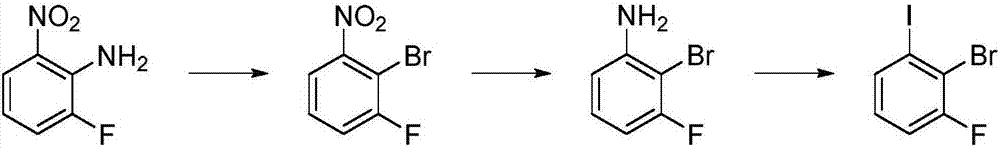
Preparation method of 1-fluoro-2-bromo-iodobenzene
A technology of iodobenzene and iodized salt, applied in the chemical field, can solve the problems of high risk, harsh conditions, and low utilization rate, and achieve the effects of low equipment requirements, mild conditions, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Preparation of 1-fluoro-2-bromo-3-iodobenzene
[0046] The synthetic route is as follows:
[0047]
[0048] 1. Preparation of 1-fluoro-2-bromo-3-nitrobenzene
[0049] Under mechanical stirring, 94.0 g of water, 102.2 g of concentrated sulfuric acid, 19.5 g of acetic acid, 19.5 g of 1-fluoro-2-amino-3-nitrobenzene, 63.2 g of concentrated hydrobromic acid (bromo 48% aqueous solution of hydrogen hydride) and cuprous bromide 9.0g. A solution made of 11.2 g of sodium nitrite and 22.4 g of water was added dropwise at room temperature, and the dripping was completed in about 5 hours, and stirring was continued for 2 hours at room temperature. It was diluted with 130.8 g of water and extracted with 39.0 g of dichloromethane. The oily layer was washed with 39.0 g of water and the solvent was concentrated to obtain 23.4 g of 1-fluoro-2-bromo-3-nitrobenzene with a yield of 85%.
[0050] 2. Preparation of 1-fluoro-2-bromo-3-aminobenzene
[0051] Into a 500 mL stain...
Embodiment 2
[0054] Example 2 Preparation of 1-fluoro-2-bromo-3-iodobenzene
[0055] The synthetic route is as follows:
[0056]
[0057] 1. Preparation of 1-fluoro-2-bromo-3-nitrobenzene
[0058] Under mechanical stirring, 76.6 g of water, 127.7 g of concentrated sulfuric acid, 19.5 g of 1-fluoro-2-amino-3-nitrobenzene, 42.1 g of concentrated hydrobromic acid (48 % aqueous solution) and cuprous bromide 9.0g. A solution made of 8.6 g of sodium nitrite and 17.2 g of water was added dropwise at room temperature, and the drop was completed in about 5 hours, and stirring was continued for 2 hours at room temperature. 130.8 g of water was added for dilution, and extracted with 39.0 g of dichloromethane. The oil layer was washed with 39.0 g of water and the solvent was concentrated to obtain 22.5 g of 1-fluoro-2-bromo-3-nitrobenzene with a yield of 82%.
[0059] 2. Preparation of 1-fluoro-2-bromo-3-aminobenzene
[0060] Into a 500 mL stainless steel autoclave, 22.0 g of 1-fluoro-2-bromo-3...
Embodiment 3
[0063] Example 3 Preparation of 1-fluoro-2-bromo-3-iodobenzene
[0064] The synthetic route is as follows:
[0065]
[0066] 1. Preparation of 1-fluoro-2-bromo-3-nitrobenzene
[0067] Under mechanical stirring, slowly add 19.5 g of acetic acid, 19.5 g of 1-fluoro-2-amino-3-nitrobenzene, 210.7 g of concentrated hydrobromic acid (48% aqueous solution of hydrogen bromide) and bromine Cuprous chloride 18.0g. A solution made of 12.9 g of sodium nitrite and 25.8 g of water was added dropwise at room temperature, and the drop was completed in about 5 hours, and stirring was continued for 2 hours at room temperature. It was diluted with 130.8 g of water and extracted with 39.0 g of dichloromethane. The oil layer was washed with 39.0 g of water and the solvent was concentrated to obtain 23.1 g of 1-fluoro-2-bromo-3-nitrobenzene, yield 84%.
[0068] 2. Preparation of 1-fluoro-2-bromo-3-aminobenzene
[0069] 22.0 g of 1-fluoro-2-bromo-3-nitrobenzene, 220.0 g of isopropanol and 3.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com