Improved L-thyroxine sodium synthesis method
A technology for sodium thyroxine and thyroxine, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of low yield of oxidative coupling reaction, affecting product cost, etc., so as to reduce unit production cost, The effect of improving production efficiency and improving catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 synthetic L-thyroxine sodium
[0029] Using L-tyrosine as raw material, L-thyroxine is obtained through iodation reaction, N-acylation reaction, esterification reaction, oxidation coupling reaction and hydrolysis reaction in sequence, and the specific process of preparing L-thyroxine sodium after salt formation The operation is as follows:
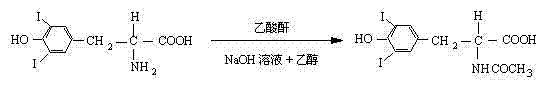
[0030] ①Iodine reaction
[0031] The main chemical reaction formula involved in the iodination reaction:
[0032]
[0033] 3,5-Diiodo-L-tyrosine
[0034] In the reaction vessel, sequentially add 700g of L-tyrosine, 750ml of concentrated hydrochloric acid and 3000ml of water, heat and dissolve under stirring, and after the temperature of the reaction system reaches 63°C, add dropwise the solution prepared by dissolving 2kg of iodine chloride in 2000ml of acetic acid The acetic acid solution of iodine chloride is subjected to the iodination reaction, after the dropwise addition is completed, after the temperature is...
Embodiment 2
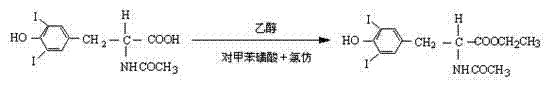
[0065] Example 2 Preparation of Oxidative Coupling Reaction Product N-acetyl-L-thyroxine ethyl ester
[0066] The esterification reaction product N-acetyl-3,5-diiodo-L-tyrosine ethyl ester obtained by the method of step 3 of Example 1 was used as an oxidative coupling reaction starter, and manganese chloride was used as a catalyst, Catalyst once adds the concrete operation of preparing oxidative coupling reaction product N-acetyl-L-thyroxine ethyl ester as follows:
[0067] In the reaction vessel, sequentially add 20 g of the esterification reaction product N-acetyl-3,5-diiodo-L-tyrosine ethyl ester prepared in step ③, and 100 ml of boric acid aqueous solution with a cocatalyst concentration of 0.05 mol / L (equivalent to 0.31g) and 200ml of solvent ethanol (equivalent to 157.9g), adjust the pH value of the reaction solution to 8-9 with a NaOH aqueous solution with a concentration of 2mol / L under stirring, add all the catalyst manganese chloride 0.16g, and heat the reaction in a...
Embodiment 3
[0071] Example 3 Preparation of Oxidative Coupling Reaction Product N-acetyl-L-thyroxine ethyl ester
[0072] The esterification reaction product N-acetyl-3,5-diiodo-L-tyrosine ethyl ester obtained by the method of step 3 of Example 1 was used as an oxidative coupling reaction starter, and manganese chloride was used as a catalyst, Catalyst is added in twice and the concrete operation of preparing coupling reaction product N-acetyl-L-thyroxine ethyl ester is as follows:
[0073] In the reaction vessel, sequentially add 20 g of the esterification reaction product N-acetyl-3,5-diiodo-L-tyrosine ethyl ester prepared in step ③, and 100 ml of boric acid aqueous solution with a cocatalyst concentration of 0.05 mol / L (equivalent to 0.31g) and 200ml of solvent ethanol (equivalent to 157.9g), under stirring, adjust the pH value of the reaction solution to 8-9 with a NaOH aqueous solution with a concentration of 2mol / L, and add 0.10g of catalyst manganese chloride, the amount of which i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com