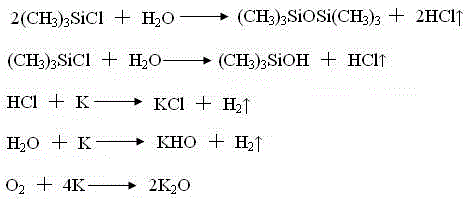Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "Trimethylsilyl iodide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
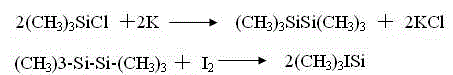
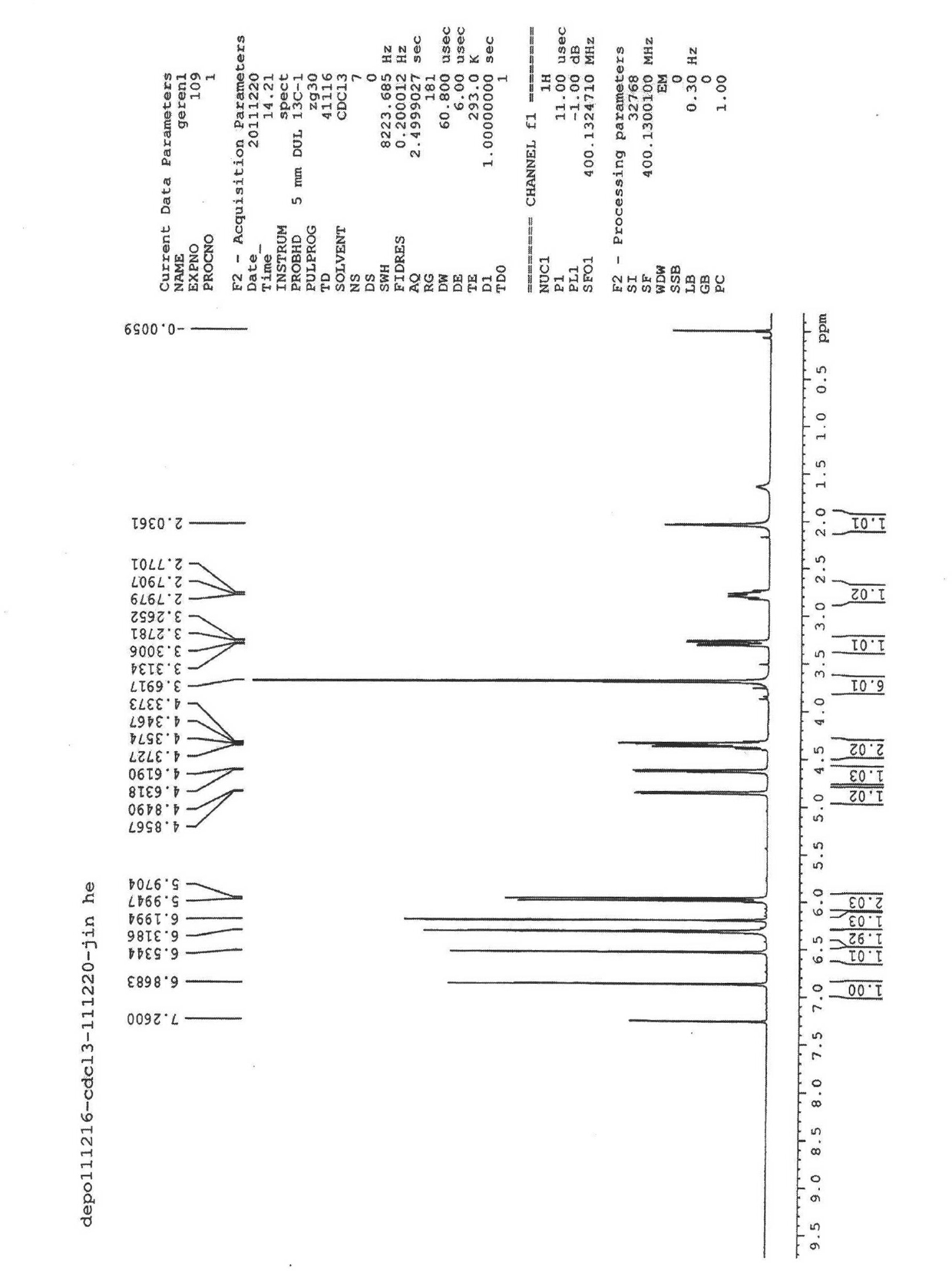
Trimethylsilyl iodide (iodotrimethylsilane or TMSI) is an organosilicon compound with the chemical formula (CH₃)₃SiI. It is a colorless, volatile liquid at room temperature.
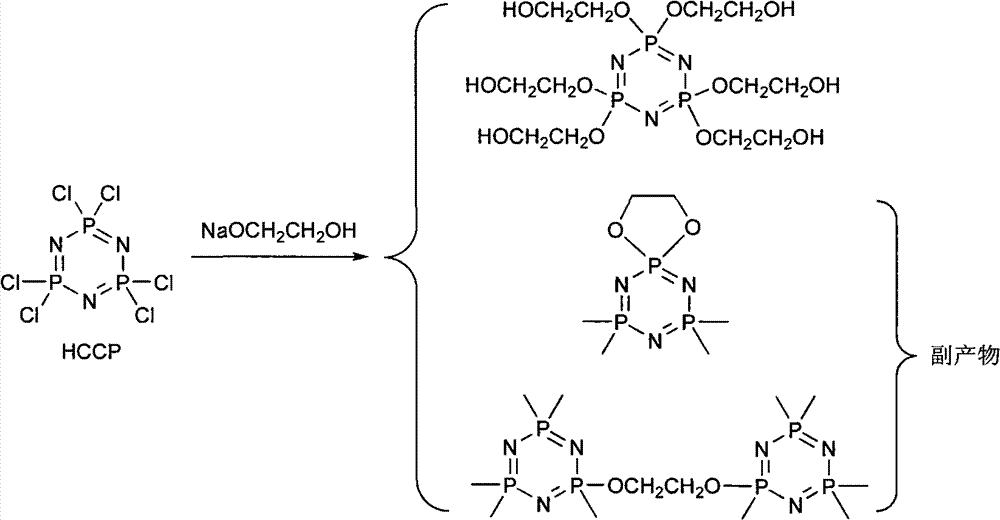
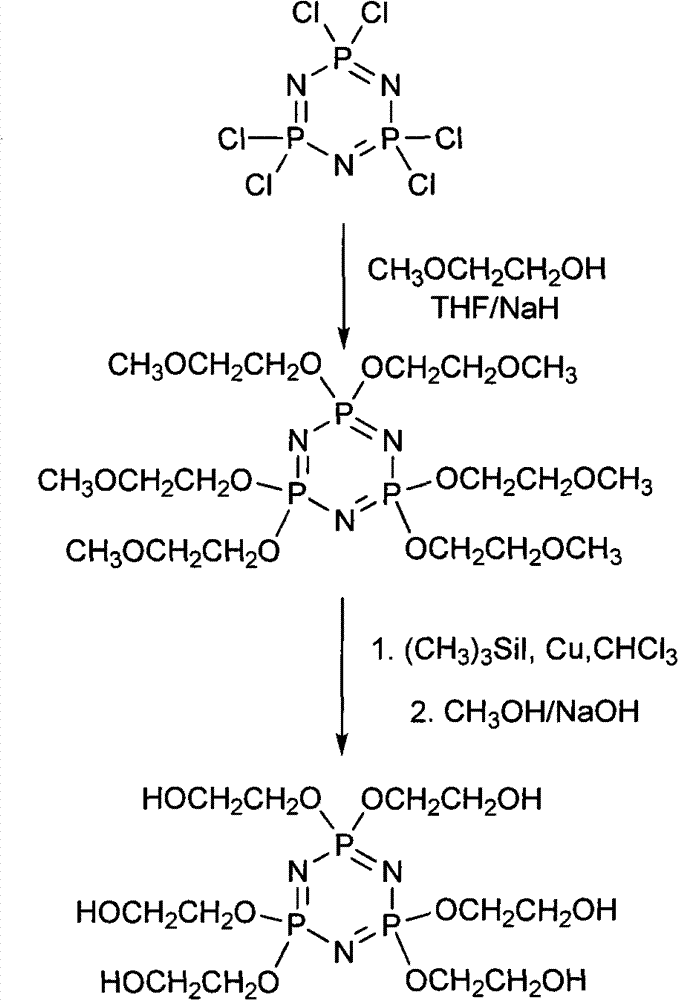
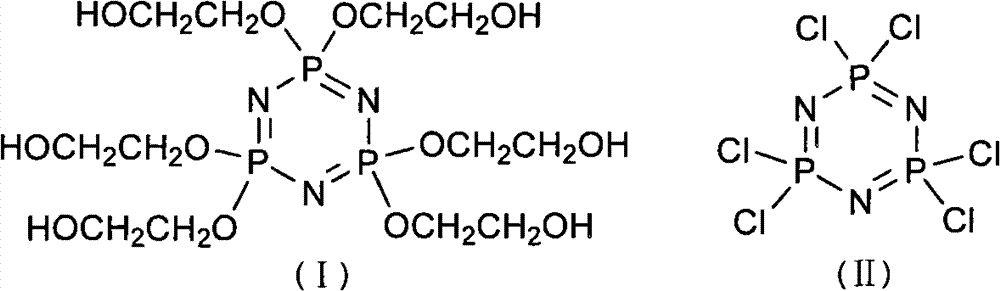
Synthetic method of 6(4-hydroxyl ethyoxyl) cyclotriphophazene
InactiveCN102766167ASimple post-processingHigh reaction yieldGroup 5/15 element organic compoundsDistillationSodium hydride
The invention discloses a synthetic method of 6(4-hydroxyl ethyoxyl) cyclotriphophazene. The method includes the following steps: (1) enabling methyl cellosolve and sodium hydride to react to obtain methyl sodium ethoxide, adding a tetrahydrofuran solution of hexachlorocyclotriphosphazene, reacting for 30min-45min at the temperature of 20 DEG C-35 DEG C, filtering, removing solvent by steaming, extracting, washing and conducting reduced pressure distillation to obtain 6(4-methoxyl ethyoxyl) cyclotriphophazene; (2) enabling the 6(4-methoxyl ethyoxyl) cyclotriphophazene and iodotrimethylsilane to react for 30h-40h at the temperature of 25 DEG C-35 DEG C under the condition of stirring, adding a methanol solution of sodium hydroxide, reacting for 6h-8h at the temperature of 15-30 DEG C under the condition of stirring, filtering, and conducting reduced pressure removing of the solvent by steaming to obtain the 6(4-hydroxyl ethyoxyl) cyclotriphophazene. The synthetic method of the 6(4-hydroxyl ethyoxyl) cyclotriphophazene is high in reacting yield and is mainly used for synthesis of the 6(4-hydroxyl ethyoxyl) cyclotriphophazene.
Owner:XIAN MODERN CHEM RES INST
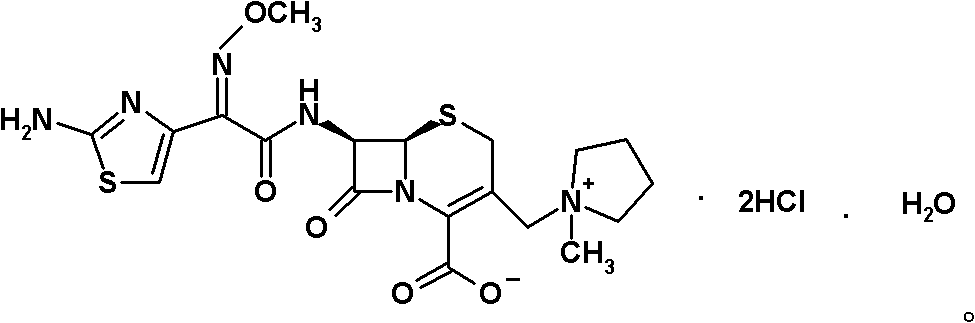
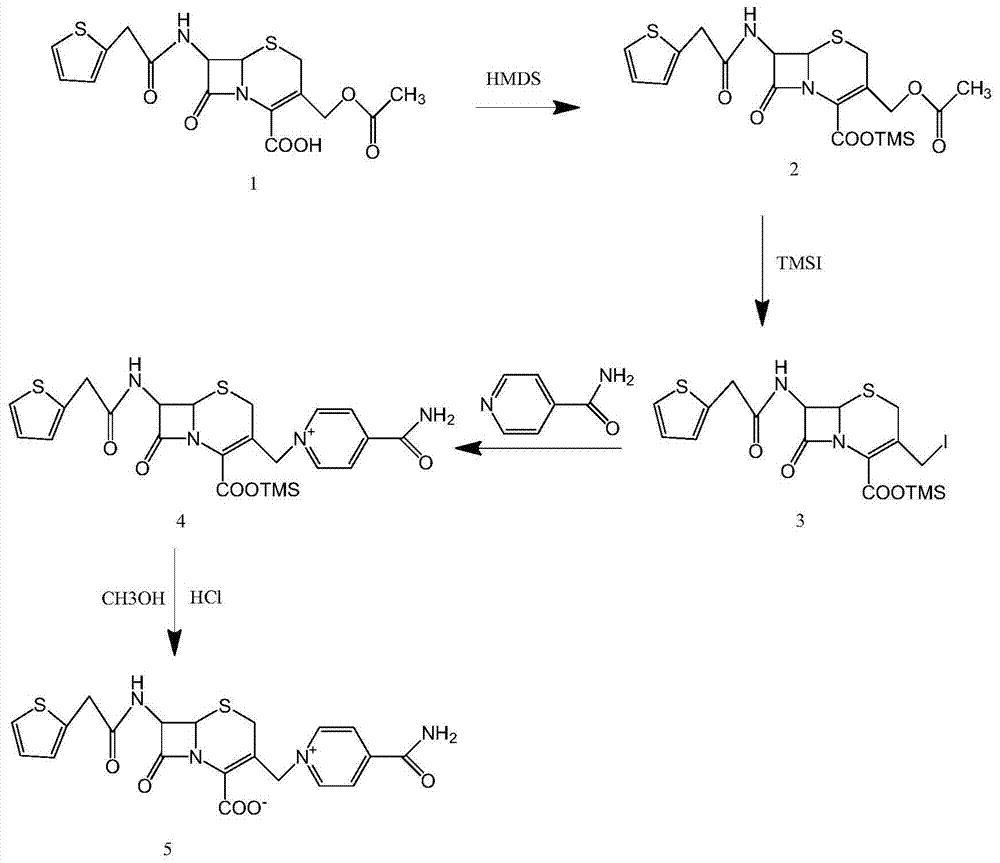
Method for synthesizing cefepime hydrochloride
InactiveCN101735251AHigh densityHigh reactivityAntibacterial agentsOrganic chemistryCefepime hydrochlorideVolumetric Mass Density
The invention relates to a method for synthesizing cefepime hydrochloride. The method comprises the following steps: taking 7-aminoce-phalosporanic acid (7-ACA) and N-methylpyrrolidine as raw materials, firstly, carrying out carboxylic and amino protection on the 7-ACA by HMDS, then preparing the N-methylpyrrolidine and iodotrimethylsilane into a quaternary ammonium salt intermediate, finally, adding the intermediate into the protected 7-ACA solution and reacting to prepare 7-MPCA; taking the 7-MPCA and AE-active ester, adding a phase transfer catalyst into an organic phase for carrying out an N-acidylating reaction, salifying and reacting to obtain the cefepime hydrochloride. The invention has the main characteristics that the quaternary ammonium salt intermediate is prepared in the step (1), the defects of high electron cloud density, strong reactivity and many side reactions of the N atom of N-methyl pyrrole are overcome, the yield is enhanced by 7%, and the product purity is enhanced. During the N-acidylating reaction in the organic phase in the step (2), the phase transfer catalyst is added, so that the conversion rate of the reaction is enhanced by 5%, and the product yield is enhanced.
Owner:YIYUAN XINQUAN CHEM
Process for synthesizing iodotrimethylsilane
ActiveCN1962668AEliminate side effectsConvenient post-processingGroup 4/14 element organic compoundsSilanesCopper
The invention discloses a synthesizing method of trimethyl iodine silane, which comprises the following steps: adding aluminium, hexamethyldisilazane and iodine in the autoclave; distilling; obtaining the rough product of trimethyl iodine silane; placing rough product in the distilling autoclave; adding copper powder; heating; collecting fraction; obtaining the refined product.
Owner:扬州三友合成化工有限公司
Preparation method of cefepime hydrochloride
ActiveCN101935325ASimple processAvoid the phenomenon of inhomogeneous crystal form and poor fluidityOrganic chemistryCefepime hydrochlorideBetaine
The invention discloses a preparation method of cefepime hydrochloride, comprising the following steps of: reacting oxalyl chloride with 2-methoxyimino-2-(2-aminothiazole-4-yl) acetic acid hydrochloride to obtain a midbody I, i.e. 2-methoxyimino-2-(2-aminothiazole-4-yl) acetyl chloride hydrochloride; mixing silanized 7-aminoce-phalosporanic acid and silanized N-methylpyrrolidine, and reacting to obtain a midbody II, i.e. hydriodic acidification (6R, 7R)-7-amino-3-[(1-methyl-1-tetrahydro pyrrolidine) methyl]-3-cephem-4-formic betaine, in the presence of trimethyl idodine silicon hydride, isopropanol and an aqueous solution of hydrogen iodide; dissolving the midbody II into dichloromethane, sequentially adding trimethylchlorosilane and hexamethyldisilazane for reaction, and then adding the midbody I and triethylamine to react to prepare the cefepime hydrochloride. The cefepime hydrochloride prepared by the method has the advantages of uniform crystal form, good flowability and simple process and is suitable for industrialized production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
'One kettle process' of preparing cefozopran and its intermediate
The present invention relates to the process of compound I or its salt. The preparation process includes the reaction between the compound II or its salt with compound A or its alt in the presence of trimethyl silane iodine (TMSI). The compound I may be used in preparing cefozopran as the fourth generation of cephalosporin.
Owner:BEIJING XINLINGXIAN MEDICAL TECH DEV CO LTD
Method for synthesizing antibiotic cefepime hydrochloride
ActiveCN101337971ASimple process conditionsEasy to operateAntibacterial agentsOrganic chemistryCefepime hydrochlorideHexamethyldisilane
The invention relates to a synthesis method of cefepime dihydrochloride that is a bacteriophage. 7-amin cethalosporanic acid (7-ACA) is used as starting material and reacts with hexamethyldisilane amine (HMDS) and iodotrimethylsilane (TMSI) first to obtain 7-ACA for protecting amino and carboxyl; then 7-ACA, amino and carboxyl of which are protected, reacts with iodotrimethylsilane and N-methylpyrrolidine to synthesize (6R, 7R)-7-amino-3-((1-methyl-1-pyrrolidine) methyl) cephalosporin-3-alkene-4-carboxylic acid hydrochloride (7-MPCA) through a one-pot method; 7-MPCA reacts with AE active ester to obtain a product of cefepime dihydrochloride through acidylation reaction and salifying reaction. Compared with the existing technical route, the synthesis method has the advantages that the process conditions are simple, the operation is convenient, the product yield is high, the product quality is stable, the method is suitable for the large-scale industrialized production, etc.
Owner:国药集团致君(苏州)制药有限公司
Process for preparing cefquinome sulfate
InactiveCN101307064AGood treatment effectLow toxicityAntibacterial agentsOrganic chemistryCefotaxime7-ACA
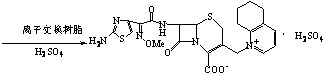
The invention discloses a method for preparing cefquinome sulfate, belonging to the cephalosporin antibiotics special for animals. The method comprises the following: A. a step of preparing 7-amino-cefquinome, during which, iodotrimethylsilane reacts with 5, 6, 7, 8-tetrahydroquinoline, and a reaction product reacts with 7-amino-cephalosporanic acid, thereby obtaining the 7-amino-cefquinome; B. a step of preparing the cefquinome sulfate, during which, the 7-amino-cefquinome reacts with AE active ester after the 7-amino-cefquinome is dissolved and is decolored by sulphuric acid, and the cefquinome sulfate is obtained after the reaction product is subject to extraction, suction filtering, leaching and drying. The method uses 7-ACA which is widely used in industry, is low in cost and is easily obtained as a raw material, without using cefotaxime acid with unstable service quality; meanwhile, the method has a mild reaction condition and simple operation, reduces the dosage of the 5, 6, 7, 8-tetrahydroquinoline which is an important intermediate, and greatly improves the product quality with the yield of 64.1 percent and the purity of 99.7 percent, thereby the method is suitable for industrial production.
Owner:崔增学
Method for preparing cefquinome sulfate
InactiveCN103275103AReduce manufacturing costMild reaction conditionsOrganic chemistryCefotaximePhosphoric acid
The invention discloses a method for preparing cefquinome sulfate. The method comprises the steps as follows: 7-aminocephalosporanic acid is taken as a raw material; a C-3 bite ester group is hydrolyzed under the action of alkali and reacts with 2-(2-Amino-4-thiazolyl)-(z)-methoxyiminoacetic, thiobenzothiazole ester, so that an intermediate A is obtained; an iodo substance 3-iodine methyl cefotaxime is obtained through the intermediate and potassium iodide under the action of phosphoric acid; the 3-iodine methyl cefotaxime reacts with 5, 6, 7, 8-tetrahydroquinoline, so that an intermediate B of cefquinome hydriodate is obtained; and finally, the intermediate B of cefquinome hydriodate reacts with sulfuric acid under the action of an alkaline anion exchange resin, so that the cefquinome sulfate is obtained. According to the method for preparing the cefquinome sulfate, the 7-aminocephalosporanic acid which is cheap and easy to obtain is taken as the raw material, so that the use of iodotrimethylsilane which is expensive and prone to decompose when contacted with light and water is avoided, and the production cost is reduced; and the reaction condition is mild, the operation is simple, the yield is high, and the cefquinome sulfate is suitable for industrial production.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Preparation process of trimethyl idodine silicon hydride
InactiveCN102718790AEnhance heat and mass transferImprove conversion rateGroup 4/14 element organic compoundsSilanesPositive pressure
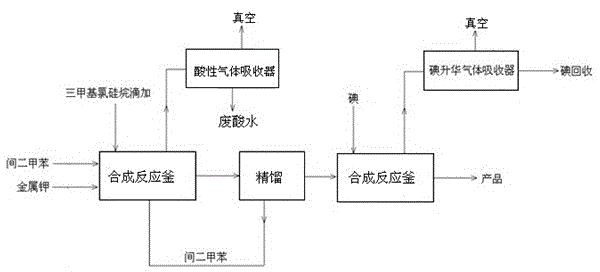
The invention discloses a preparation process of trimethyl idodine silicon hydride, which comprises the following specific steps of: (1) hexamethyldisiloxane preparation: firstly adding m-xylene and metallic potassium into a stirring kettle, mixing and agitating; and then dropwise adding trimethylchlorosilane into the stirring kettle, stop agitating after reaction and leading materials in the kettle into a washing kettle; (2) washing: adding water into the washing kettle, standing and settling, rectifying generated liquid and recovering solid residues; (3) rectification: adding clear liquid into a rectifying tower kettle, intermittently recovering hexamethyldisilane and intermediate fractions and then recovering the m-xylene; and (4) iodine silane synthesis: adding the rectified hexamethyldisiloxane into a reaction kettle, and adding iodine to generate a trimethyl idodine silicon hydride product. The preparation process has the beneficial effects that the reaction is finished in a micro-positive pressure system, so that the mass transfer and heat transfer effects in the reaction are better, the generated residues in the reaction are less, the product conversion rate is greatly improved, the control is easy, and the operating cost is reduced.
Owner:XINYAQIANG SILICON CHEM JIANGSU
Iodotrimethylsilane preparation method
ActiveCN104926850AQuick responseHigh reaction yieldGroup 4/14 element organic compoundsDistillationPhysical chemistry
The invention provides an iodotrimethylsilane preparation method. The preparation method comprises the following steps that superfine aluminum power and half hexamethyldisiloxane are put in a reaction flask, elementary substance iodine and half hexamethyldisiloxane are put in a constant pressure dropping funnel, vacuumizing and nitrogen replacing are performed on a reaction system, and all the steps are repeated for three times; stirring is started, the temperature rises to 60 DEG C, and hexamethyldisiloxane solutions of the iodine are dropwise added; after dropwise adding is finished, the outer temperature rises to 130 DEG C for backflow, and therefore after being cooled, steam enters the constant pressure dropping funnel to dissolve the undissolved iodine in the constant pressure dropping funnel; after the iodine is dissolved completely and dropwise added in the reaction system, reaction is performed for 2 hours until the reaction is complete; heating is stopped, the temperature is cooled below 30 DEG C, a reaction device is changed into a distillation device to collect main distillate fractions, and then colorless liquid iodotrimethylsilane competitive products are obtained.
Owner:山东博苑医药化学股份有限公司
Novel method for preparing trimethyliodosilane
ActiveCN106928268ASimple processEasy to operateGroup 4/14 element organic compoundsCyclic processLithium chloride
The invention relates to a preparation process of trimethyliodosilane, which has the advantages of moderate reaction conditions, simple process, safety in operation, high yield and extremely few three wastes. The preparation process takes anhydrous sodium iodide, anhydrous lithium chloride and trimethylchlorosilane as raw materials and the raw materials react in a dried nitrogen atmosphere to synthesize the trimethyliodosilane. According to the method provided by the invention, a traditional complicated process of preparing trimethyliodosilane from hexamethyldisilane and hexamethyldisiloxane is changed; reaction conditions are moderate and operation is safe; dangers of utilizing high-danger chemicals including metal potassium and sodium are avoided; meanwhile, a high-temperature iodization difficulty is also avoided; in a whole production circulating process, only the trimethyliodosilane product and a byproduct sodium chloride are produced and other three wastes are not generated, so that the process is green and environmental-friendly.
Owner:山东博苑医药化学股份有限公司
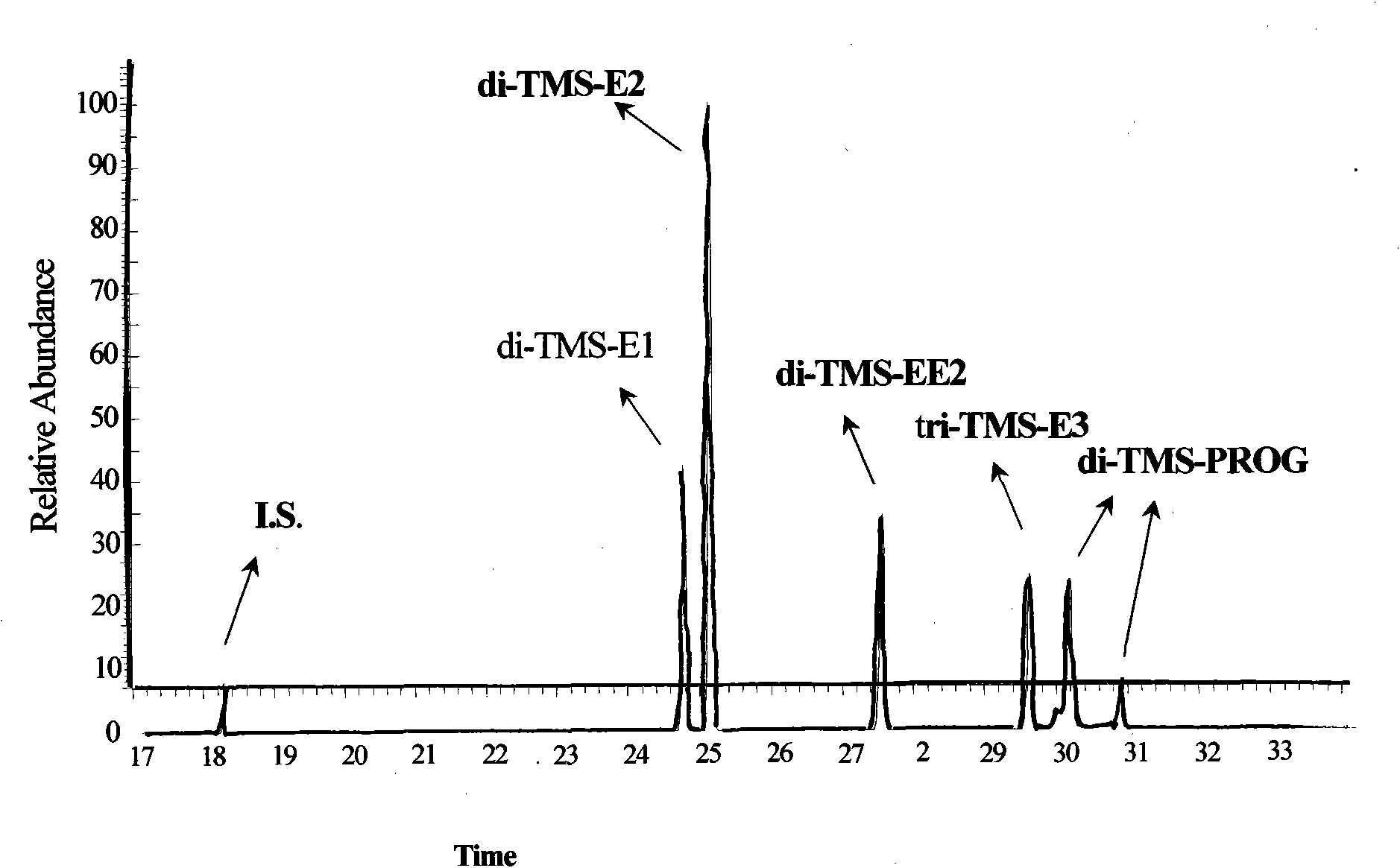
Hydroxyl group/keto group synchronous derivatization method of steroid environment endocrine disturbing chemicals
InactiveCN101942006AImplementing Synchronous DerivatizationReduce polarityComponent separationSteroids preparationEstriolLinear correlation
The invention discloses a steroid environment endocrine disturbing chemicals hydroxyl group / keto group synchronous derivatization method of steroid environment endocrine disturbing chemicals (oestrone, 17 beta-estradiol, 17 alpha-ethinyl estradiol, estriol and progesterone). The method comprises the following steps: first fetching standard mixed solution containing the steroid environment endocrine disturbing chemicals and internal standard and introducing high-purity nitrogen at normal temperature so as to dry the standard mixed solution; and adding derivatization reagent prepared from N-methyl-N-trimethylsilyl trifluoroacetamide, iodotrimethylsilane and dithiothreitol based on a proportion of 1,000 muL:5 to 10 muL:5 mg and reacting at 20 to 60 DEG C for 5 to 10 min so as to obtain the derivatization product shown in figure. The method has the advantages of simple and time-saving operation, low energy consumption and cost and the like. The hydroxyl group and the keto group can be derivatized synchronously, the linear correlation among the derivatization products is good, and the detection limit of instrument can reach 0.01 to 1 pg / mu L. The method can be used for the GC-MS detection of the steroid environment endocrine disturbing chemicals in samples such as water, bottom sediment, organisms and the like.
Owner:KUNMING UNIV OF SCI & TECH
Preparation method of cefquinome sulfate
The invention relates to a method for synthesizing cefquinome sulfate and relates to a preparation method of a medicament for animals. The preparation method comprises the following steps: taking cefotaxime, 2,3-cyclohexyl pyridine and trimethyliodosilane as initial raw materials; preparing cefotaxime hydriodate from the cefotaxime; carrying out decoloring and silica column chromatography on the cefotaxime; and reacting the treated cefotaxime with sulphuric acid to prepare a cefquinome sulfate product. Compared with the prior art, the invention has the advantages of high yield of products, stable production quality and suitability for industrial production.
Owner:PU LIKE BIO ENG
Method for preparing cefepime hydrochloride
InactiveCN102675345AHigh crystal purityImprove liquidityOrganic chemistryCefepime hydrochlorideReflux
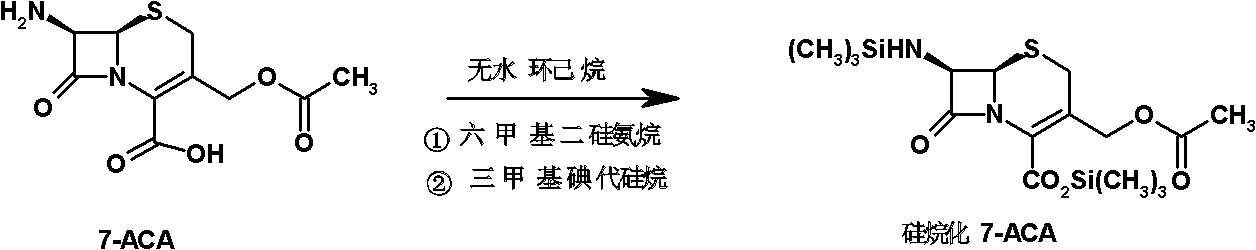
The invention discloses a method for preparing cefepime hydrochloride. The method comprises the following steps of: adding hexamethyldisilazane and trimethylsilyl iodide into 7-aminocephalosporanic acid (ACA), performing vacuum reflux at the temperature of 55 DEG C for 12h, reducing the temperature, diluting, adding N-methylpyrrolidone and trimethylsilyl iodide, reacting at the temperature of 40 DEG C for 23h, after-treating and recrystallizing to obtain 7-ACMP.HCl, dissolving the 7-ACMP.HCl into a solvent at the temperature of 0 to 5 DEG C, adding a small amount of AE-active ester and triethylamine for multiple times, and treating to obtain the cefepime hydrochloride after the reaction is finished. The method for preparing the cefepime hydrochloride is high in reaction speed, low in production cost, and high in product purity and flowability.
Owner:苏州盛达药业有限公司
Trimethyliodosilane preparing method
ActiveCN105237559ANo low generationRapid responseGroup 4/14 element organic compoundsDistillationIodine
The invention discloses a trimethyliodosilane preparing method. The method comprises the steps of adding aluminum chloride anhydrous and hexamethyldisiloxane to a reaction container in the presence of protective gas, conducting stirring, increasing the temperature to 40-50 DEG C, then adding the iodine elementary substance, increasing bath temperature to 125-140 DEG C, conducting reflux reaction for 1-1.5 h, changing the device to be a distilling device, conducting atmospheric distillation, and collecting 106-107 DEG C cut fraction to obtain trimethyliodosilane. The method has the advantages that the yield of trimethyliodosilane is increased, purification is easy, conditions are mild, and large-scale production and popularization are facilitated.
Owner:佛山市南海区波尔有机硅有限公司
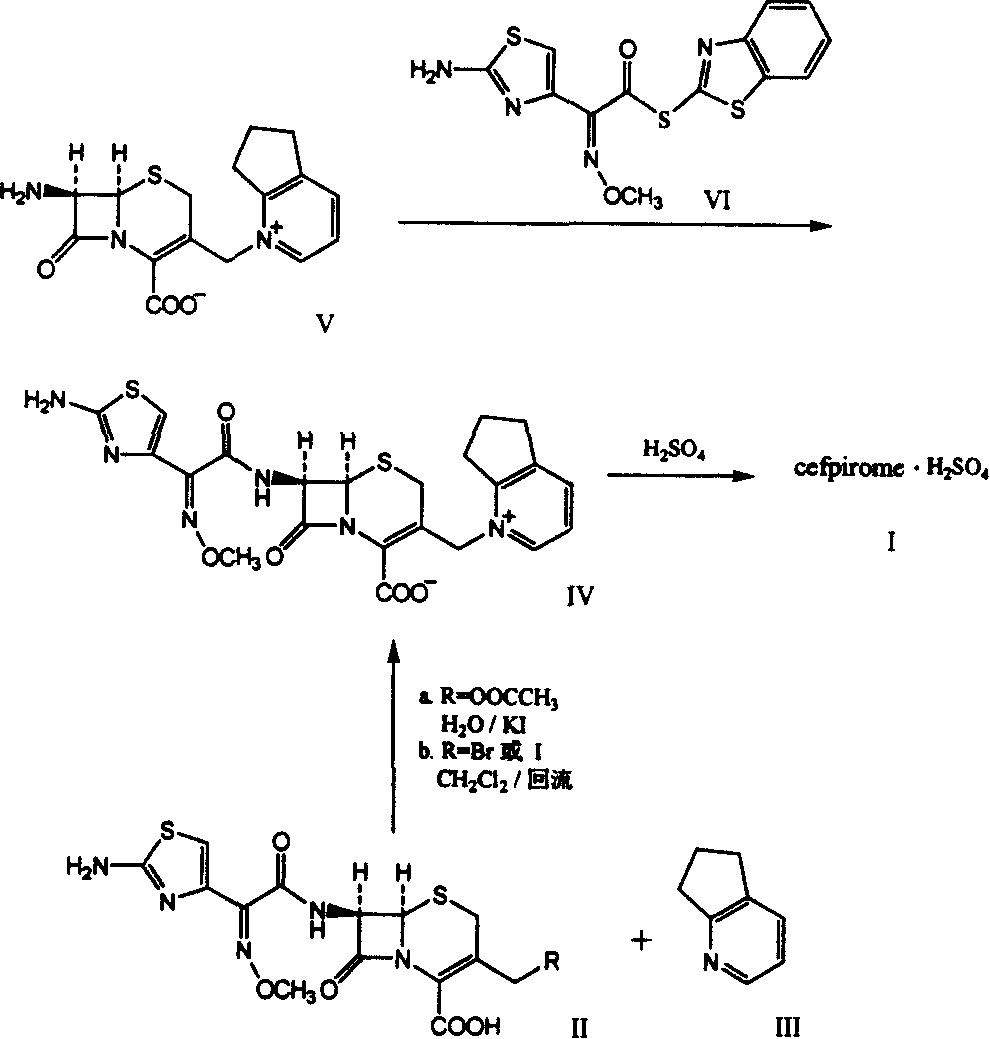
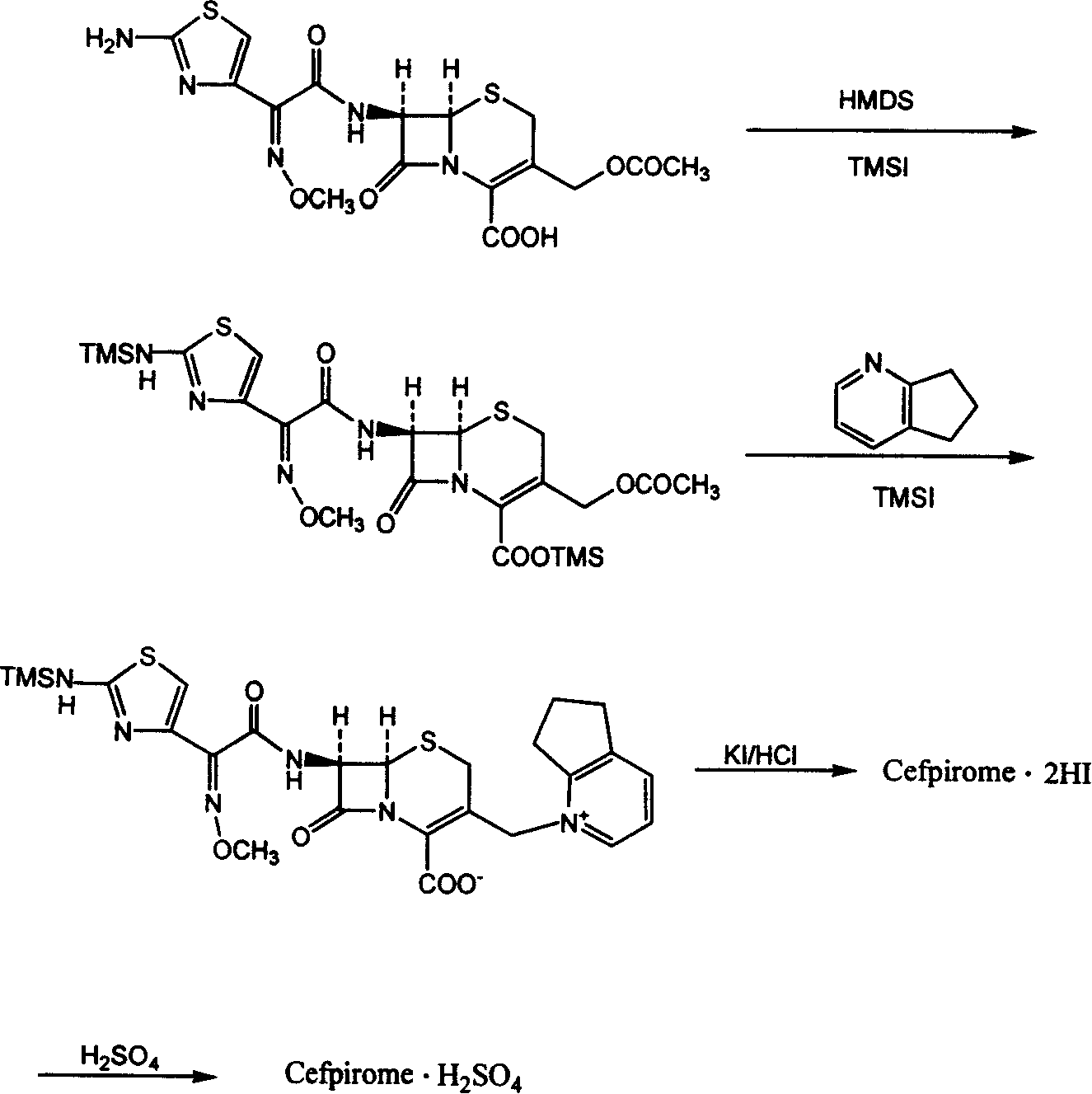
Synthetic method of cefpirome sulfate
ActiveCN101284840ALow costHigh yieldAntibacterial agentsOrganic chemistryTrimethylsilyl chlorideCefotaxime
The invention relates to a synthesis method for making cefpirome sulfate. The method comprises the following steps that: cefotaxime and 2, 3-cyclopenopyridine, with the molar ratio of between 1:7 and 1:12, are mixed with trimethyl bromosilicane used as protecting agent inside organic solvent to carry out substitution reaction, thereby generating cefpirome dihydrobromide; and the salt-forming reaction of the cefpirome dihydrobromide is carried out to make cefpirome sulfate. According to the synthesis method of cefpirome sulfate provided by the technical proposal of the invention, cefotaxime and trimethyl bromosilicane are respectively used as raw material and protecting agent to make cefpirome sulfate. Because enough supply and technical maturity of cefotaxime are realized in China and trimethyl bromosilicane is more active than trimethylchlorosilane and is cheaper than trimethyliodosiliane, the synthesis method has the advantages of low cost of raw material, high yield, simple synthetic route and convenient operation.
Owner:GUANGDONG JIUMING PHARMA
Preparation method for etoposide intermediate
The invention discloses a preparation method for an etoposide intermediate, which comprises the following steps: (1) adding a compound A into a reaction system with dichloromethane as a solvent, then adding iodotrimethylsilane in the reaction system, cooling the reaction solution to a temperature in a range of -10 DEG C to -5 DEG C, adding n-hexane after the reaction materials are completely consumed, stirring to precipitate powdery solid, and filtering to obtain a compound B; (2) adding the compound B in dichloromethane solvent, uniformly stirring at room temperature, freezing the dissolved solution to a temperature in a range -5 DEG C to -8 DEG C, dropping pyridine, rapidly dropping dichloroacetyl chloride, and keeping stirring until the reaction is complete to obtain a compound C; (3) adding acetone, water and sodium thiosulfate into the solution obtained in the step (2), keeping stirring at 25-30 DEG C until the reaction is complete, adding water to precipitate white solid, and recrystallizing to obtain a compound D; and the specific reactive mode is as follows:. The preparation method provided by the invention is low in cost, high in efficiency, environment-friendly, moderate in reaction, high in safety and the like.
Owner:SHANGHAI JINHE BIO TECH
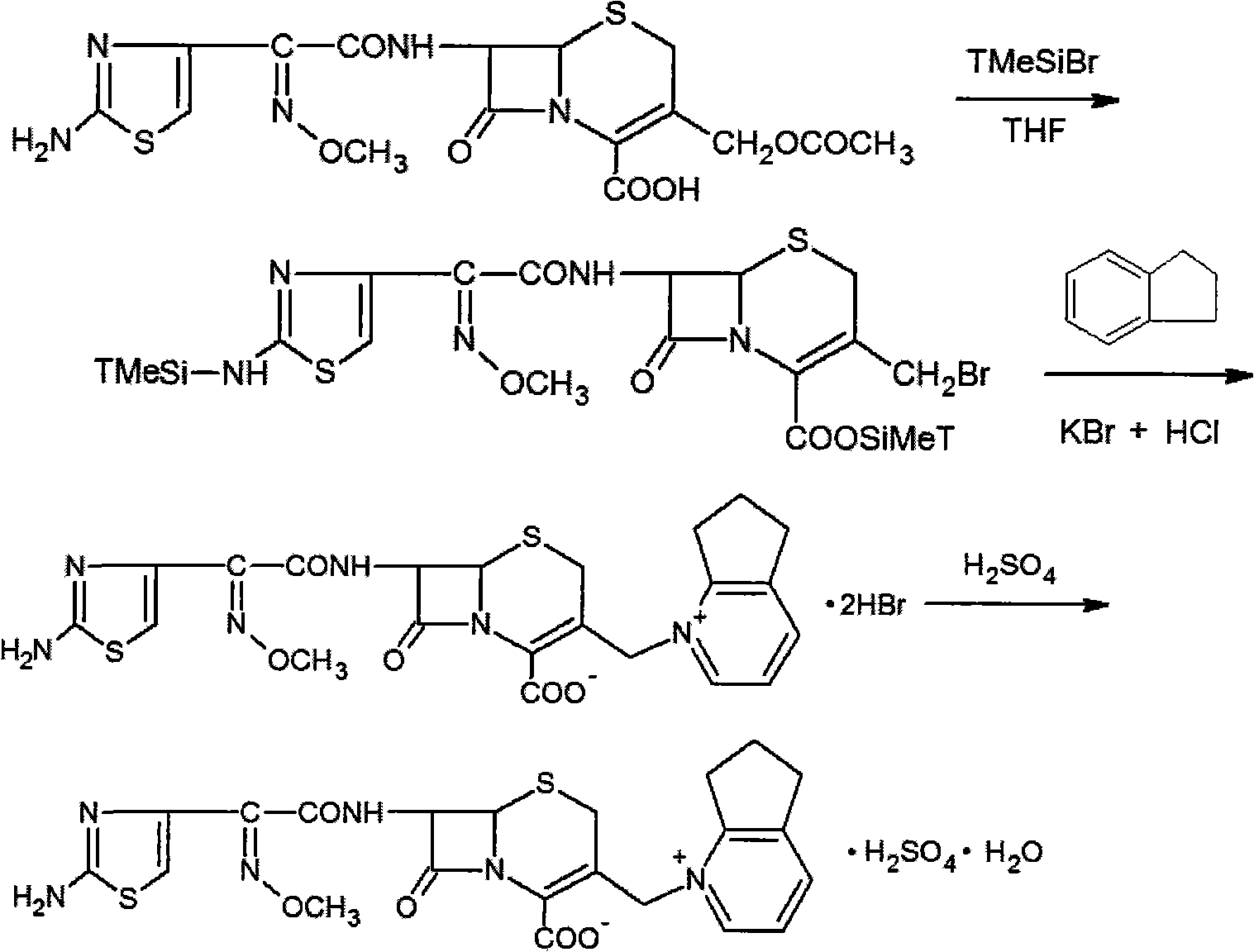
Process for preparing pure cephalosporine intermediates
The present invention relates to a process for preparing key intermediates for cephalosporin antibiotics substantially free of undesired Δ2 isomer. Thus, 7-aminocephalosporanic acid (7-ACA) is silylated with hexamethyldisilazane in cyclohexane at reflux temperature. (6R,7R)-3-[(Acetyloxy)methyl]-7-(trimethylsilyl)aminoceph-3-em-4-oic acid obtained is reacted with the mixture of N-methylpyrrolidine and trimethylsilyl iodide in cyclohexane, desilylated with isopropyl alcohol and treated with hydrochloric acid to obtain [6R-(6α,7β)]-1-[[7-Amino-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-1-methylpyrrolidinium inner salt hydrochloride. [6R-(6α,7β)]-1-[[7-Amino-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-1-methylpyrrolidinium inner salt hydrochloride is N-acylated with syn-2-(2-aminothiazol-4-yl)-2-methoxyimino acetic acid 2-benzothiazolyl thioester (MAEM) followed by treatment with hydrochloric acid to give cefepime dihydrochloride monohydrate.
Owner:HETERO DRUGS LTD
Preparation method of cefalonium
ActiveCN104725403ARaw materials are easy to getEasy to operateOrganic chemistryIodination reactionSolvent
The invention relates to a preparation method of cefalonium. According to the preparation method, raw materials (cefalotin and pyrazinamide) react at a low temperature to obtain the product cefalonium. The preparation method particularly comprises the following steps: dissolving cefalotin acid into an organic acid, carrying out carboxyl protection by using a silanization protection reagent and then carrying out iodination reaction on reaction products and iodotrimethylsilane; then carrying out amination reaction on the reaction product from the former step and pyrazinamide; and finally carrying out deprotection by alcoholysis, regulating the pH value at a low temperature and crystalizing to obtain cefalonium. According to the preparation method of cefalonium, cefalotin acid is protected by the silanization protection reagent in an organic solvent and then reacts with iodotrimethylsilane; the reaction time is short, the reaction conditions are mild, the reaction is complete and no side reaction is almost generated; due to adoption of a mixed solvent crystallization method, the characteristics of high drying speed, light color and high yield can be achieved; in addition, the used solvent can be recycled and the amount of generated sewage can be reduced; therefore, the preparation method of cefalonium has remarkable economic and environmental benefits and facilitates industrial production.
Owner:QILU SYNVA PHARMA
Iodotrimethylsilane preparing by dissolving and adding iodine
ActiveCN104926851ANo pollutionQuick responseGroup 4/14 element organic compoundsHexamethyldisilaneDistillation
The invention provides an iodotrimethylsilane preparation method. The preparation method comprises the following steps that half hexamethyldisilane is put in a reaction flask, a layer of rectifying column packing glass springs is added in a constant pressure dropping funnel, then elementary substance iodine and half hexamethyldisilane are added in the constant pressure dropping funnel, vacuumizing and nitrogen replacing are performed on a reaction system, and all the steps are repeated for three times; stirring is started, the temperature rises to 65 DEG C, and hexamethyldisilane solutions of the iodine are dropwise added; after dropwise adding is finished, the outer temperature rises to 130 DEG C for backflow, and therefore after being cooled, steam enters the constant pressure dropping funnel to dissolve the undissolved iodine in the constant pressure dropping funnel; after the iodine is dissolved completely and dropwise added in the reaction system, reaction is performed for 2 hours until the reaction is complete; heating is stopped, the temperature is cooled below 30 DEG C, a reaction device is changed into a distillation device to collect main distillate fractions, and then colorless liquid iodotrimethylsilane competitive products are obtained.
Owner:山东博苑医药化学股份有限公司
Preparation method of cefquinome sulfate
The invention relates to a method for synthesizing cefquinome sulfate and relates to a preparation method of a medicament for animals. The preparation method comprises the following steps: taking cefotaxime, 2,3-cyclohexyl pyridine and trimethyliodosilane as initial raw materials; preparing cefotaxime hydriodate from the cefotaxime; carrying out decoloring and silica column chromatography on the cefotaxime; and reacting the treated cefotaxime with sulphuric acid to prepare a cefquinome sulfate product. Compared with the prior art, the invention has the advantages of high yield of products, stable production quality and suitability for industrial production.
Owner:PU LIKE BIO ENG
Method for synthesizing cefpirome sulfate
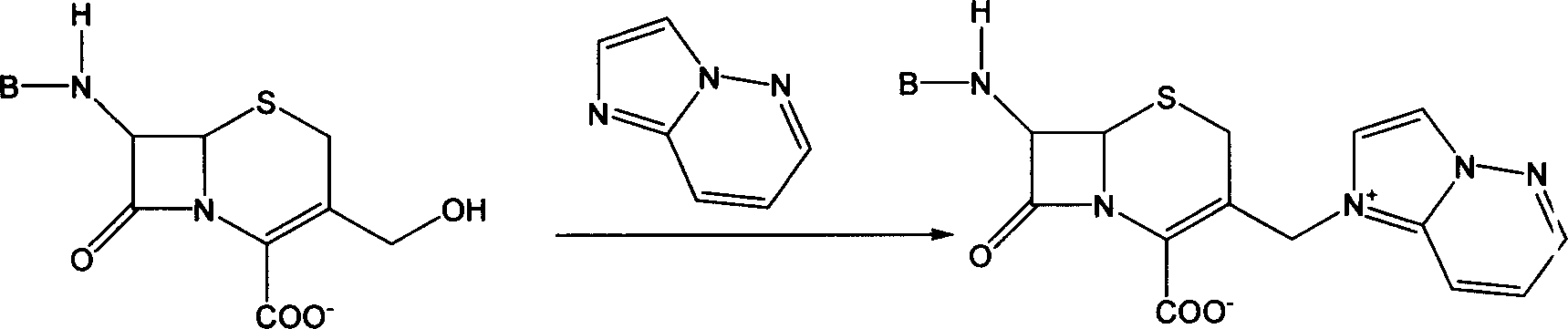
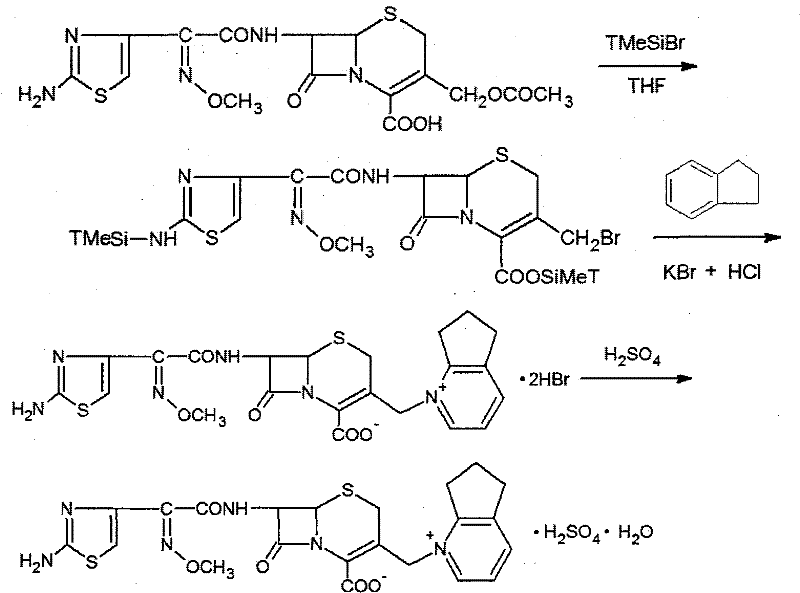
InactiveCN101747349AOvercome the disadvantage of azeotropic separationOvercome the disadvantages of strong alkalinity and more side reactionsOrganic chemistryChemical recyclingAlkalinityQuaternary ammonium cation
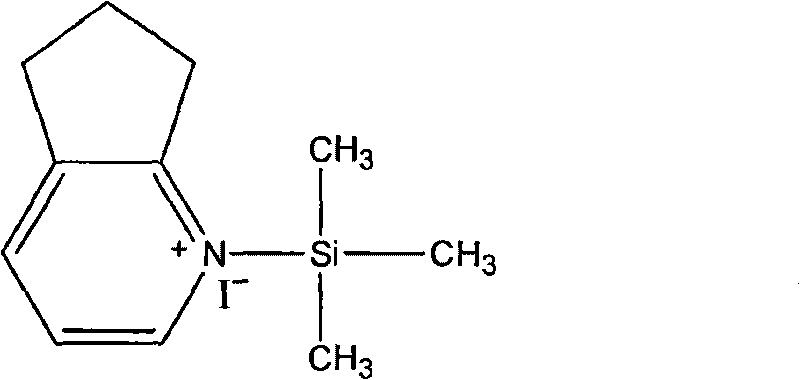
The invention relates to a method for synthesizing cefpirome sulfate. The method comprises the following steps: firstly, preparing 2,3-cyclopentenopyridine and trimethylsilyl iodide into quaternary ammonium salt intermediate compound; Secondly, performing carboxyl and amido protection for 7-ACA through hexamethyl disilylamine, and finally adding quaternary ammonium salt intermediate compound into the well protected 7-ACA solution for rapid reaction to obtain cefpirome mother nuclide 7-ACP; Thirdly, performing N- acidylation reaction to 7-ACP and AE active ester in the mixed phase of DMF and water, and obtaining cefpirome sulfate after salt forming reaction. By using step one to obtain quaternary ammonium salt intermediate compound, not only N,N- diethylaniline is saved, but also the side chain 2,3-cyclopentenopyridine is easy to rectify and recycle, thereby greatly reducing the production cost; simultaneously, the disadvantages of strong alkalinity and a large amount of side reaction of 2,3-cyclopentenopyridine are overcome. By using the method, the yield rate and the purity of the product can be improved.
Owner:YIYUAN XINQUAN CHEM
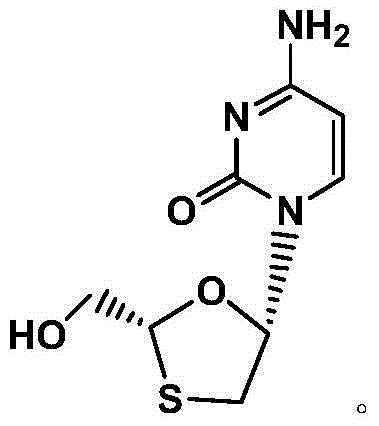
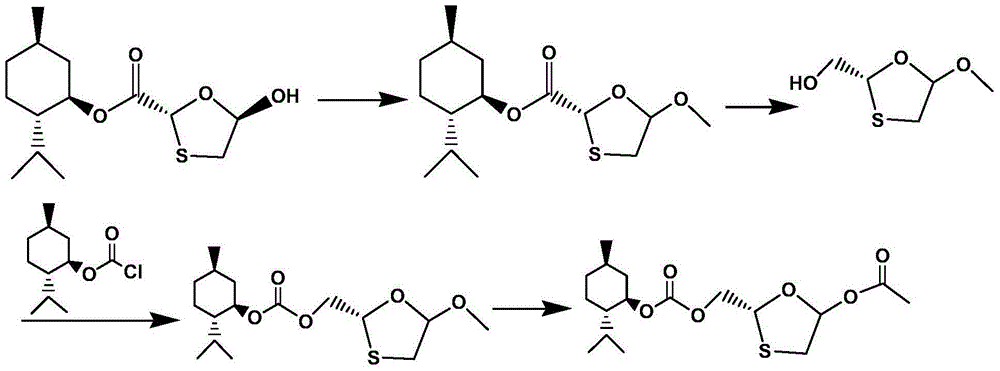
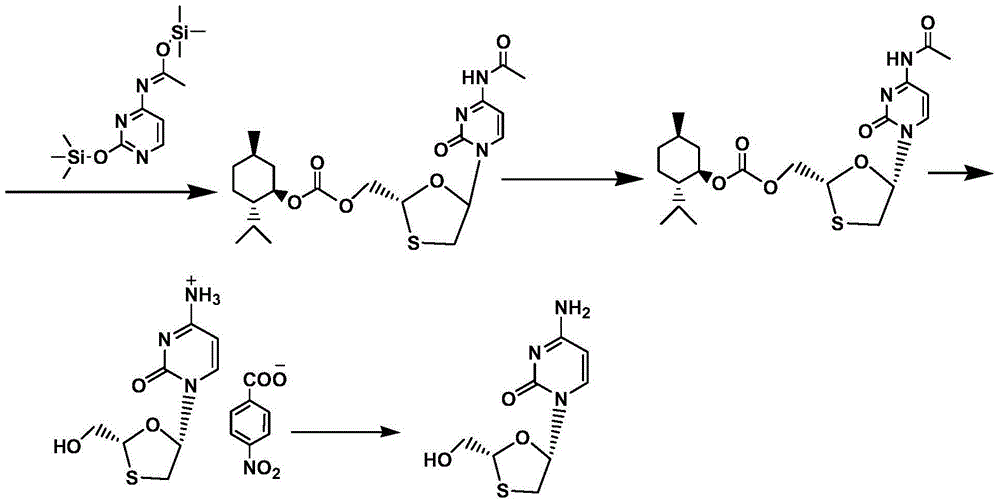
Synthetic method of lamivudine intermediate
The present invention discloses a synthetic method of lamivudine intermediate, the method comprises the following steps: a compound of formula V is obtained by methoxylation reaction of a compound of formula VI and a hydrochloric acid methanol solution; a compound of formula IV is obtained by reduction reaction of the compound of formula V and sodium borohydride in ethanol; a compound of formula III is obtained by esterification reaction of the compound of formula IV and chlorinated carbonate under the effect of an organic base for formation of bicyclic carboxylic ester; a compound of formula II is obtained by acylation reaction of the compound of formula III and acetic anhydride under the acid catalysis; a meso compound of the formula I' is obtained by glycosylation reaction of the compound of formula II and silane protected N4-acetamido cytosine under the Iodotrimethylsilane catalysis; and the lamivudine intermediate as shown in the formula I is obtained by recrystallization resolution of the meso compound of the formula I'. The method has the advantages of being simple in operation, low in cost, high in product purity, and the like.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
Preparation method of cefpirome hydriodate
The invention discloses a preparation method of cefpirome hydriodate. According to the method, under a condition of the presence of excess trimethyliodosilane in a reaction system, dilute hydrochloric acid is used for replacing a mixed liquid of potassium iodide and hydrochloric acid in the prior art for crystallization, thus cefpirome hydriodate residues are reduced to zero, and at the same time, the product yield is ensured.
Owner:HARBIN PHARMA GRP CO LTD GENERAL PHARMA FACTORY
2',3'- di-O-acetyl-5'-deoxy-5-fulurocytidineonium compound and preparation method thereof
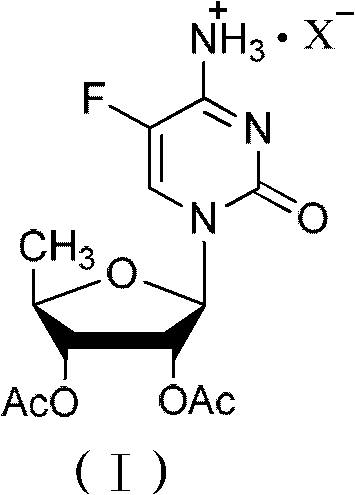
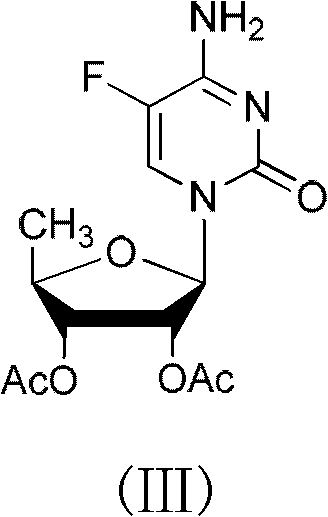
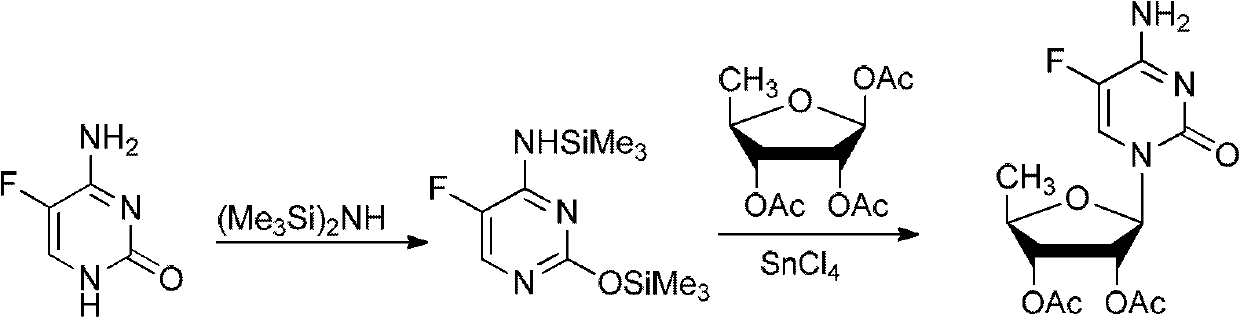
ActiveCN102424697AReduce usageClear structureSugar derivativesSugar derivatives preparationCombinatorial chemistryBiological activation
The invention relates to a 2',3'- di-O-acetyl-5'-deoxy-5-fulurocytidineonium compound of formula (I) or a solvate thereof. The compound is prepared by using 5-deoxy-1,2,3-tri-O-acetyl-beta-D-ribofuranose as a raw material, carrying out condensation reaction on 5-deoxy-1,2,3-tri-O-acetyl-beta-D-ribofuranose and protected 5-flucytosine under the activation of iodotrimethylsilane to obtain a reaction product, and carrying out deprotection, acidification and crystallization. According to the invention, a novel intermediate for synthesizing Capecitabine is developed, one pot process is adopted for reacting to obtain the 2',3'- di-O-acetyl-5'-deoxy-5-fulurocytidineonium compound, and high-purity Capecitabine can be further synthesized by using the 2',3'- di-O-acetyl-5'-deoxy-5-fulurocytidineonium compound as a key intermediate. The compound has the characteristics of clear structure, high purity and stable quality. The use of the compound to synthesize Capecitabine can reduce reaction steps, control process cost, and reduce environmental pollution.
Owner:山东安信制药有限公司
Synthetic method of cefpirome sulfate
ActiveCN101284840BLow costHigh yieldAntibacterial agentsOrganic chemistryTrimethylsilyl chlorideCefotaxime
The invention relates to a synthesis method for making cefpirome sulfate. The method comprises the following steps that: cefotaxime and 2, 3-cyclopenopyridine, with the molar ratio of between 1:7 and 1:12, are mixed with trimethyl bromosilicane used as protecting agent inside organic solvent to carry out substitution reaction, thereby generating cefpirome dihydrobromide; and the salt-forming reaction of the cefpirome dihydrobromide is carried out to make cefpirome sulfate. According to the synthesis method of cefpirome sulfate provided by the technical proposal of the invention, cefotaxime and trimethyl bromosilicane are respectively used as raw material and protecting agent to make cefpirome sulfate. Because enough supply and technical maturity of cefotaxime are realized in China and trimethyl bromosilicane is more active than trimethylchlorosilane and is cheaper than trimethyliodosiliane, the synthesis method has the advantages of low cost of raw material, high yield, simple synthetic route and convenient operation.
Owner:GUANGDONG JIUMING PHARMA
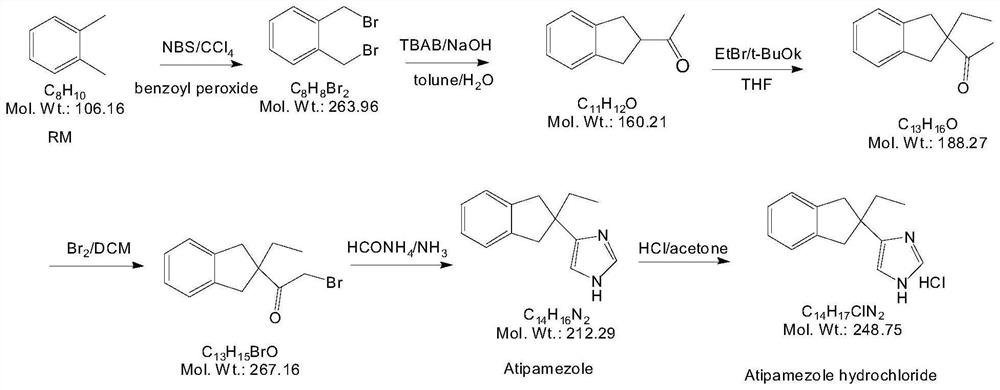
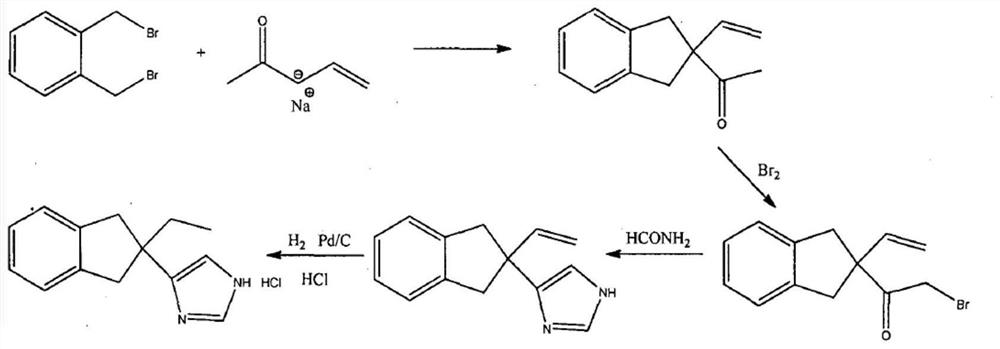
Preparation method of atipamezole
PendingCN112225700AMild reaction conditionsMeet the requirementsOrganic chemistryBulk chemical productionOrganosolvVeterinary Drugs
The invention belongs to the technical field of veterinary drug preparation methods, and particularly relates to a preparation method of atipamezole. The method comprises the following steps: carryingout two-step reduction on a compound 1 to prepare atipamezole, wherein the reaction route is shown in Figure 6, in the second step, a compound 2 is dispersed in an organic solvent, a reducing agent trimethyliodosilane is added, the reaction is performed to obtain the atipamezole, the reaction conditions of the preparation method are mild, the weight yield can reach 74% or above, and the purity is99% or above.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
Method for synthesizing cefpirome sulfate
InactiveCN101161655AReduce degradation damageReduce dosageOrganic chemistryBis(trimethylsilyl)amineIon exchange
The present invention relates to a preparation method of cefpirome sulfate, and is characterized in that hexamethyldisilazane (HMDS) with low price combined with a water-ethanol mixture solvent is added as the ion exchange medium, so that the needed dosage of 2,3-cyclopentenopyridine and odotrimethylsilane is reduced, as well as the fabrication cost. The synthetic technics provided by the present invention has the advantages of feasibility, low cost, and stable quality of the product.
Owner:上海慈瑞医药科技股份有限公司
A kind of preparation method of cefuroxime
ActiveCN104725403BRaw materials are easy to getEasy to operateOrganic chemistrySolventIodination reaction
The invention relates to a preparation method of cefalonium. According to the preparation method, raw materials (cefalotin and pyrazinamide) react at a low temperature to obtain the product cefalonium. The preparation method particularly comprises the following steps: dissolving cefalotin acid into an organic acid, carrying out carboxyl protection by using a silanization protection reagent and then carrying out iodination reaction on reaction products and iodotrimethylsilane; then carrying out amination reaction on the reaction product from the former step and pyrazinamide; and finally carrying out deprotection by alcoholysis, regulating the pH value at a low temperature and crystalizing to obtain cefalonium. According to the preparation method of cefalonium, cefalotin acid is protected by the silanization protection reagent in an organic solvent and then reacts with iodotrimethylsilane; the reaction time is short, the reaction conditions are mild, the reaction is complete and no side reaction is almost generated; due to adoption of a mixed solvent crystallization method, the characteristics of high drying speed, light color and high yield can be achieved; in addition, the used solvent can be recycled and the amount of generated sewage can be reduced; therefore, the preparation method of cefalonium has remarkable economic and environmental benefits and facilitates industrial production.
Owner:QILU SYNVA PHARMA
A kind of preparation method of iodotrimethylsilane
The invention discloses a preparation method of iodotrimethylsilane, which comprises: adding anhydrous aluminum chloride and hexamethyldisiloxane into a reaction vessel in the presence of a protective gas, starting to stir, and raising the temperature to 40‑50°C, then add iodine elemental substance, after the addition of iodine elemental substance, the bath temperature rises to 125‑140°C, and the reaction is refluxed for 1‑1.5 hours. Methyl iodosilane. The method can improve the yield of iodotrimethylsilane, has simple purification, mild conditions, and is suitable for large-scale production and popularization.
Owner:佛山市南海区波尔有机硅有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com