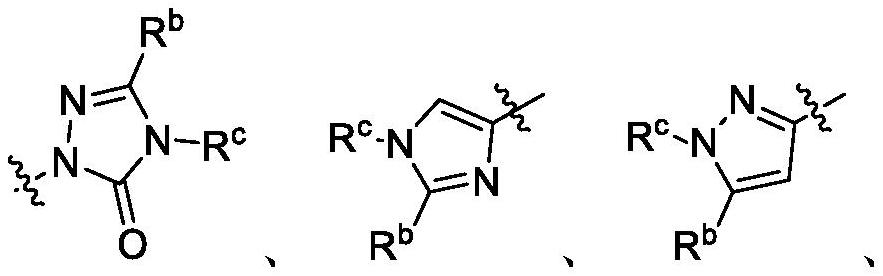
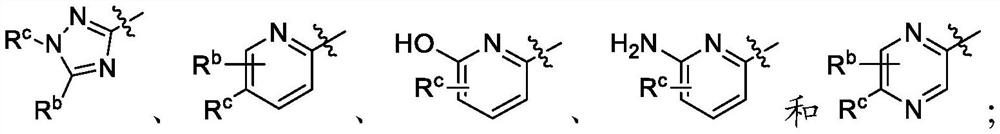
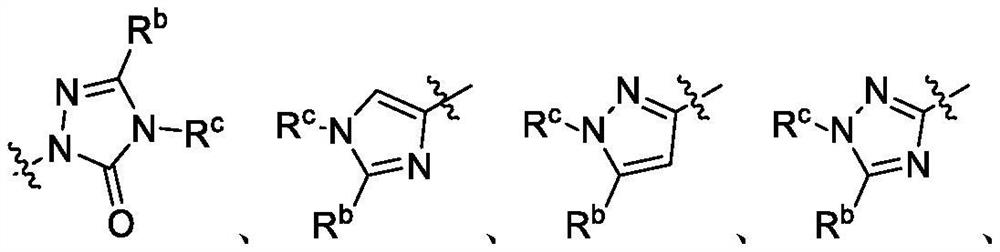
Fluorinated quinoline and quinoxaline derivatives as dihydroorotate dehydrogenase (DHODH) inhibitors for treatment of cancer, autoimmune and inflammatory diseases
A solvate and compound technology, applied in the field of new compounds, can solve problems such as limited effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0344] Exemplary compounds useful in the methods of the present invention will now be described with reference to exemplary synthetic schemes for their general preparations below and specific examples that follow.
[0345] plan 1
[0346]
[0347] According to Scheme 1, the 1,2,4-triazol-5(4H)-one compound of formula (IV), wherein PG is Bn, was prepared from ethyl 2-(benzyloxy)acetate in three steps. In the first step, 2-(benzyloxy)acetate is prepared by reacting ethyl 2-(benzyloxy)acetate with hydrazine hydrate in a suitable solvent such as EtOH etc. at a temperature ranging from 70°C to 85°C Acetylhydrazine. Hydrazides and isocyanates of formula (III) (wherein R c for C 1-6 alkyl) in a suitable solvent such as water etc. to give the corresponding semicarbazides. Subsequent cyclization of the semicarbazide with a suitable base such as NaOH in a suitable solvent such as water provides compounds of formula (IV) (wherein PG is Bn).
[0348] Scenario 2
[0349]
...
Embodiment 1
[0528] Example 1: 4-ethyl-1-(7-fluoro-4-isopropyl-2-(2-methoxyphenyl)quinolin-6-yl)-3-(hydroxymethyl) base)-1H-1,2,4-triazol-5(4H)-one.
[0529]
[0530] Step A. 3-((Benzyloxy)methyl)-4-ethyl-1-(7-fluoro-4-isopropyl-2-(2-methoxyphenyl)quinoline- 6-yl)-1H-1,2,4-triazol-5(4H)-one. in N 2 (2-Methoxyphenyl)boronic acid (39 mg, 256.7 μmol), [1,1-bis(di-tert-butylphosphino)ferrocene]dichloride palladium(II) (Pd( dtbpf)Cl 2 ) (15 mg, 23.33 μmol) and K 2 CO 3 (97 mg, 701.9 μmol) added to 3-((benzyloxy)methyl)-1-(2-chloro-7-fluoro-4-isopropylquinolin-6-yl)-4-ethyl-1H -1,2,4-Triazol-5(4H)-one (Intermediate 2, 160 mg, 233.3 μmol) in dioxane / H 2 O mixture (v / v, 5 / 1, 6 mL) in solution. Put the mixture in N 2 was stirred at 85°C for 16 hours. Mix the mixture with H 2 Diluted with 0 (20 mL) and extracted with DCM (20 mL x 3). The organic layer was passed through Na 2 SO 4 Dry, filter and evaporate under reduced pressure. by flash column chromatography (SiO 2 , 0-80% e...
Embodiment 2
[0532] Example 2: 1-(2-(3-Chloro-5-methyl-1H-pyrazol-4-yl)-7-fluoro-4-isopropylquinolin-6-yl)-4-ethyl yl-3-(hydroxymethyl)-1H-1,2,4-triazol-5(4H)-one.
[0533]
[0534] Step A. 3-((Benzyloxy)methyl)-1-(2-(3-chloro-5-methyl-1H-pyrazol-4-yl)-7-fluoro-4-isopropyl Quinolin-6-yl)-4-ethyl-1H-1,2,4-triazol-5(4H)-one. in N 2 Next, to 3-chloro-5-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaboran-2-yl)-1H-pyrazole (middle body 3, 250 mg, 496 μmol), 3-((benzyloxy)methyl)-1-(2-chloro-7-fluoro-4-isopropylquinolin-6-yl)-4-ethyl-1H -1,2,4-Triazol-5(4H)-one (Intermediate 2, 235 mg, 413.33 μmol) and K 2 CO 3 (114 mg, 826.65 μmol) in dioxane / H 2 Add Pd(dppf)Cl to a solution in O(v / v, 5 / 1, 2 mL) 2 (33.8 mg, 41.33 μmol). The reaction mixture was stirred at 80°C overnight, then cooled to room temperature. The reaction mixture was diluted with ethyl acetate (20 mL), washed with brine (10 mL), and the aqueous layer was extracted with ethyl acetate (20 mL x 3). The organic layer was pas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com