Nitrilase, encoding genes, carrier and application
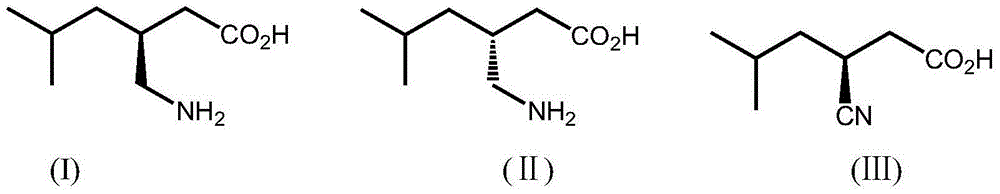
A nitrilase and coding gene technology, which is applied in the application field of preparing -3-cyano-5-methylhexanoic acid, can solve the problems of difficult separation and purification, environmental pollution and the like, and achieves high atom economy and environmental friendliness. , mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: the acquisition of nitrilase
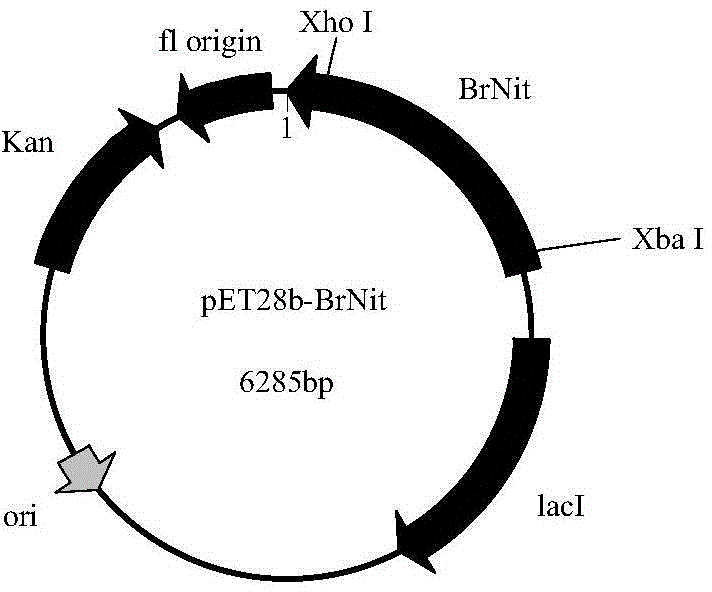
[0053] A nitrilase gene sequence was screened using protein PDB and NCBI database data to obtain a nitrilase gene (NCBI Reference Sequence: NP_001288810.1). The nitrilase is derived from Brassica rapa, a Brassicaceae plant. According to the amino acid sequence of the nitrilase, codon optimization is carried out according to the codon preferred by Escherichia coli, and according to the expression vector pET28b(+) Features The enzyme cutting sites Xho I and Xba I were designed, and the nitrilase gene BrNit (shown in SEQ ID NO: 2) was synthesized by the method of total synthesis through the conventional operation of genetic engineering. The amino acid sequence of the encoded enzyme is shown in SEQ ID NO: 2. ID NO: 1.
Embodiment 2
[0054] Example 2: Construction of recombinant expression vector pET28b(+)-BrNit and construction of recombinant engineering bacteria
[0055] Use Xho I and Xba I restriction endonucleases to carry out double digestion and recycling of the BrNit gene fragment, and use T4 DNA ligase to combine the fragment with the commercial vector pET28b(+) treated with the same restriction endonuclease. Ligation was carried out at 16°C for 16 hours, thereby constructing the intracellular recombinant expression vector pET28b(+)-BrNit. Transform the constructed intracellular expression vector pET28b(+)-BrNit into E.coli BL21(DE3) (Invitrogen) recipient bacteria, spread on LB agar plates containing kanamycin (final concentration: 50 μg / mL) On the plate, cultivate overnight at 37°C, and on the next day, clones were randomly selected from the colonies grown on the plate, and the plasmids were extracted for agarose gel electrophoresis identification, and the recombinant genetically engineered bacte...
Embodiment 3
[0056] Embodiment 3: the preparation that contains nitrilase BrNit bacterial cell
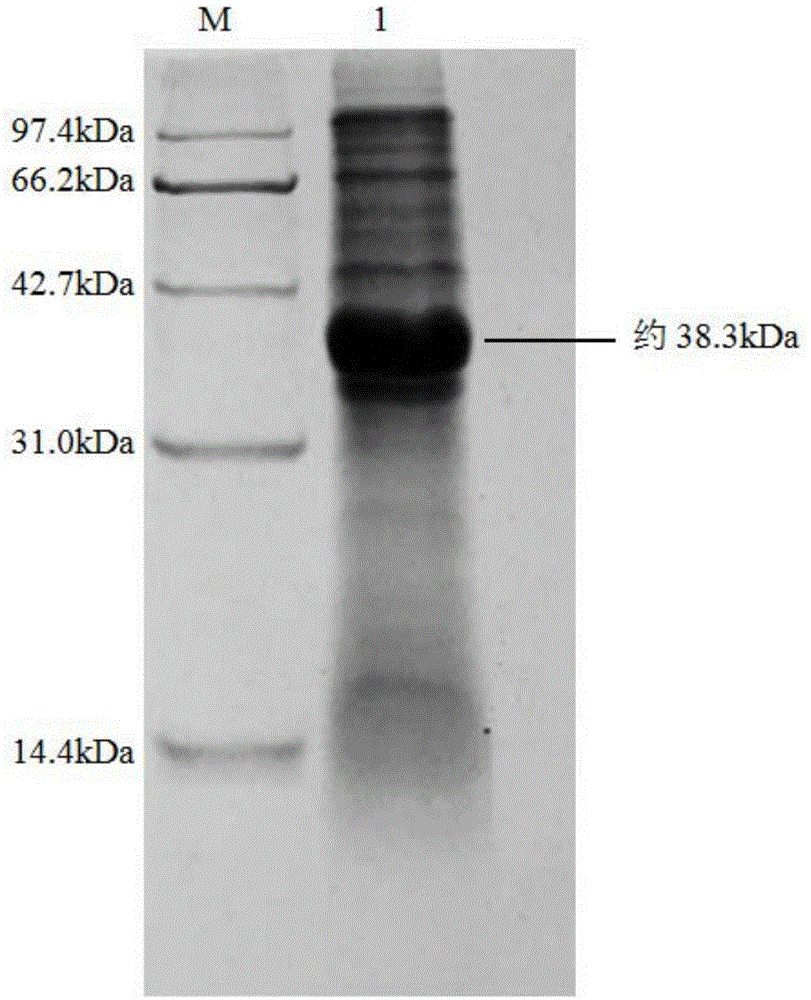
[0057] The genetically engineered bacteria E.coli BL21(DE3) / pET28b(+)-BrNit constructed in Example 2 was inoculated into LB liquid medium containing 50 μg / mL kanamycin, cultivated at 37°C for 12h, and then inoculated with 2% The amount (v / v) was inoculated into fresh LB liquid medium containing 50 μg / mL kanamycin, and cultured at 37°C to the cell concentration OD 600 About 0.6, then add IPTG with a final concentration of 0.2mM to the LB liquid medium, and after inducing culture at 28°C for 10h, centrifuge the culture solution at 4°C and 12000rpm for 5min, discard the supernatant, and collect the recombinant nitrilase-containing The wet cells of Escherichia coli BL21(DE3) / pET28b-BrNit containing intracellular expression of recombinant nitrilase. Then SDS-PAGE electrophoresis was carried out to verify the size and expression of the target protein (see figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com