Preparation method of lorlatinib intermediate compound
A technology of lorlatinib and intermediates, applied in the field of organic synthesis of intermediates, can solve the problems of poor atom economy, unpredictable yield and the like, and achieve the effects of reducing production cost and unit price, simple purification method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
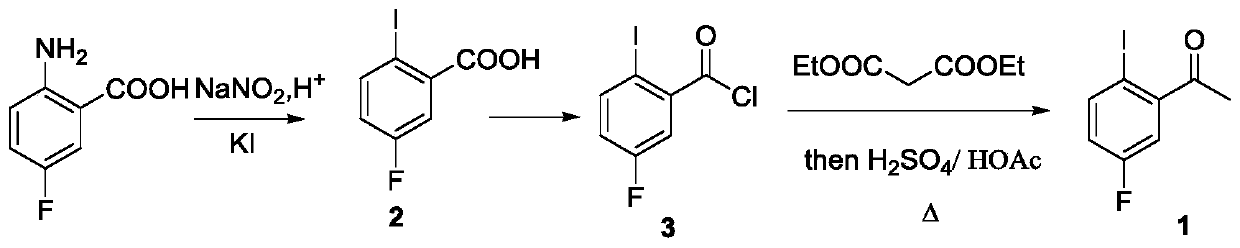
[0034] A preparation method of lorlatinib intermediate compound, comprising the steps of:
[0035] (1) Preparation of 2-amino-5-fluoroacetophenone:
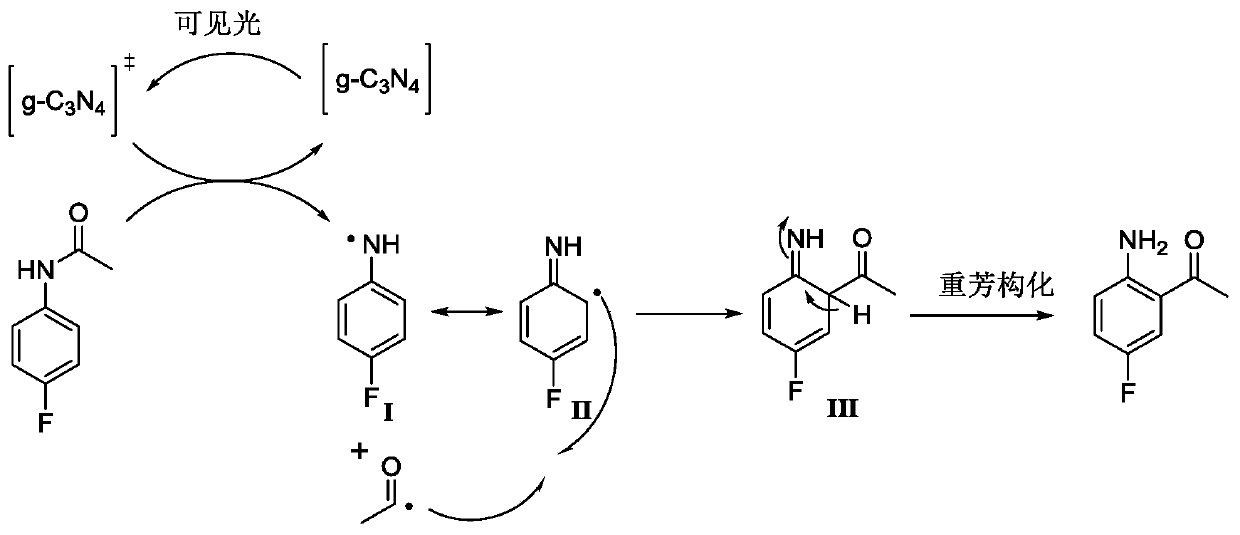
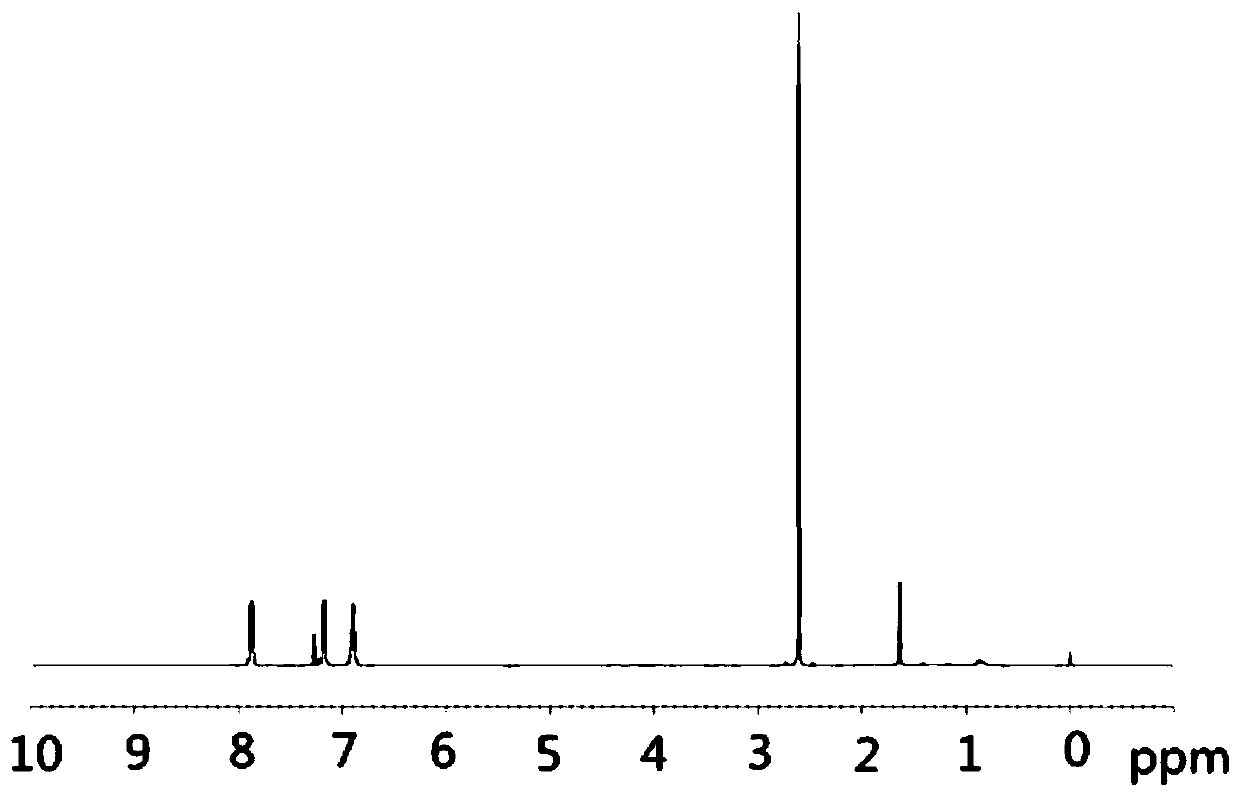
[0036]Under the protection of argon atmosphere, add 15.3g (100mmol) 4-fluoroacetanilide (compound 4) and 200mL dioxane solvent in a 500mL single-necked bottle with a magnetic stirrer, and then add 306mg visible light catalyst to nitride Carbon (g-C 3 N 4 ) (w / w=2.35%), the temperature was raised to 40°C under stirring, and at the same time, a LED lamp with a power of 36 watts was used to illuminate the reaction system for photocatalytic Fries rearrangement reaction, and the reaction system was kept under light for 44 hours. After tracking the complete reaction of the raw materials, cool down to room temperature, filter the reaction solution with diatomaceous earth to remove the visible light catalyst and filter it with suction, wash the filter residue with 50mL of dioxane, collect the filtrate, and concentrate the filtrate unde...
Embodiment 2
[0044] (1) Preparation of 2-amino-5-fluoroacetophenone:
[0045] Under the protection of argon atmosphere, 23.0g (150mmol) of 4-fluoroacetanilide (compound 4) and 150mL of methyl tert-butyl ether solvent were added to a 500mL single-necked bottle with a magnetic stirrer, and then 338mg of visible light catalyst nitrogen carbon dioxide (g-C 3 N 4 ) (w / w=1.47%), heated up to 35°C under stirring, and at the same time, a LED lamp with a power of 72 watts was used to illuminate the reaction system for photocatalytic Fries rearrangement reaction, and the reaction system was kept under light for 48 hours. After tracking the reaction of the raw materials, it was lowered to room temperature, and the reaction solution was filtered with diatomaceous earth to remove the visible light catalyst and suction filtered, and the filter residue was washed with 50 mL of dioxane, and the filtrate was collected, and the filtrate was concentrated under reduced pressure to remove the solvent dioxane ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com