Recombinant escherichia coli for producing cyclic adenosine monophosphate with high yield and application thereof in synthetization of cyclic adenosine monophosphate
A technology for recombining Escherichia coli and cyclic adenosine monophosphate, which is applied to the application field of synthesizing cyclic adenosine monophosphate, can solve problems such as inability to meet large-scale industrial production, complex enzyme preparation, etc., and achieve the effects of cost saving and process simplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Construction of BL21(DE3)-PET22b-CYA446 Expression Strain
[0026] Using the whole genome of Escherichia coli MG1655 as a template, the 1-446 nucleotide coding sequence of adenylyl cyclase protein was amplified by conventional PCR.
[0027] The upstream primer used has an Nde I restriction site, and the sequence is: GGAATTCCATATGatgTACCTCTATATTGAGACTCTGAAAC.
[0028] The downstream primer has an Xho I restriction site, and the sequence is CCGCTCGAGTTCCGAGAGATCGGGTGAA.
[0029] The reaction conditions are: 95°C for 2min, 95°C for 20s, 50°C for 20s, 72°C for 50s, a total of 30 cycles; 72°C for 5min. The obtained sequence was subjected to 1% agarose gel electrophoresis and the corresponding fragments were recovered. The sequence and the expression vector pET22b were digested with Nde I and Xho I of Takara Company. The enzyme digestion reaction system was: 10×buffer H 2 μl, Nde I 0.5 μl, Xho I 0.5 μl, gene fragment or pET22b vector 3 μl, H 2 O14 μl. The enzyme...
Embodiment 2
[0031] Example 2 Induced expression and cell disruption of BL21(DE3)-PET22b-CYA446
[0032] 1. Induced expression of BL21(DE3)-PET22b-CYA446
[0033]The positive strain BL21(DE3)-PET22b-CYA446 was inoculated into 100ml LB / Amp liquid medium, and cultured with shaking at 37°C and 200rpm until OD600≈1. Inoculate in 500mL fresh LB / Amp liquid medium at a ratio of 10:100, shake and culture at 37°C and 200rpm until OD600≈0.5~0.7 (such as image 3 As shown, the enzyme activity is better between 0.5 and 0.7, but it can be seen from the figure that when the OD is 0.5, the enzyme activity of the enzyme is also the strongest). Add IPTG to a final concentration of 0.5‰~1‰ (eg Figure 4 As shown, the enzyme activity is better when the concentration is between 0.5‰~1‰, but the IPTG with a concentration of 1‰ has the best effect); at 20~25°C (such as Figure 5 As shown, the enzyme activity is better between 20~25°C, and 20°C is the best), shake culture at 200rpm for 18~20h. Centrifuge at ...
Embodiment 3
[0036] Example 3 Catalytic synthesis of CAMP
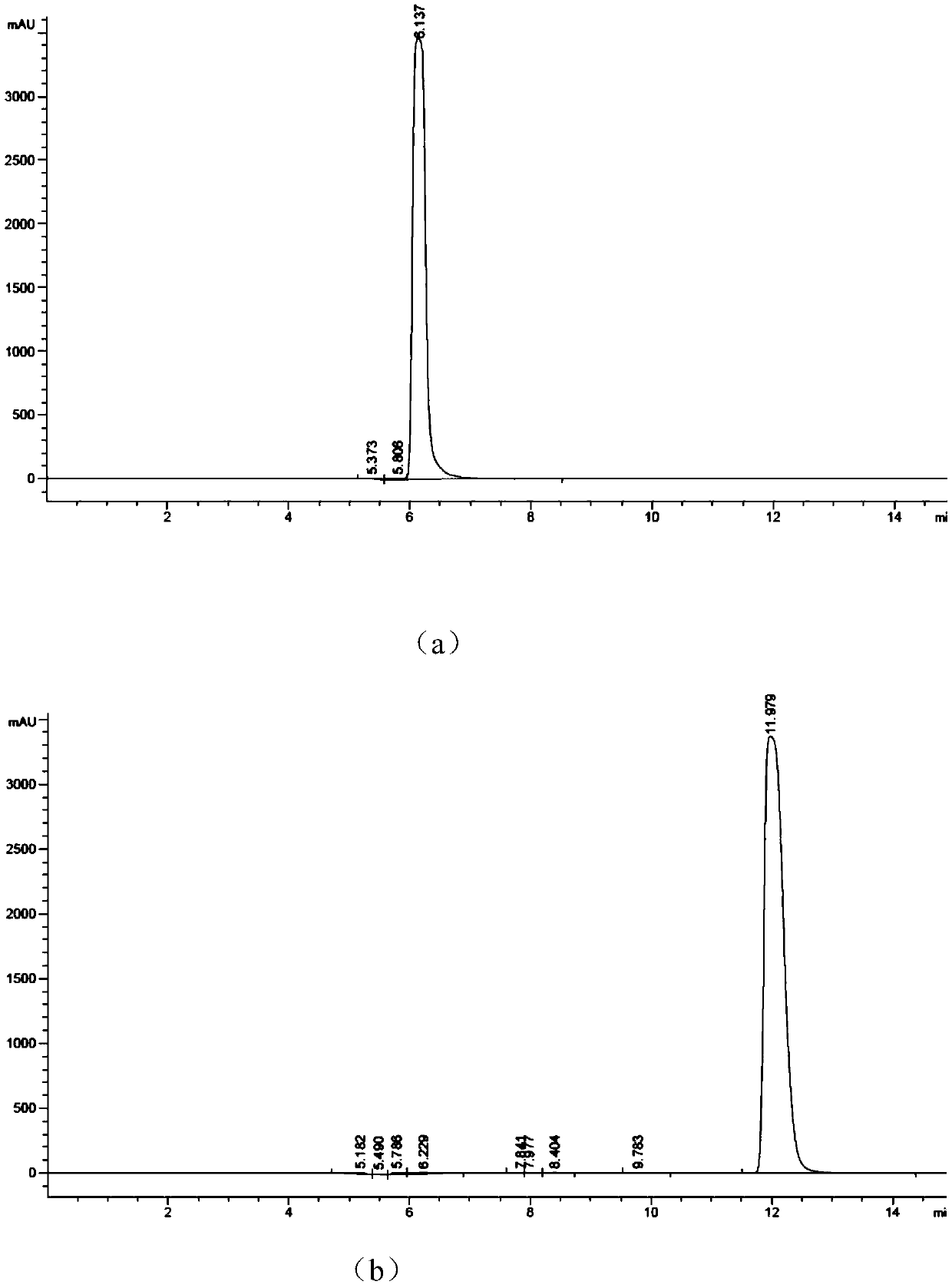
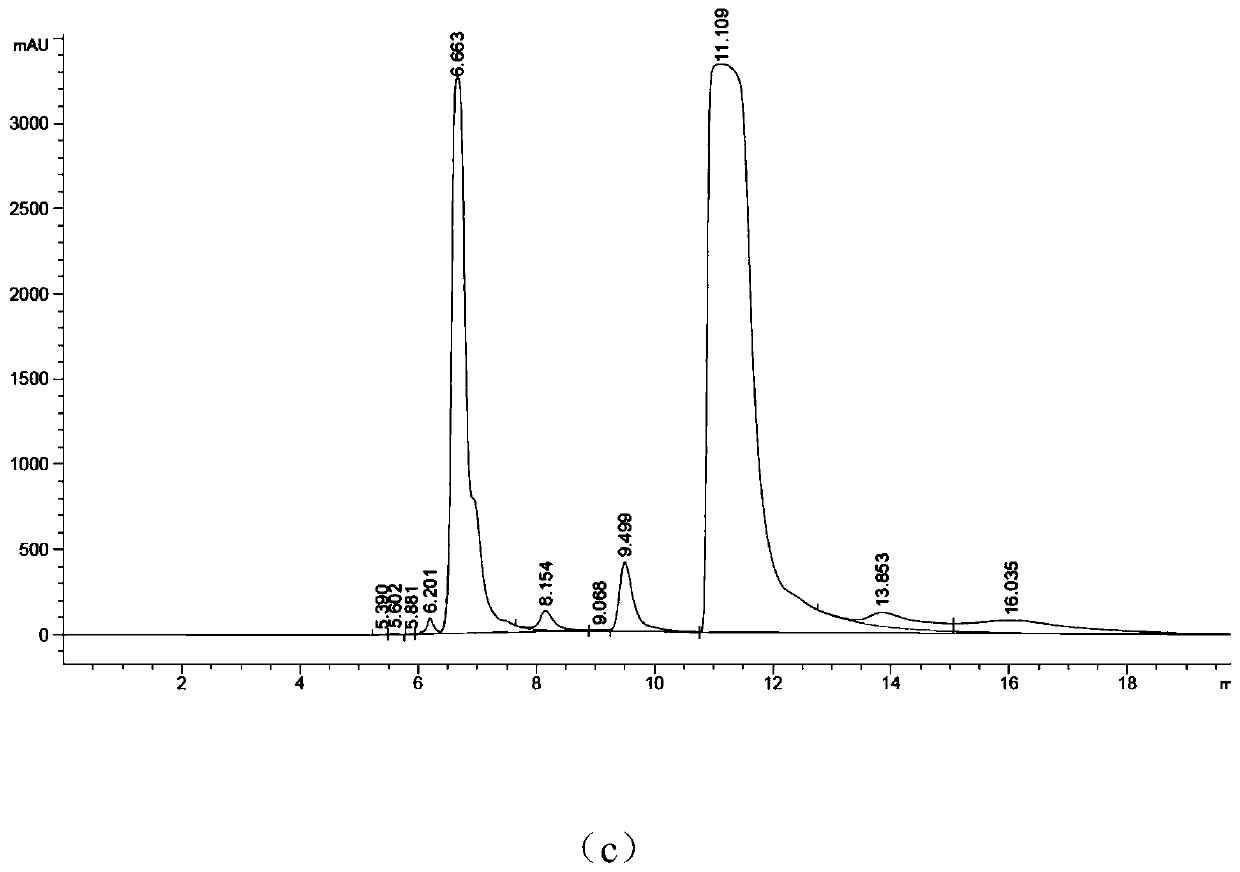
[0037] Mix the following reaction system in the test tube: add adenosine triphosphate with a concentration of 10-50mM, 10-20mM ZnCl 2 (like Image 6 As shown, the enzyme activity between 10-20mM is better, 10 mM is the best), Tris HCl (pH8.0), after stirring evenly, it will be at pH7.0-9.0 (such as Figure 7 As shown, the enzyme activity is better between pH7.0~9.0, and pH8.0 is the best), 30~35℃ (such as Figure 8 As shown, the enzyme activity is better between 30~35°C, and 30°C is the best) to react for 4~6h to complete the enzymatic reaction to synthesize cyclic adenosine monophosphate. Agilent HC-C18 chromatographic column was used to detect the generation of cAMP in the supernatant, the mobile phase was phosphoric acid + triethylamine (6mL+18.4mL to 1L):methanol=75:25, the flow rate was 0.4ml / min, and the detection wavelength was 254nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com