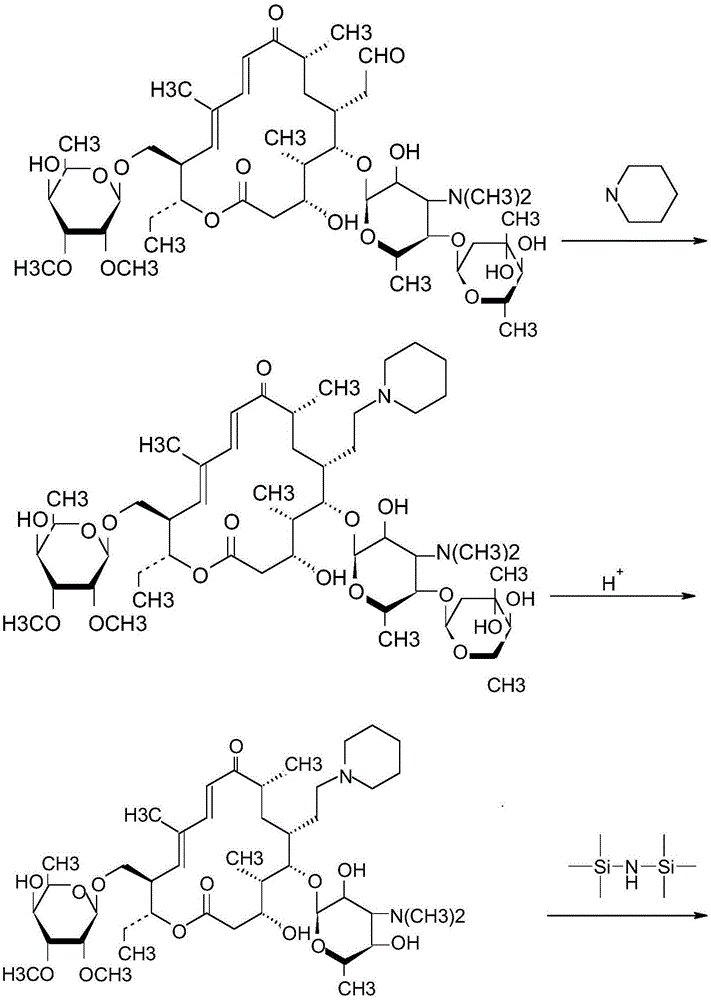
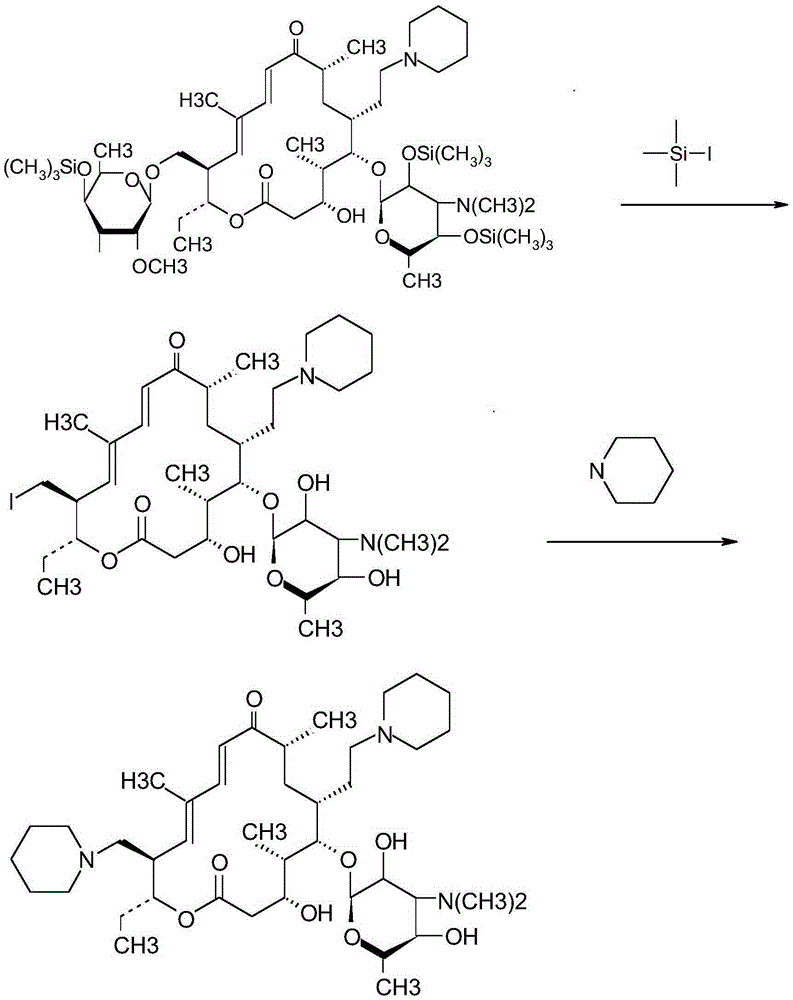
Synthetic method for tildipirosin
A synthetic method, a new technology of Tylenol, applied in the direction of chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of structural damage of raw materials, difficulty in obtaining Tylenolactone, and low yield, and achieve Reduce energy consumption, facilitate industrial operation, and control the reaction easily
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] (1) Preparation of 20-piperidinyl-5-O-mycaminosyl-tylonolide
[0059] Dissolve 18.3g of tylosin, 4.2g of piperidine, and 1.4g of formic acid in 50ml of toluene, heat up to 75°C, react for 1 hour, cool down to room temperature, add 60ml of water and heat up to 35°C, adjust with 60% sulfuric acid solution pH = 2.5, hydrolysis reaction for 1 hour.
[0060] After the reaction, the reaction solution was cooled to room temperature, 40ml of dichloromethane was added, and the pH was adjusted to 10 with 10N sodium hydroxide solution, the layers were separated, the aqueous phase was washed twice with 10ml of dichloromethane, and the organic phases were combined and dried over anhydrous sodium sulfate. spare.
[0061] (2) Preparation of 20-piperidinyl-23-I-5-O-mycaminosyl-tylonolide
[0062] Add 5.2g of hexamethyldisilazane to the dried dichloromethane solution, react at 30°C for 2.5 hours (no ammonia gas is released at this time), lower the reaction solution to room temperature...
Embodiment 2
[0066] (1) Preparation of 20-piperidinyl-5-O-mycaminosyl-tylonolide
[0067] Dissolve 18.3g of tylosin, 3.4g of piperidine, and 1.8g of formic acid in 40ml of toluene, heat up to 70°C, react for 1 hour, cool down to room temperature, add 40ml of water and heat up to 40°C, adjust with 60% sulfuric acid solution pH = 3.0, hydrolysis reaction for 1 hour.
[0068] After the reaction, the reaction solution was cooled to room temperature, 40ml of dichloromethane was added, and the pH was adjusted to 10 with 10N sodium hydroxide solution, the layers were separated, the aqueous phase was washed twice with 10ml of dichloromethane, and the organic phases were combined and dried over anhydrous sodium sulfate. spare.
[0069] (2) Preparation of 20-piperidinyl-23-I-5-O-mycaminosyl-tylonolide
[0070] Add 4.8g of hexamethyldisilazane to the dried dichloromethane solution, react at 35°C for 2 hours (no ammonia gas is released at this time), lower the reaction solution to room temperature, ...
Embodiment 3
[0074] (1) Preparation of 20-piperidinyl-5-O-mycaminosyl-tylonolide
[0075] Dissolve 18.3g of tylosin, 4.2g of piperidine, and 1.8g of acetic acid in 50ml of toluene, heat up to 80°C, react for 50min, cool down to room temperature, add 60ml of purified water and heat up to 45°C, with a mass fraction of 60% The sulfuric acid solution was used to adjust the pH to 2.5, and the hydrolysis reaction was carried out for 1 hour.
[0076] After the reaction, the reaction solution was cooled to room temperature, 40ml of dichloromethane was added, and the pH was adjusted to 10 with 10N sodium hydroxide solution, the layers were separated, the aqueous phase was washed twice with 10ml of dichloromethane, and the organic phases were combined and dried over anhydrous sodium sulfate. spare.
[0077] (2) Preparation of 20-piperidinyl-23-I-5-O-mycaminosyl-tylonolide
[0078] Add 6.4g of hexamethyldisilazane to the dried dichloromethane solution, reflux at 40°C for 2.5 hours (no ammonia gas i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com