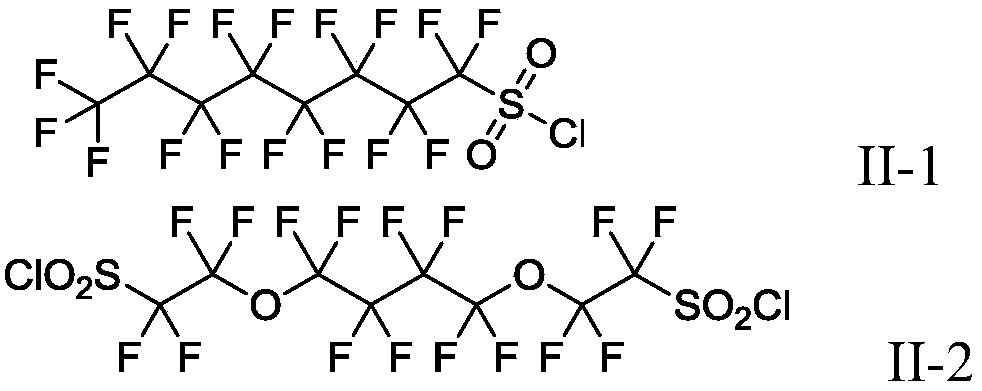
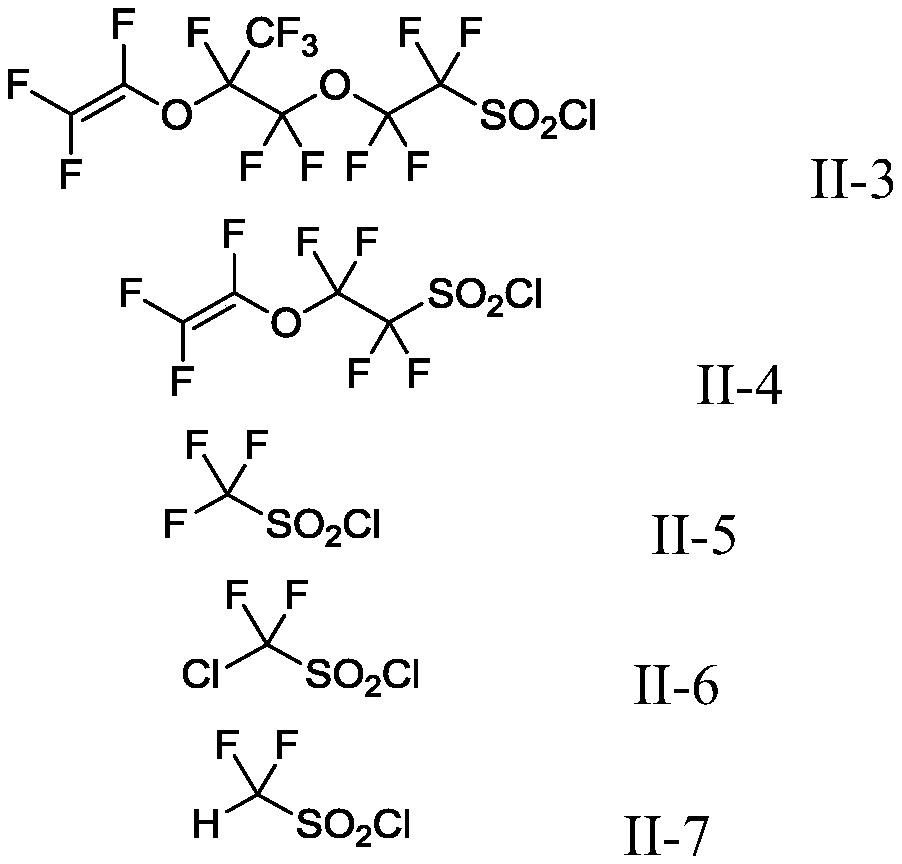
Production method of fluoroalkyl iodide
A technology of fluoroalkyl iodide and alkyl, which is applied in the field of preparation of fluoroalkyl iodide, and can solve the problems of high cost, low cost, and difficult availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087]
[0088] Add 100mL of THF solution to a 250mL open beaker containing a stirring bar, weigh 1.14g (0.03mol) of sodium borohydride and add it; adjust the rotor speed to about 800 rpm (too low to produce foam that affects the reaction effect), Load 15g (0.03mol) of the sulfonyl fluoride raw material represented by the formula (IV-1) weighed on the automatic injection pump of the 10mL syringe; After the addition was completed, the reaction was carried out at room temperature for 2 h. After the fluorine spectrum detection reaction is complete, add SO 2 Cl 2 (0.06mol) was reacted at room temperature for 30 minutes, and the reaction was complete by fluorine spectrum detection. Add water 50mL to wash subsequently, add 5g KI reaction again, heat to 80 ℃ reflux 2h, get the perfluorooctyl iodide shown in formula (I-1) after distilling off solvent tetrahydrofuran, total yield is 75%, purity>92 %.
Embodiment 2
[0090] (1)
[0091]
[0092] Add 2Kg (3.34mol) of the substrate represented by formula (IV-2) and 4.5Kg of acetonitrile into a 20L four-necked reaction flask. Heat up while stirring, control the internal temperature to 70°C, add 279g (7.34mol) NaBH in batches 4 , observe the change of internal temperature, control the internal temperature not to exceed 75°C, react at room temperature for 3 hours, detect the reaction by fluorine spectrum, after the reaction is completed, add 108g of water dropwise to quench the excess NaBH 4 . After the reaction, filter with suction, wash the filter cake with DCM, and dry at 40°C to obtain the sodium salt of sulfinic acid represented by formula (III-2), with a yield of about 90%.
[0093] (2)
[0094]
[0095]Add 3000mL of sulfuryl chloride into the 10L reaction flask, cool down to about 10°C in a dry ice bath, add 1960g (3.23mol) of sulfinic acid sodium salt shown in formula (III-2) in batches while stirring, a large amount of gas is ...
Embodiment 3
[0100] (1)
[0101]
[0102] Add 89.2g (0.2mol) of the sulfonyl fluoride raw material represented by formula (IV-3) and 200mL of ethanol into a 500mL three-neck flask, cool down in an ice-water bath, and when the internal temperature is lower than 5°C, add NaBH in batches 4 , Control the internal temperature not to exceed 10°C. Add 7.56g (0.2mol) NaBH 4 Then take a sample for F-spectrum detection, add 0.76g (0.02mol) corresponding amount of NaBH 4 Complete reaction of raw materials while avoiding NaBH 4 excess. Ethanol was distilled off under reduced pressure. The product represented by formula (III-3) was obtained with a fluorine spectrum yield of 97%.
[0103] (2)
[0104]
[0105] Add 200mL dichloromethane and 32mL (0.4mol) SO 2 Cl 2 , fully stirred to make the reaction system into a uniform white emulsion, react at room temperature for 2 hours, and take samples to check whether the reaction of the raw materials is complete. After all the raw materials have b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com