Synthetic method of cefpirome sulfate
A cefpirome sulfate and synthetic method technology, applied in organic chemistry, antibacterial drugs, etc., can solve the problems of low yield and high synthesis cost, and achieve the effects of convenient operation, low raw material cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
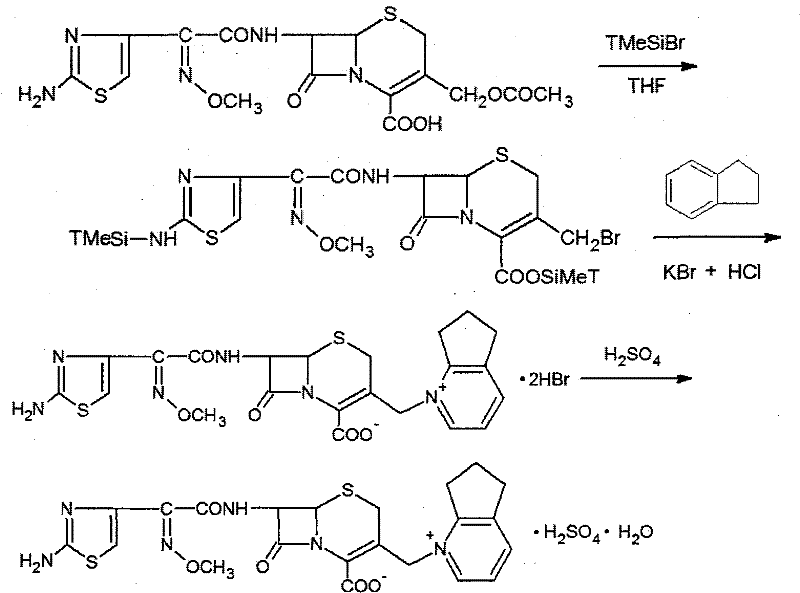
[0033] The synthetic method of cefpirome sulfate that this specific embodiment provides, at first needs to prepare the dihydrobromide of cefpirome, then prepare target product cefpirome sulfate with the dihydrobromide of cefpirome produced, Its synthetic route is as follows:
[0034]
[0035] The specific steps of its synthesis are described as follows:
[0036] Preparation of cefpirome dihydrobromide comprises the following three steps:
[0037] Prepare the solution: After fixing the reaction flask in an ice-water bath, add 200 ml of dry tetrahydrofuran (THF) as an organic solvent to the reaction flask; then add the protective agent bromotrimethylsilane (21.42 g, 18.5mL, 140mmol), the temperature was controlled below 5°C during the addition process, and nitrogen was passed through to remove oxygen for 20 minutes.
[0038] Carry out substitution reaction: add 2,3-cyclopentenopyridine (20.23 g, 20 mL, 170 mmol) to the prepared solution, and keep the system temperature near...
specific Embodiment approach 2
[0047] Using dimethylformamide (DMF) instead of tetrahydrofuran in Embodiment 1, and then using benzene instead of carbon tetrachloride, the same target product can be obtained, but the yield is lower.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com