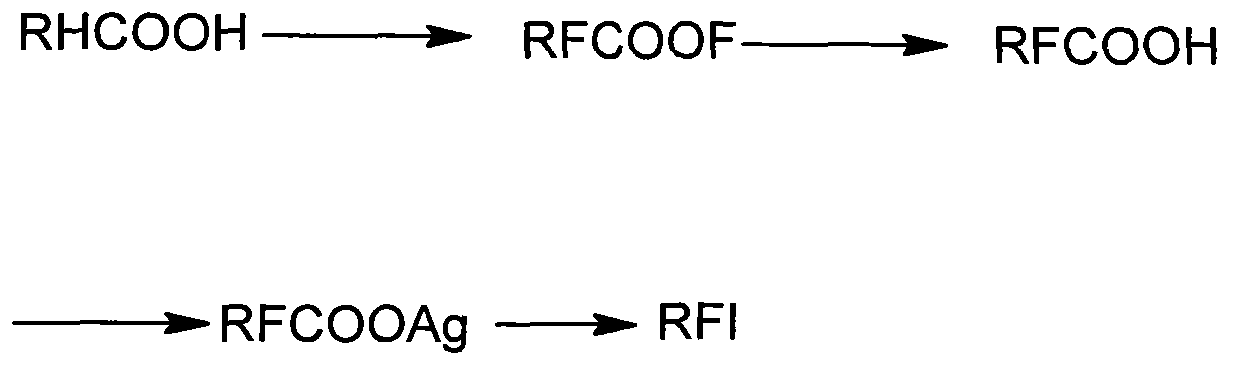
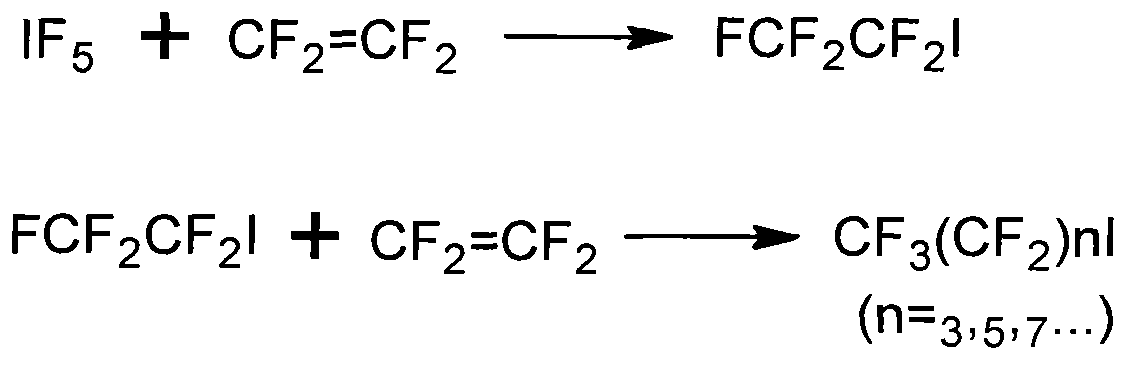
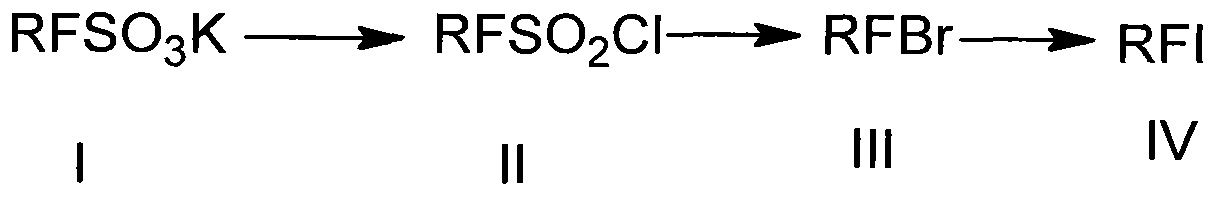Preparation method of heptadecafluorooctyl iodoalkane
A technology of fluorooctyl iodide and fluorooctyl bromide, which is applied in the field of chemical synthesis, can solve the problems restricting the industrial application of electrolysis and telomerization, and achieve the effects of convenient industrial production, simple synthesis route and mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037] The present invention will be further described below in conjunction with embodiment.
[0038] According to this embodiment image 3 The synthesis route shown is to prepare heptadecafluorooctyl iodane, using commercially available potassium perfluorooctane sulfonate (potassium heptadecafluorooctane sulfonate) as a raw material, and the other reagents described below are all analytically pure.
[0039] Step 1: Preparation of heptadecafluorooctanesulfonyl chloride (II) from potassium perfluorooctanesulfonate (I)
[0040] Add (I) and the sulfonylating reagent into the reaction flask, stir and heat up to the reaction temperature, and maintain the temperature until the reaction is completed. Distill under reduced pressure, intercept 108°C-114°C / 100mmHg fraction to obtain the crude product, the crude product is washed with sodium bicarbonate aqueous solution to neutral or near neutral, stand to separate layers, take the lower liquid, dry, and crystallize to obtain Heptadeca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com