActiveCN104961850AIncrease intakeHigh transfection efficiencyOther foreign material introduction processesCholesterolCell membrane
The invention discloses a cation gene carrier-constructed niosomes cation gene carrier with excellent performance such as high transfection and low toxicity, obtained by combining a commonly used activity polymerization method based on lipids (cholesterol, phosphatidylinositol and the like). A method is easy and feasible, a polymerization method ensures effective controllable molecular weight, compared with a common cation gene carrier, the gene carrier has a characteristic of being effectively compatible with a cytomembrane by a lipid matrix, or a characteristic of establishing into lipidosome per se to increase intake of cells, so the transfection efficiency of the cells is increased. The cation gene carrier of a series of niosomes constructed by the method has well transfection efficiency in cell lines of HepG2, COS7, C6, SL, H9C2 and the like, the transfection efficiency is higher than the transfection efficiency of the internationally commercially used lipidosome lipfect2000, and the niosomes cation gene carrier effectively solves the difficult problem of poorer in-vivo transfection efficiency of the lipidosome lipfect2000, and has higher commercial potential.
Owner:BEIJING UNIV OF CHEM TECH
Modifications of solid 3-sn-phosphoglycerides
InactiveUS20090017119A1Improvements in enzymatic modification of 3-sn-phosphoglyceride moleculesLow costBiocidePowder deliveryLipid formationMonoglyceride
Owner:BIOMOLECULAR PROD
Process and apparatus for cooling and atomizing liquid or pasty-like substances
InactiveUS20070079629A1Rapid cooling and atomizationGood reproducibilityDomestic cooling apparatusLighting and heating apparatusLipid formationPharmaceutical industry
A process and an apparatus for the rapid cooling and atomization of liquid or pasty substances, in particular even relatively small amounts of liquid or pasty substances being able to be cooled and atomized economically. Both process and apparatus are particularly useful in the pharmaceutical industry for preparing injectable microparticles of polymer or lipid matrices suitable for the incorporation of active ingredients.
Owner:LAB SERONO SA
SOLID DISPERSIONS CONTAINING 20-O-beta-D-GLUCOPYRANOSYL-20(S)-PROTOPANAXADIOL
InactiveUS20120322752A1High dissolution rateBiocideOrganic active ingredientsD-GlucopyranoseProtopanaxadiol
The present invention provides solid dispersion, comprising: 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol which is a pharmacologically active ingredient; and a saturated polyglycolized glyceride which is a lipid matrix. The solid dispersion of the present invention has effects of increasing dissolution rate of 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol.
Owner:IL HWA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com