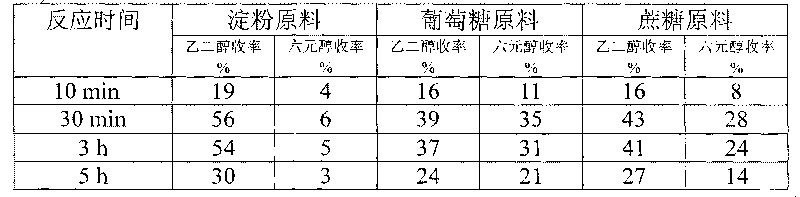
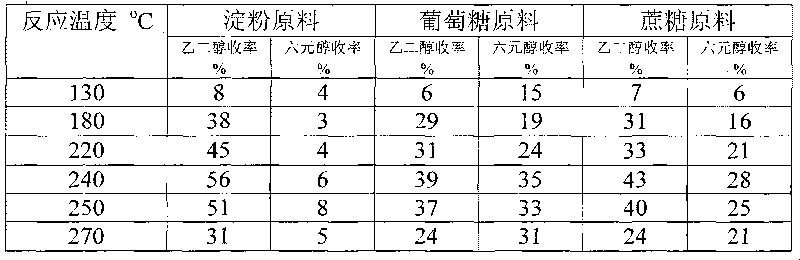
Method for preparing ethanediol from polyhydroxy compounds
一种多羟基化合物、乙二醇的技术,应用在羟基化合物制备、有机化合物的制备、化学仪器和方法等方向,能够解决制备过程繁琐等问题,达到反应过程简单、好应用前景、高原子经济性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of metal tungsten catalyst: impregnate the ammonium metatungstate solution (mass concentration: 0.4g / ml) on the activated carbon carrier, dry it in an oven at 120°C for 12 hours, and place it under H 2 Reduction was carried out at 700°C for 1 h in the atmosphere to obtain W / AC with a tungsten loading of 20 wt%.
Embodiment 2
[0020] Preparation of metal nickel-tungsten catalyst: The tungsten catalyst prepared as described in Example 1 is further impregnated with nickel nitrate solution, dried at 120°C, and reduced at 400°C for 2 hours to obtain Ni-W / AC (5wt%Ni-18wt%W) catalyst.
Embodiment 3
[0022] Metal catalyst Ni / AC, Ni / SiO 2 , the preparation of Pt / AC, Ru / AC: impregnate the activated carbon support with nickel nitrate, chloroplatinic acid, and ruthenium trichloride aqueous solution respectively, and after drying at 120°C for 12h, reduce it in a hydrogen atmosphere at 450°C for 1h to obtain the catalyst Ni / AC (5wt% Ni), Pt / AC (0.5wt% Pt), Ru / AC (5wt% Ru). Replace the activated carbon support with SiO 2 , the same method can be used to prepare Ni / SiO 2 (15 wt% Ni) catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com