Chirality dihydrogen silane compound and synthetic method and application thereof
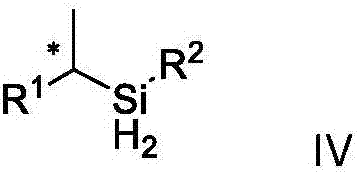
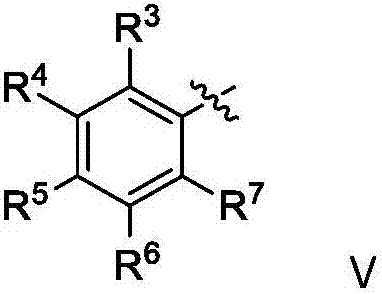
A dihydrosilane and compound technology, applied in the field of chiral dihydrosilane compound and its synthesis, can solve problems such as inapplicability and inapplicability of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Chiral CoX 2 -OIP Complex Catalyzed Hydrosilation of Alkenes and Silanes
[0071] Add chiral CoX to a dry reaction tube at room temperature 2 -OIP complex (0.01mmol), alkene (1.0mmol) shown in formula I, silane (1.0mmol) shown in formula II, ether (2mL), sodium tert-butoxide (0.03mmol), then at room temperature or After stirring at 0° C. for 1 hour, the product was obtained by column chromatography (elution solvent was petroleum ether or a mixture of petroleum ether and ethyl acetate).
[0072]
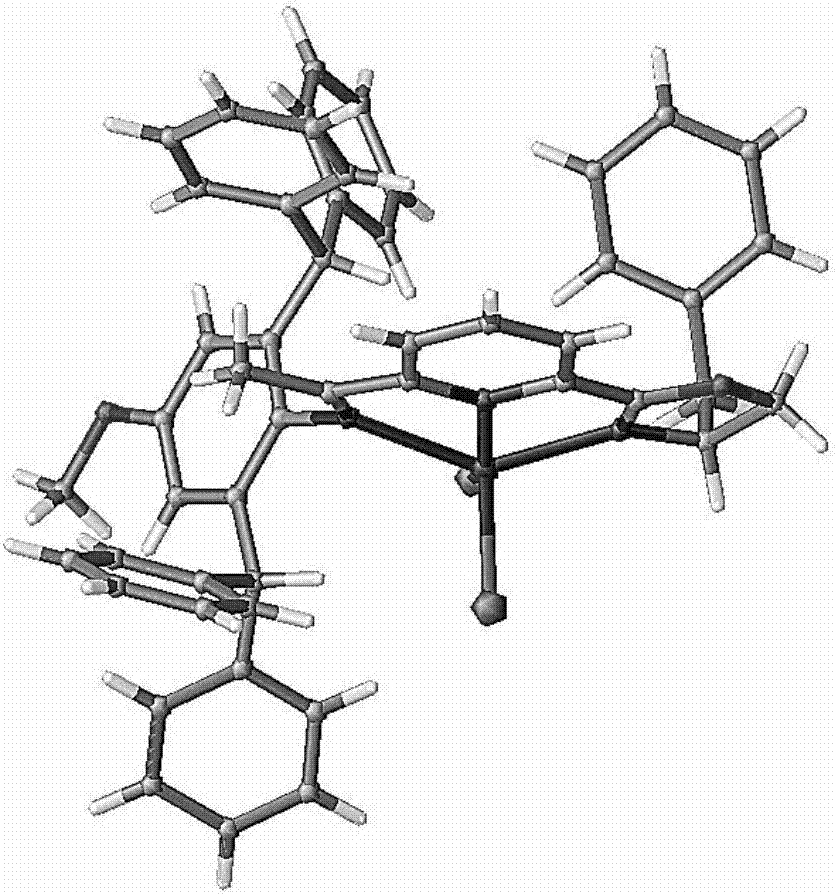
[0073] In Example 1, chiral CoX 2 The chemical formula of the -OIP complex is as shown in formula III-1:
[0074]
[0075] Preparation Method References: (a) Dai, S.; Sui, X.; Chen, C.Angew.Chem.Int.Ed.2015, 54, 9948-9953. (b) Greenhalgh, M.D.; Thomas, S.P.J.Am. Chem. Soc., 2012, 134, 11900-11903. (c) Leonard, W.R.; Romine, J.L.; Meyers, A.I.J. Org. Chem. 1991, 56, 1961-1963. ; Lu, Z. Org. Lett. 2015, 17, 5939-5941. (e) Guo, J.; Shen, X. Z.; Lu, Z. Angew. ...
Embodiment 2
[0208] Embodiment 2: product oxidation synthesis chiral alcohol compound (application example)
[0209]
[0210] In a 20mL reaction tube, add 1a (0.087g, 0.3mmol), dichloromethane (15mL), stir at 0°C and add HBF 4 ·Et 2 O (0.35g, 1.6mmol, 40%Wt). Stir for 3h, spin off the solvent, then add tetrahydrofuran (3mL), methanol (3mL), potassium fluoride (0.07g, 1.2mmol), potassium bicarbonate (0.30 g, 3.0 mmol), H 2 o 2 (1.5mL, 30%wt). Stir at room temperature for 15h, dilute with water, extract 3 times with ether, wash with saturated brine, dry over anhydrous sodium sulfate, spin dry, pass PE / EtOAc=4 / 1 to get 0.048g (0.24mmol , 81% yield) target product. white solid, [α] 20 D =-42 (c 0.27, CHCl 3 ),99%ee, 1 H NMR (CDCl 3 ,400MHz):δ7.55-7.61(m,4H),7.40-7.47(m,4H),7.31-7.37(m,1H),4.95(q,J=6.4Hz,1H),1.86(br,1H ), 1.54 (d, J=6.4Hz, 3H). (References for experimental procedures: Bergens, S.H.; Noheda, P.; Whelan, J.; Bosnich, B.J.Am.Chem.Soc.1992,114,2121-2128 .Compound data...
Embodiment 3
[0216] Embodiment 3: product oxidation synthesis silanol compound (application example)
[0217]
[0218] 2a (0.1046g, 0.5mmol), BCl was added to the 20mL reaction tube 3 (0.5mmol, 0.5mL, 1.0M inDCM), H 2 O (1.2 mL), dichloromethane (2.0 mL). Stir overnight, dilute with water, extract 3 times with dichloromethane, wash with saturated brine, and dry over anhydrous sodium sulfate. PE / EtOAc=10 / 1 was passed through the column to obtain 0.0790 g (0.35 mmol, 70% yield) of the target product. Oily liquid, 1:1dr. 1 H NMR (CDCl 3 ,400MHz):δ7.45-7.51(m,2H),7.19-7.44(m,12H),7.03-7.15(m,6H),4.93(d,J=1.8Hz,1H),4.90(d,J =2.4Hz, 1H), 2.48-2.57(m, 2H), 2.17(br, 2H), 1.41(d, J=7.8Hz, 3H), 1.38(d, J=7.8Hz, 3H).(compound data Consistent with the literature: Visco, M.D.; Wieting, J.M.; Mattson, A.E.Org. Lett. 2016, 18, 2883-2885.)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com