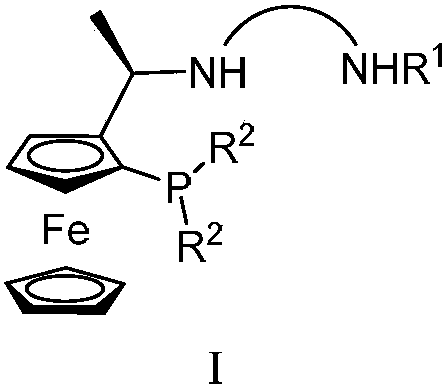
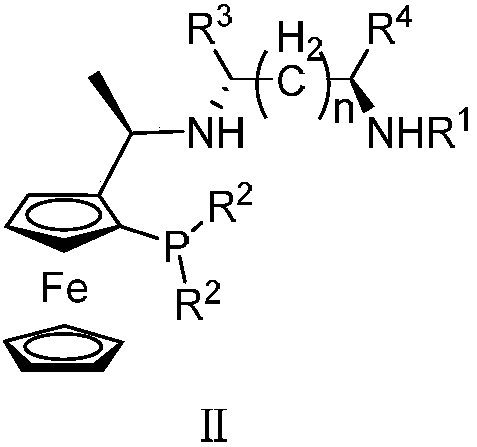
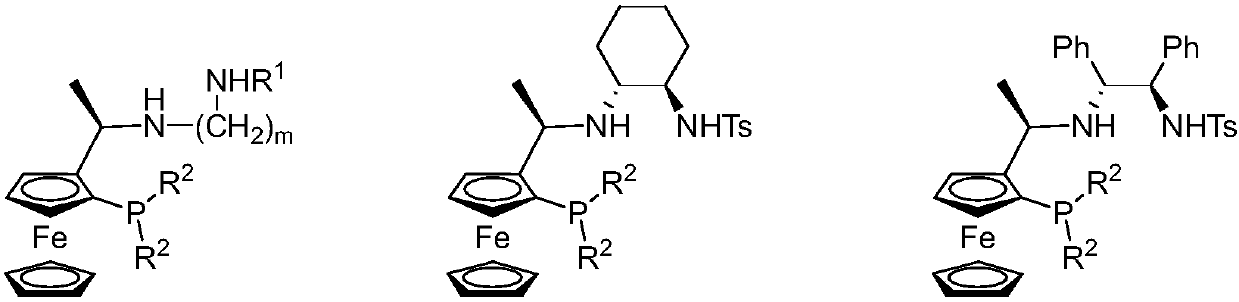
Tridentate nitrogen phosphine ligand and complex and application thereof in asymmetric catalytic hydrogenation of ketone
A technology of tridentate nitrogen and phosphine ligands, which can be used in organic compound/hydride/coordination complex catalysts, catalytic reactions, preparation of organic compounds, etc. Use, good catalytic effect, high enantioselectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of tridentate nitrogen phosphine ligand
[0043]
[0044] 0°C, N 2 Add 7mL of tBuLi's n-hexane solution (1.6mol / L, 11.2mmol) dropwise into anhydrous ether (20mL) solution of compound 1 (2.57g, 10mmol) under stirring, and naturally rise to room temperature and stir 2h. Then the temperature was lowered to -78°C, and redistilled PCl was slowly added dropwise. 3 (11.46 mmol, 1 mL), the mixture was warmed to room temperature and reacted overnight. Then the temperature was lowered to -78°C again, and R was slowly added dropwise with a constant pressure funnel. 2 MgBr solution (by 30mmol R 2 Br and 0.8g, 33.3mmol magnesium chips were prepared in tetrahydrofuran). After the dropwise addition, the temperature was slowly raised to react overnight, and then 20 mL of saturated NH 4 Cl solution. The oily phase was extracted three times with ether, each time with 20 mL of ether. The oil phase was dried with anhydrous sodium sulfate, spin-dried, and chromatographe...
Embodiment 2
[0060] Preparation of 1-phenylethyl alcohol from acetophenone (S / C=10 000)
[0061]
[0062] Under high-purity argon atmosphere, [Ir(COD)Cl] 2 (3.4mg, 0.005mmol) and chiral ligand L6 (9.2mg, 0.011mmol) were dissolved in isopropanol (1mL) and stirred at room temperature for 3 hours to obtain a clear orange solution. Take 20 μL (0.001 mol%) of the orange solution with a microsyringe and add it to a mixed system of acetophenone (2 mmol), isopropanol (2 mL) and lithium tert-butoxide (1 mol %). The reaction system was placed in an autoclave at room temperature and H 2 (20atm) and stirred for 12 hours. The solvent was removed under reduced pressure and separated by column chromatography (using silica gel column, eluent: ethyl acetate) to obtain pure product 1-phenylethanol. The product was analyzed by HPLC and the measured ee value was 98%. Determination of enantiomeric excess by HPLC, Chiralcel OD-H column, n-hexane:isopropanol=95:5; flow rate=1.0mL / min; UV detection at 210nm...
Embodiment 3
[0064] Preparation of 1-phenylpropanol from propiophenone (S / C=10 000)
[0065]
[0066] Under high-purity argon atmosphere, [Ir(COD)Cl] 2 (3.4mg, 0.005mmol) and chiral ligand L6 (9.2mg, 0.011mmol) were dissolved in isopropanol (1mL) and stirred at room temperature for 3 hours to obtain a clear orange solution. Take 20 μL (0.001 mol%) of the orange solution with a microsyringe and add it to a mixed system of propiophenone (2 mmol), isopropanol (2 mL) and lithium tert-butoxide (1 mol %). The reaction system was placed in an autoclave at room temperature and H 2 (20atm) and stirred for 12 hours. The solvent was removed under reduced pressure and separated by column chromatography (using silica gel column, eluent: ethyl acetate) to obtain pure 1-phenylpropanol. The product was analyzed by HPLC, and the measured ee value was 99%. Determination of enantiomeric excess by HPLC, Chiralcel OJ-H column, n-hexane:isopropanol=95:5; flow rate=1.0mL / min; UV detection at 210nm; t R (S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com