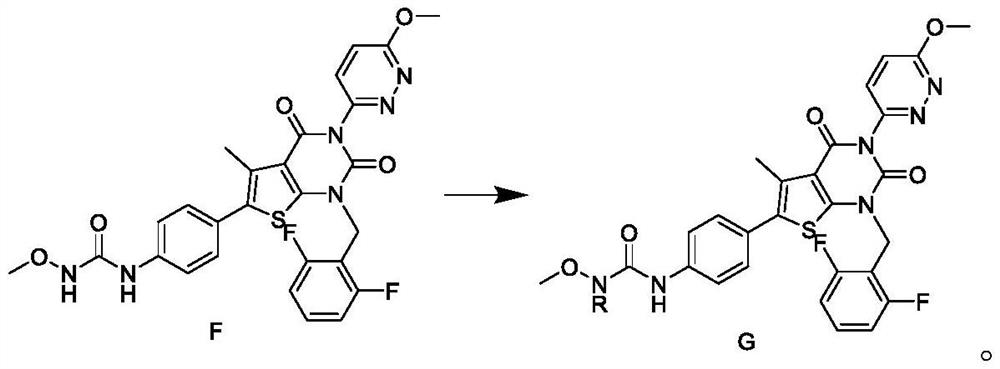
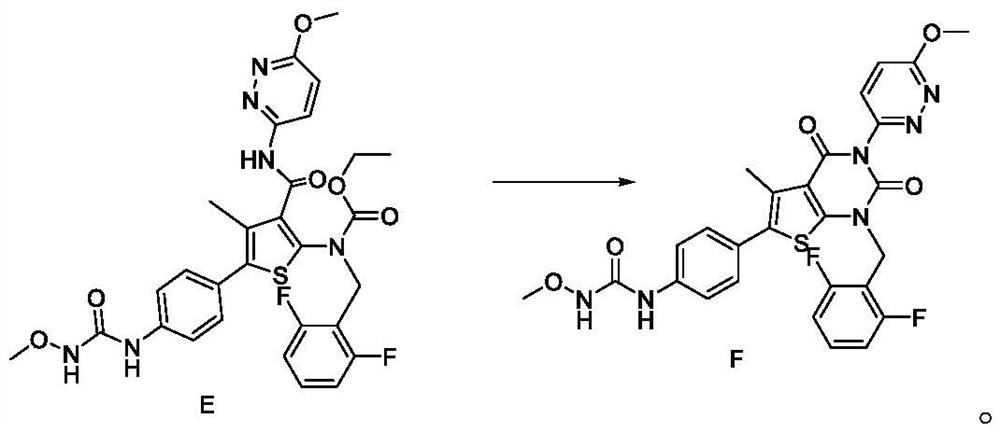
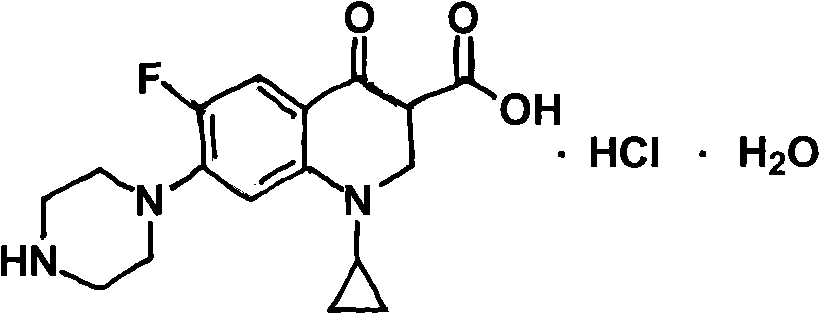
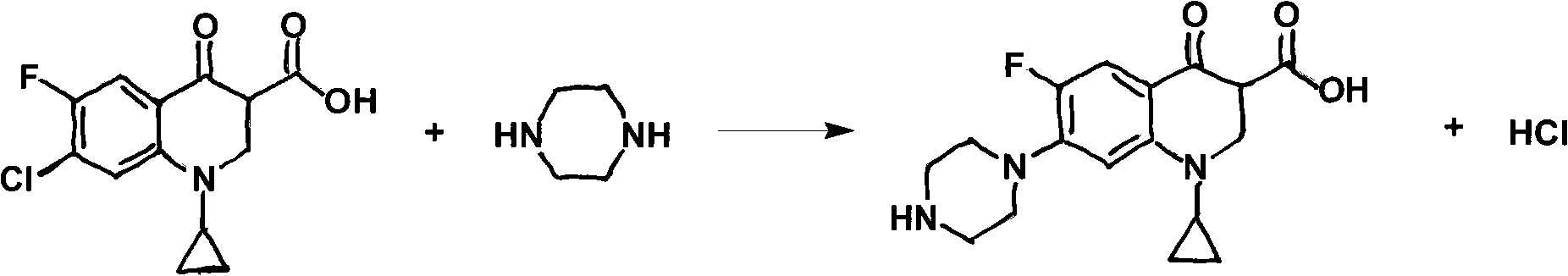
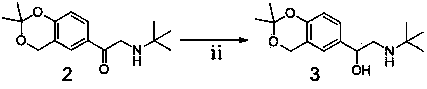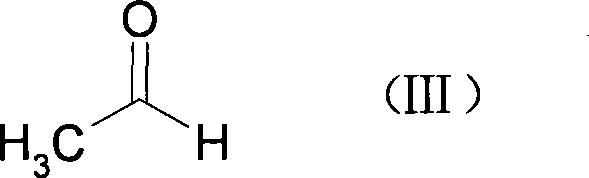Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
189results about How to "Simplified post-processing steps" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method for particle diameter controllable millimeter-scale polyurea monodisperse microsphere
InactiveCN104151516AMeet particle size requirementsNo pollutionMicroballoon preparationMicrocapsule preparationMicrospherePollution
The invention relates to a preparation method for a particle diameter controllable millimeter-scale polyurea monodisperse microsphere. The preparation method comprises the following steps: isocyanate compound is adopted as a monomer and is squeezed through syringe needles or capillary tubes to form liquid drops and the liquid drops are added into water or a polyamine aqueous solution for sedimentation and polymerization; during the settlement process of the liquid drops, the monomers on the surfaces of the liquid drops react with the water or the polyamine aqueous solution rapidly to form a gelatin layer or callus and the stability of particles is maintained. According to the method, as water is adopted as the dispersion medium, no pollution is generated; the polymerization reaction can be conducted under lower temperature, the equipment is simple and the manufacturing cost is low; the obtained microsphere is free from residual monomer and the post-processing is simple; no surface modified agent or pore-foaming agent is needed, porous polyurea microsphere with rich amino on the surface is prepared in one step; the method can be used in the fields of enzyme immobilization, chemocatalysis, dye, adsorption and separation of heavy metal ions and the like.
Owner:UNIV OF JINAN
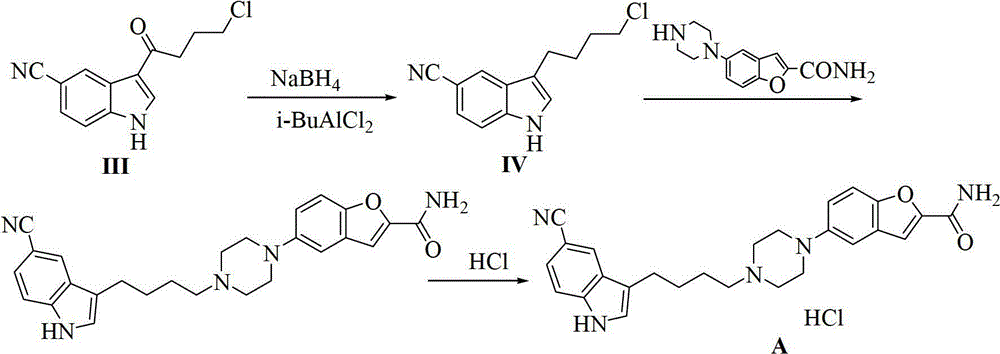
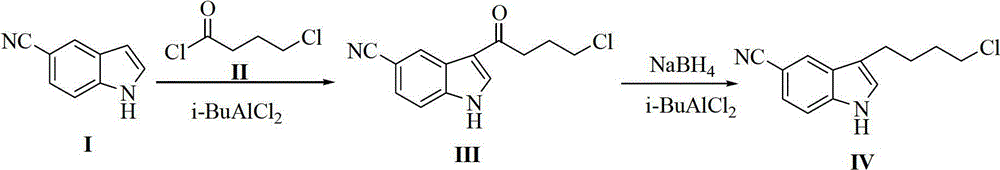
Preparation method of 3-(4-chlorobutyl)-5-cyanoindole
ActiveCN102875440AReduce usageOperational securityOrganic chemistryPotassium borohydrideLewis acid catalysis
The invention discloses a preparation method of 3-(4-chlorobutyl)-5-cyanoindole which is shown as the formula IV. The preparation method comprises the steps as follows: carrying out following carbonyl reduction reaction on a compound III and a hydroboron reducing agent in the solvent under the catalyzing of Lewis acid to obtain the product, wherein Lewis acid is one or more of aluminium trichloride, magnesium chloride, zinc chloride and ferric chloride; and the hydroboron reducing agent is one or more of sodium borohydride, potassium borohydride, lithium borohydride and borane. The preparation method disclosed by the invention is safe in operation, low in requirement on equipment, low in cost, simple in post-processing steps, high in yield of product, high in purity, and is suitable for industrialization.
Owner:CHIRAL QUEST (SUZHOU) CO LTD +1
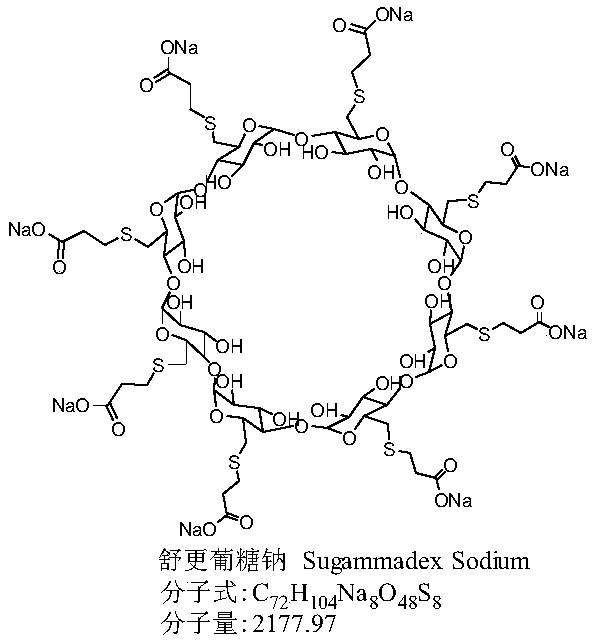
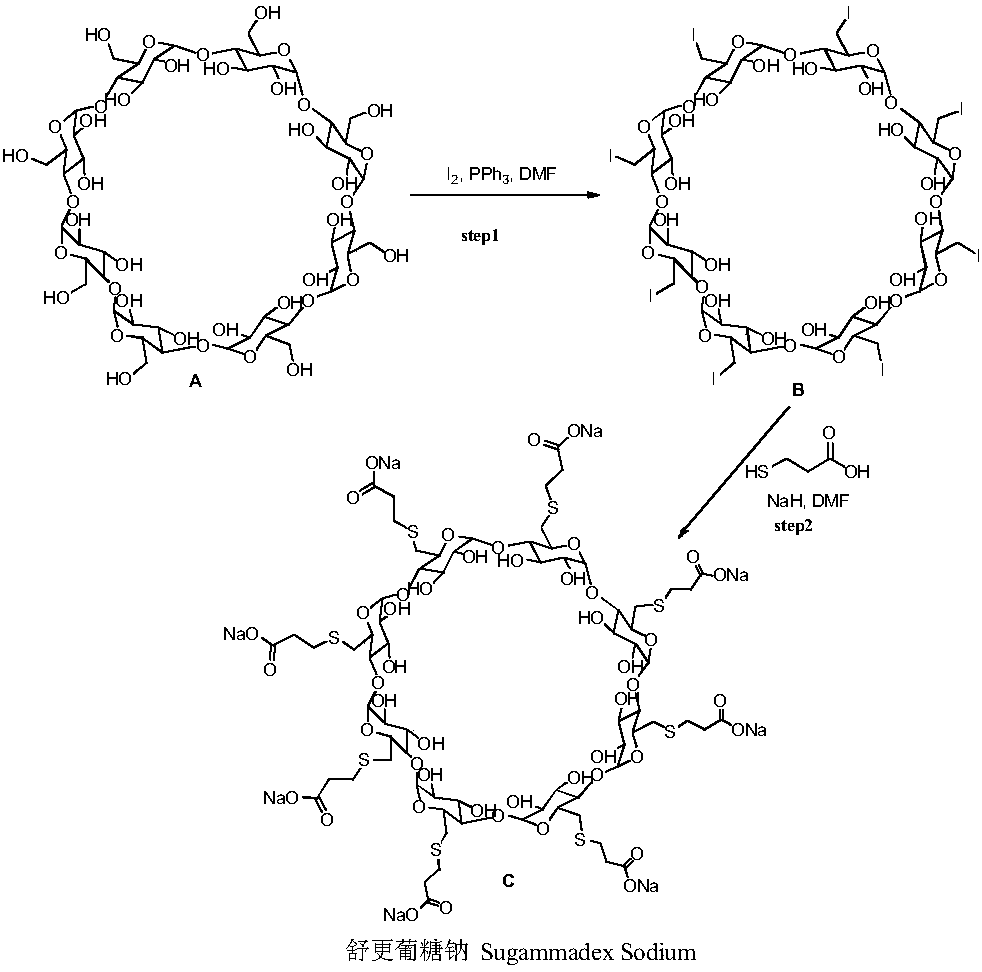
Preparation method for green and environmentally friendly sugammadex sodium
The invention belongs to the field of pharmacy, and especially relates to a preparation method for green and environmentally friendly sugammadex sodium. The preparation method obtains a crude productof sugammadex sodium through preparation for the first time by taking 3-mercaptopropionic acid, alkali and 6-deoxy-6-perhalogeno-gamma-cyclodextrin as raw materials; and high purity sugammadex sodiumcan be obtained after desolvation through recrystallization and column chromatography purification. Compared with the prior art, a reaction solvent of the preparation method can replace organic solvents like N,N-dimethylformamide used by traditional methods by only using water, so that disadvantages of hard recovery and large pollution caused by large amount of high boiling organic solvents can beimproved; and the sugammadex sodium is clean and environmentally friendly, green and efficient, has advantages of being mild in reaction condition, high in product purity, simple in post-treatment step, high in product yield, low in cost and convenient for industrialization, and has good application prospects.
Owner:HEFEI BOSIKC PHARMTECH CO LTD
Covalent organic framework material, preparation method therefor and application of covalent organic framework material in synthesis of hindered amines
ActiveCN109280179ANo significant decrease in activityIncrease profitOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsOrganic solventHindered amine light stabilizers
The invention relates to a covalent organic framework material, a preparation method therefor and a method for synthesizing N,N'-di(2,2,6,6-tetramethyl-4-piperidyl)-1,3-benzenediformamide by using thecovalent organic framework (COFs) catalyst. The method comprises the specific steps: subjecting dimethyl isophthalate and 2,2,6,6-tetramethyl-4-aminopiperidine to a heterogeneous catalytic reaction with stirring in an organic solvent by taking the covalent organic framework material (COFs) as a catalyst, and carrying out aftertreatment after the reaction ends up, thereby obtaining the product. The catalyst has sufficient alkaline loci and can be used for effectively catalyzing the reaction and remarkably increasing yield; and after the catalyst is used, treatment steps are simpler, the emission of salt-bearing wastewater is reduced, heavy-metal pollution is avoided, and the catalyst is environmentally friendly.
Owner:天罡新材料(廊坊)股份有限公司
Method for synthesizing azoxystrobin intermediate
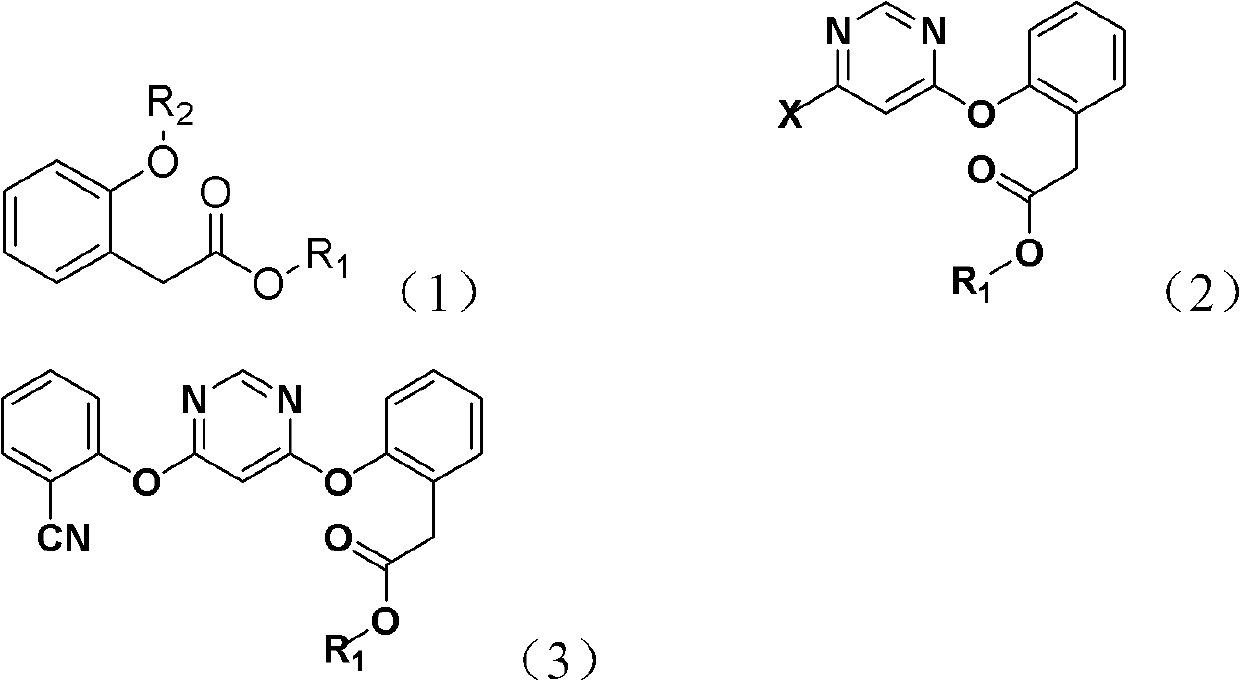
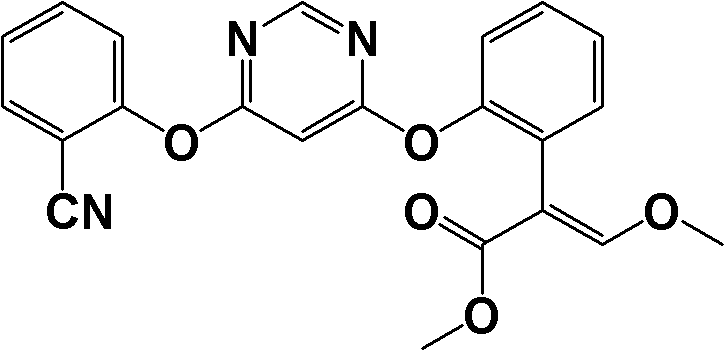
The invention discloses a method for synthesizing an azoxystrobin intermediate. The synthesizing azoxystrobin intermediate has the structure shown as a formula (3). The method comprises the following steps of: under the alkaline condition, performing first contact reaction on 4,6-dihalogenopyrimidine and a compound shown as a formula (I) to obtain a product containing a compound with a structure shown as a formula (2); and under the alkaline condition, performing second contact reaction on the compound with the structure shown as the formula (2) or the product containing the compound with the structure shown as the formula (2) and 2-hydroxybenzonitrile in the presence of a catalyst to obtain the product containing the azoxystrobin intermediate with the structure shown as the formula (3), wherein the catalyst is 1,4-diazabicyclo[2.2.2]octane and / or 2-methyl-1,4-diazabicyclo[2.2.2]octane. The method for synthesizing the azoxystrobin intermediate has mild reaction conditions, is simple, has less Vilsmeier rearrangement reaction and ensures high reaction yield.
Owner:NUTRICHEM LAB CO LTD
Preparative circulating chromatography apparatus
ActiveCN104422735AExtended service lifeSmall dead volumeComponent separationChromatographic separationNatural product
A preparative circulating chromatography apparatus can be applied in chromatographic separation preparation of target components in a complex sample and is mainly advantaged in that: (1) with pre-treatment and concentration of an on-line sample, large-volume sampling on the premise of a high column efficiency can be achieved; (2) through position exchange between a separation column and a trapping column, circulating separation and purification of the target components are achieved and the preparative circulating chromatography apparatus is less in circulating dead volume and column efficiency loss; and (3) through concentration-elution of a finish product through the trapping column, a separation-concentration integrated process is achieved, wherein the three functions are achieved through adjustment of an adsorption performance of the trapping column and valve exchange. Compared with a conventional preparative chromatography method, the apparatus has significant advantages on separations of natural products, drugs and chemical products, especially on separation and preparation of micro-scale targeted components and unstable components in a mixture.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Levalbuterol intermediate and levalbuterol hydrochloride synthesis method
ActiveCN104557572ASimplified post-processing stepsImprove protectionOrganic compound preparationAmino-hyroxy compound preparationSynthesis methodsStructural formula
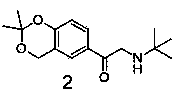
The invention provides a levalbuterol intermediate and a levalbuterol hydrochloride synthesis method, and relates to a levalbuterol intermediate and a method for preparing levalbuterol hydrochloride from the intermediate. The method comprises steps as follows: 2-halogenate-1-(2,2-dimelthyl-4-hydrogen-benzo [d][1,3] dioxane)-butanone and organic amine have a Hoffman alkylation reaction to prepare a compound in the formula 2, the structural formula of the compound is shown in the specification, and the compound in the formula 2 is subjected to a reduction reaction, optically pure organic acid resolution and deprotection by hydrochloric acid to obtain levalbuterol hydrochloride. The method does not need processes of protection or deprotection and the like of hydroxyl groups on a benzene ring, protection, deprotection and purification processes are reduced, the synthesis route is short, operation is simple, meanwhile, borane-thioether does not need to be used as a reduction agent, and safety and environmental protection are realized.
Owner:SHANGHAI DINGYA PHARM CHEM CO LTD
Preparation method of 2,5-furandicarboxylic acid
ActiveCN111039906AAvoid adsorptionSimplified post-processing stepsOrganic chemistryChemical recyclingFuranPtru catalyst
The invention provides a preparation method of 2,5-furandicarboxylic acid, which comprises the following steps: under air and / or oxygen conditions, putting 5-hydroxymethylfurfural and a catalyst intoa mixed solution of water and an organic solvent, and carrying out catalytic oxidation reaction to obtain the 2,5-furandicarboxylic acid, wherein the catalyst comprises a carrier and an active component loaded on the carrier; the active component is one or more selected from ruthenium, palladium, platinum and rhodium; the carrier is one or more selected from activated carbon, graphite, fullerene and graphene oxide. The mixed solution of water and the organic solvent is used as the solvent so that solubility of the product 2,5-furandicarboxylic acid is increased, efficient conversion of 5-hydroxymethylfurfural is achieved without adding an alkaline additive to increase the solubility, high-selectivity 2,5-furandicarboxylic acid is obtained, and the method is simple in operation method, mildin reaction condition, environmentally friendly, free of pollution and good in industrial application prospect.
Owner:CHINA PETROLEUM & CHEM CORP +1
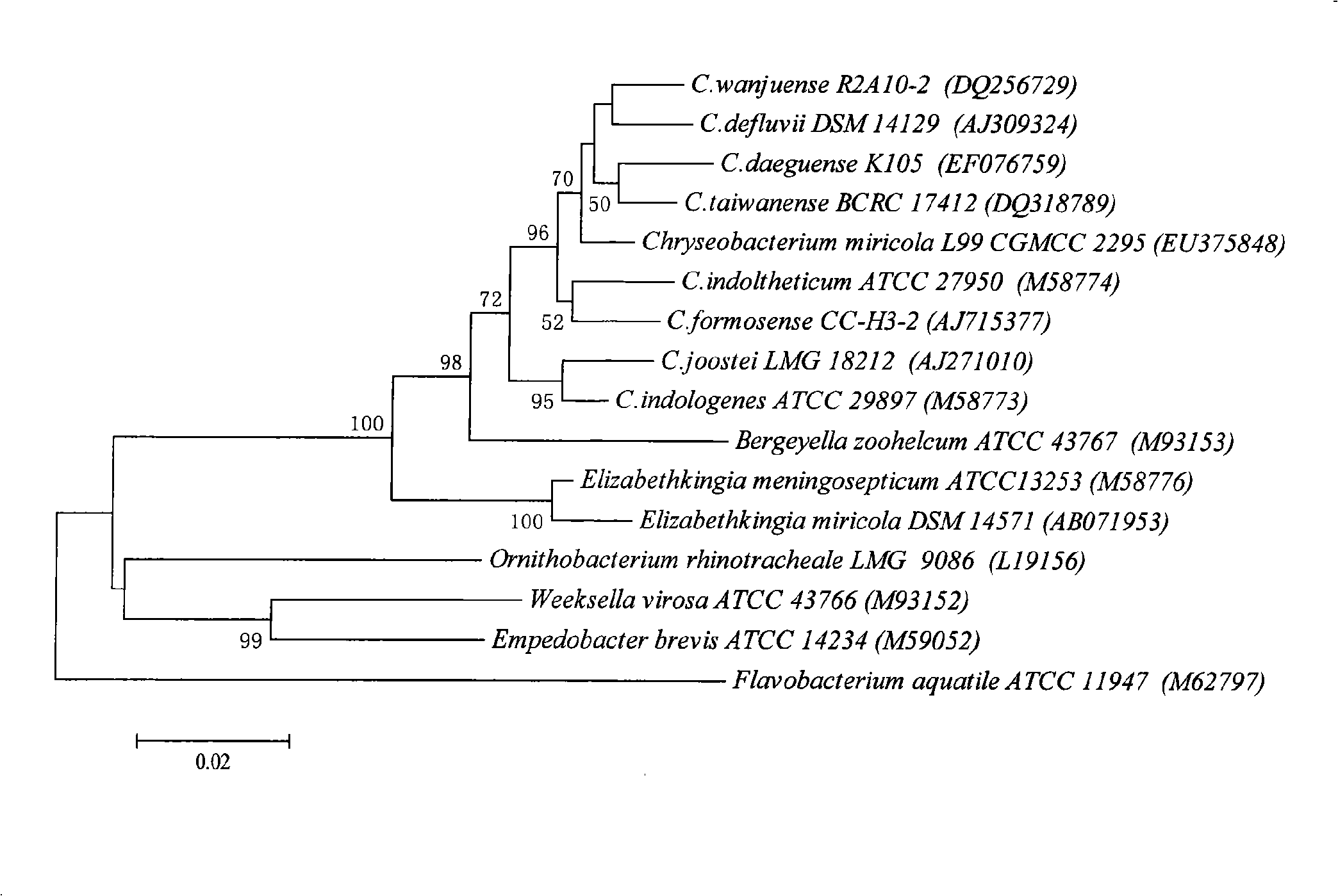
Chryseobacterium gleum for producing keratinase and separation method thereof
The invention discloses a chryseobacterium gleum of keratinase and a method for separating the chryseobacterium gleum. The Latin name of the chryseobacterium gleum is Chryseobacterium L99; the chryseobacterium gleum is stored in CGMCC with the collecting number of CGMCC No.2295. The invention discloses the morphological properties of the bacterium: the bacterium body has a rod shape, no spores are produced, the bacterium does not have motility and the Gram pigmentation shows negative. The invention discloses whole-cell fatty acid of the bacterium. The main components in weight percent are as follows: 47.59 percent of 13 methyl 14 acid, 15.72 percent of dihydroxy-13-methyl myristic acid and / or Omega-7-cis-13 methyl-15 acid, 13.91 percent of trihydroxy-15-methyl 16 acid, and 9.48 percent of Omega-9-cis-14 methyl hexadecanoic acid. The invention also discloses the whole series of a 16 S rDNA of the bacterium. The separating method comprises the following steps that: the common nutritional medium is bred in enrichment, the keratin is taken as the only SiCN source solid culture medium prescreening operation and is taken as the secondary screening of the only SiCN source solid culture medium. The method is effective and rapid and the strain has strong ability to produce enzyme.
Owner:ZHEJIANG UNIV
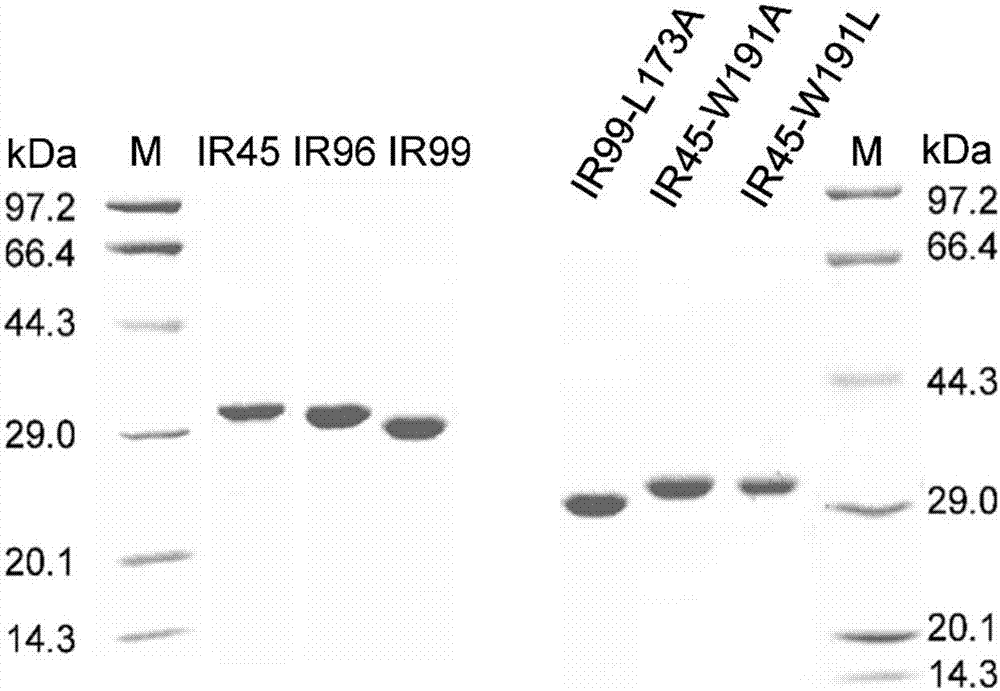
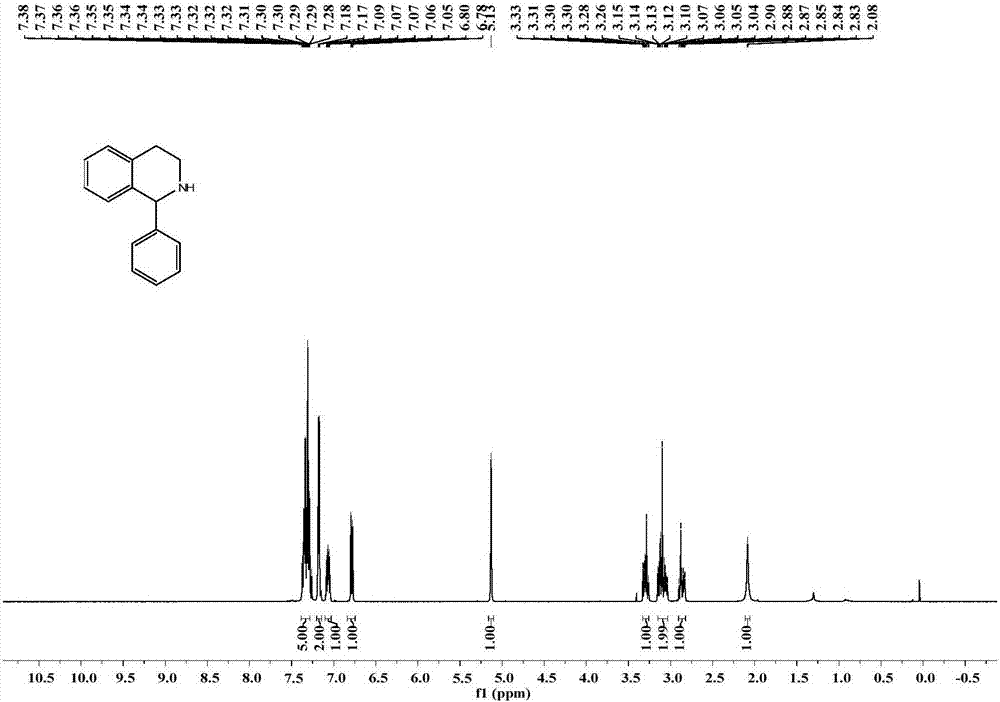
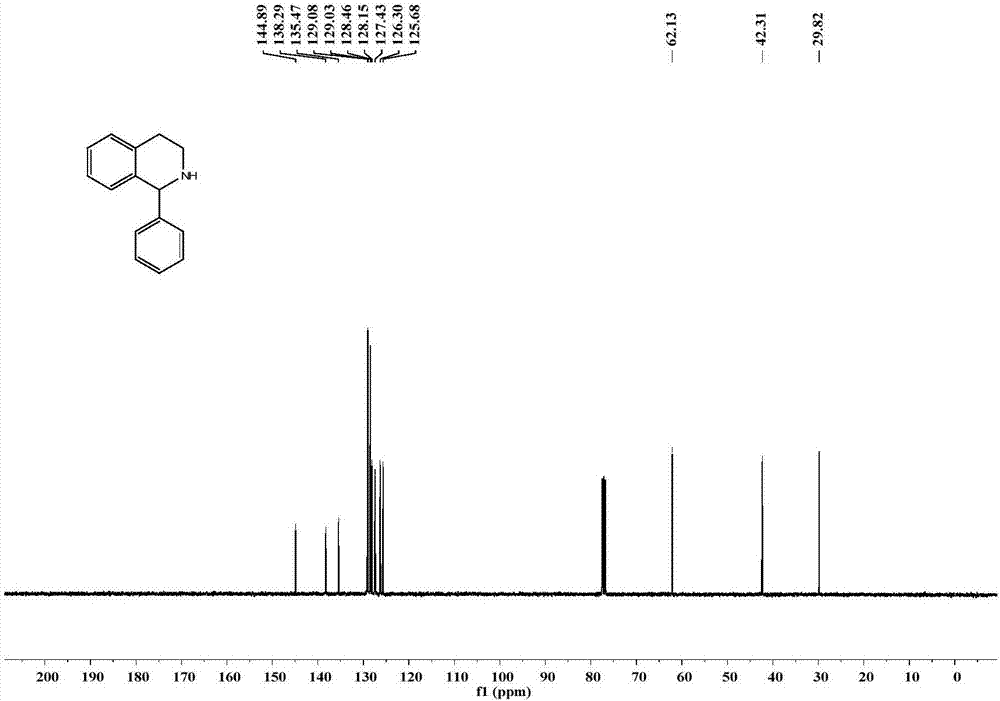
Application of imine reductase and mutant thereof in synthesis of (S)-1-aryl-1, 2, 3, 4-tetrahydroisoquinoline
The invention discloses application of imine reductase and a mutant thereof in synthesis of (S)-1-aryl-1, 2, 3, 4-tetrahydroisoquinoline, and belongs to the field of enzyme engineering. The imine reductase provided by the invention is one of the following enzymes: IR45, IR96 and IR99 as shown in SEQ ID NO. 1-3, having corresponding coding nucleotides preferably as shown in SEQ ID NO. 1-3; the mutant of the imine reductase is one of mutants IR45-W191A and IR45-W191L of IR45 and mutant IR99-L173Aof IR99. The imine reductase or the mutant thereof can catalyze a compound of formula I to synthesize a compound of formula II, wherein R in the formula I and the formula II is hydrogen, methoxy or halogen; the catalysis efficiency is high; the obtained target product has high optical purity, simple post-processing and good environmental-protection and safety.
Owner:WUHAN UNIV
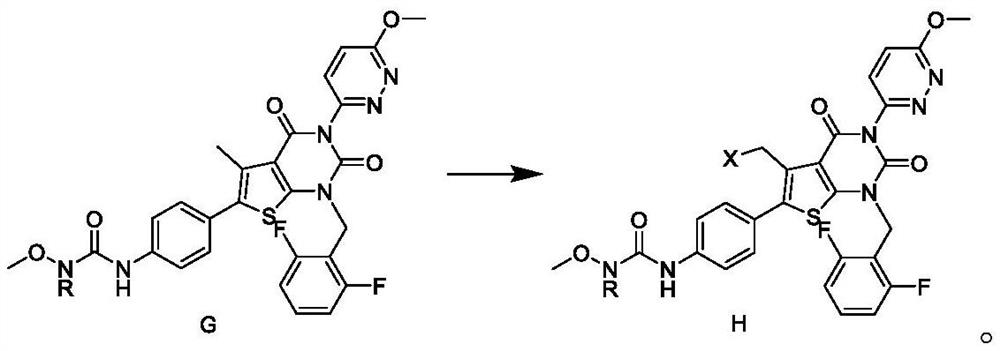
Relugolix intermediate and preparation method thereof
ActiveCN113563304AHigh purityReduce manufacturing costOrganic chemistryBulk chemical productionBiochemical engineeringOrganosolv
The invention discloses a Relugolix intermediate and a preparation method thereof. The invention provides a preparation method of a compound H. The preparation method comprises the following step of: carrying out nucleophilic substitution reaction on a compound G and a halogenating reagent in an organic solvent in the presence of an initiator to obtain the compound H. The preparation method disclosed by the invention is simple and safe to operate, simple in post-treatment step, environment-friendly and high in total yield, and the Relugolix product prepared from the intermediate disclosed by the invention is high in purity, low in heavy metal element content, capable of reaching the standard of raw material medicines, low in production cost and suitable for industrial production.
Owner:上海新礼泰药业有限公司
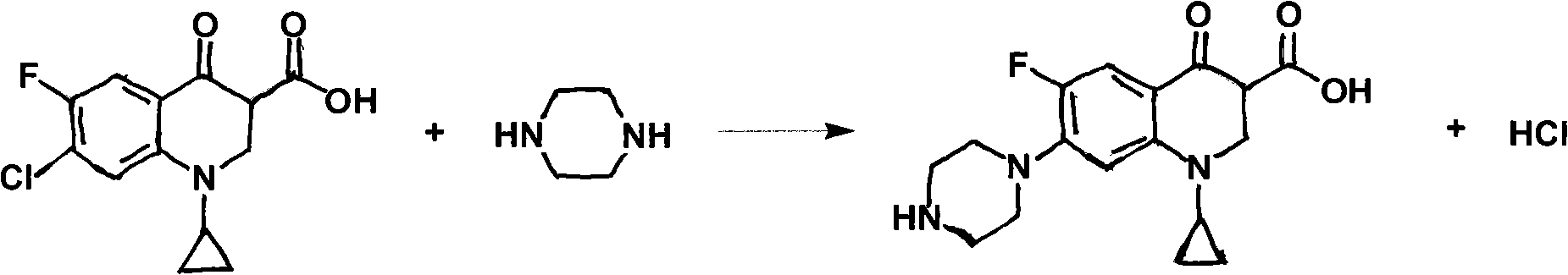
Preparation method of ciprofloxacin hydrochloride
InactiveCN101851198AReduce pollutionReduce generationOrganic chemistryAntiinfectivesIsoamyl alcoholCarboxylic acid
The invention discloses a preparation method of ciprofloxacin hydrochloride. The existing synthesis method generally uses isoamyl alcohol and the like as solvent, thus having the problems of strong smell, difficult biodegradation and higher environmental pollution. If the solvent such as the isoamyl alcohol and the like is used, more alkali insoluble substances and acidic insoluble substances are generated in recovery after the reaction, so that the insoluble substances are needed to be treated by purification through the steps such as alkali-dissolving heat-filtering call-back throw filter, acidic-dissolving heat-filtering call-back throw filter, salifying decoloration heat-filtering and the like, the process route is longer, and the product yield is lower. The preparation method of the ciprofloxacin hydrochloride selects the proper solvent as reaction medium, firstly leads cyclopropane carboxylic acid and piperazine to have piperazine reduction reaction and then leads the reaction product to be salified together with hydrochloric acid. In the reaction process, less alkali insoluble substances and acidic insoluble substances are generated in the reaction process, and the ciprofloxacin hydrochloride can be obtained by salifying decoloration heat-filtering, separation by crystallization, throw filter, rinsing and drying after being directly cooled and leached, so that the time for occupying equipment and the time of working procedures are shortened, and the production efficiency of a workshop is improved.
Owner:ZHEJIANG JINGXIN PHARMA +1
Lactobacillus salivarius capable of inhibiting Candida albicans growth, and separation method thereof
ActiveCN103555604AShort fermentation cycleGood antibacterial effectAntimycoticsBacteriaBiotechnologySporeling
The present invention discloses a strain of Lactobacillus salivarius capable of inhibiting Candida albicans growth, and a separation method thereof, wherein the Latin name is Lactobacillus salivarius LI01, the Lactobacillus salivarius LI01 is preserved in the China General Microbiological Culture Collection Center, and the preservation number is CGMCC No.7045. The present invention discloses morphological characteristics of the Lactobacillus salivarius LI01, wherein the morphological characteristics comprise rod-shaped bacteria, no spore production, no motility, and positive Gram staining. The present invention discloses main components of the whole-cell fatty acid of the Lactobacillus salivarius LI01, wherein the main components comprise: about 38.48% of 16:0, about 8.29% of 18:1w9c, about 14.68% of 19:0cyclow10c / 19w6, about 5.94% of 18:0, about 12.75% of 19:0cyclow10c / 19w6, about 11.55% of 19:0cyclow8c, about 12.75% of 19:1w6c, and about 14.68% of 18:1w7c. The invention further discloses the whole sequence of 16S ribosomal DNA (16SrDNA) of the Lactobacillus salivarius LI01. The separation screening method of the present invention comprises three steps: carrying out enrichment culture with a MRS nutrient culture medium, adopting a Candida albicans covering layer to carry out primary screening, and adopting liquid co-culture to carry out re-screening. According to the present invention, the method is rapid and efficient, and the obtained Lactobacillus salivarius LI01 has strong Candida albicans resistance ability.
Owner:ZHEJIANG UNIV
Relugolix intermediate and preparation method thereof
ActiveCN113563303AHigh purityReduce manufacturing costOrganic chemistryBulk chemical productionBiochemical engineeringOrganosolv
The invention discloses a Relugolix intermediate and a preparation method thereof. The invention provides a preparation method of a compound D. The preparation method comprises the following steps of: by taking an organic solvent and / or water as a solvent, carrying out hydrolysis reaction on a compound C under the action of alkali to obtain the compound D. The preparation method disclosed by the invention is simple and safe to operate, simple in post-treatment step, environment-friendly and high in total yield, and the Relugolix product prepared from the intermediate disclosed by the invention is high in purity, low in heavy metal element content, capable of reaching the standard of raw material medicines, low in production cost and suitable for industrial production.
Owner:上海新礼泰药业有限公司
Improved process for extracting sterol from vegetable oil asphalt
The invention discloses an improved method for extracting phytosterol from asphalt of vegetable oil. The method has a simple process, high product purity and low product cost. The improved method comprises the following steps: (1) the asphalt of the vegetable oil is heated and melted at a temperature of between 80 and 100 DEG C, is added with alkali and water, is kept at the temperature, is continuously stirred and saponified for 0.5 hour, is added with hot water, is continuously heated, reflows and reacts for 1.5 to 2.5 hours; a saponification reaction solution is cooled and delaminated; the collected unsaponified layer is added with alcohol with 2 to 3 times of the weight of the raw material of the asphalt, is added with alkali, is heated to a temperature of between 75 and 85 DEG C, reflows for 3 to 5 hours and is stopped to heat and stir; after the solution is cooled to the temperature of 50 DEG C, the solution is added with hot water and continuously reacts and reflows for 10 to 30 minutes; and after the solution is fully cooled, the phytosterol is precipitated out and filtered to obtain a coarse product of the phytosterol; and (2) the coarse phytosterol is eluted through an organic solvent; a filtrate evaporation solvent is put to a vacuum dry box and is dried at a temperature of between 50 and 60 DEG C to obtain the phytosterol product.
Owner:NANJING UNIV OF TECH
Preparation method of apixaban and intermediates thereof
ActiveCN107955002AHigh purityAtom utilization is highOrganic chemistryOrganic solventP-fluoronitrobenzene
The invention discloses a preparation method of apixaban and intermediates thereof. The invention provides a preparation method of an apixaban intermediate I. The preparation method of the apixaban intermediate I comprises the step of performing nucleophilic substitution reaction on an apixaban intermediate II and p-fluoronitrobenzene in an organic solvent in the presence of an alkali to obtain the apixaban intermediate I. The preparation method has short steps, simple and safe operation, simple post-treatment steps, environmental friendliness and high total yield, and the obtained product hashigh purity, low production cost and high atomic utilization, and is suitable for industrial production. The formula is shown in the description.
Owner:SHANGHAI BOCIMED PHARMA CO LTD
Method for preparing polythioether polymer grafted carbon fibers on basis of thiol-ene photopolymerization
The invention discloses a method for preparing polythioether polymer grafted carbon fibers on the basis of thiol-ene photopolymerization. The method specifically includes the steps that firstly, carbon fibers are subjected to acidification to obtain acidified carbon fibers; secondly, the acidified carbon fibers further react with mercapto-alcohol to obtain sulfhydrylated carbon fibers; thirdly, the sulfhydrylated carbon fibers, dimercapto monomers and diene monomers initiate a thiol-ene photopolymerization reaction under irradiation of ultraviolet, and the polythioether polymer grafted carbon fibers are obtained. The method has the advantages that by means of the thiol-ene photopolymerization reaction, the consumption of solvents is reduced, after-treatment steps are simplified, large-scale production is benefited, and meanwhile the toxic and harmful acylating chlorination process existing in a traditional carbon fiber surface grafting and modifying process is avoided; polythioether polymer is grafted on the surfaces of the carbon fibers so that active functional groups on the surfaces of the carbon fibers are increased, wettability of the carbon fibers with resin is easily improved, and interface performance between the carbon fibers and a resin matrix is improved.
Owner:江西国计纳米科技有限公司
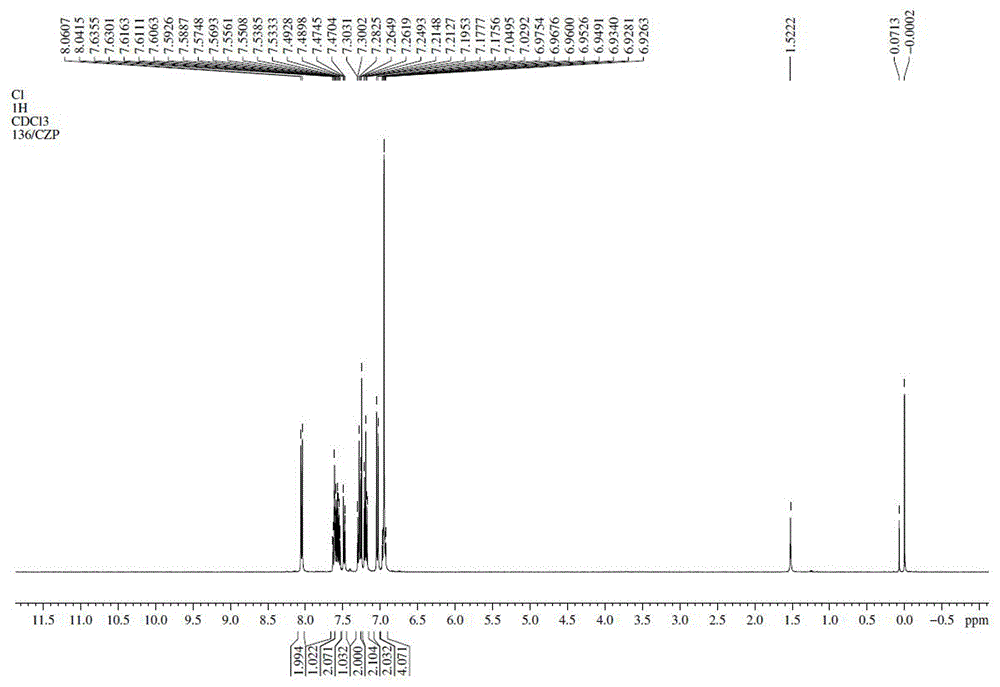
Synthesis method of photoelectric material intermediate 9-(4'-chlorobiphenyl-2-yl) carbazole
The invention discloses a synthesis method of a photoelectric material intermediate 9-(4'-chlorobiphenyl-2-yl) carbazole, and belongs to the field of organic chemical synthesis. According to the method, 9-(2-bromophenyl) carbazole is synthesized from carbazole and o-dibromobenzene as raw materials, and then reacts with 4-chlorophenylboronic acid to synthesize a target compound. In synthesis of the intermediate 9-(2-bromophenyl) carbazole, the o-dibromobenzene is taken as a reaction material and is also taken as a reaction solvent, and L-lysine is taken as a catalyst ligand, so that the reaction rate is improved, the post-treatment step is simplified, the production cost is reduced, and industrial production is facilitated.
Owner:PUYANG HUICHENG ELECTRONICS MATERIAL
Synthesis method of 1,2-benzoisothiazolinyl-3-one compound
The invention belongs to the technical field of fine chemical engineering, and particularly relates to a synthesis method of a 1,2-benzoisothiazolinyl-3-one compound. The method comprises the following steps: reacting a 2-halogenated cyanophenyl compound with a mercaptan compound to obtain a 2-alkylthiocyanophenyl compound; and in the presence of water, reacting with a halogenating agent to obtain the 1,2-benzoisothiazolinyl-3-one compound. The byproduct alkyl halide in the method can further react with sulfide to obtain the mercaptan compound which can be used repeatedly as a reactant. On one hand, the raw materials used by the technique have low smell and volatility, so the technique is green and environment-friendly and generates small environmental pollution; and on the other hand, the method has the advantages of mild reaction conditions, simple technical steps, recyclable byproduct and low reaction cost, and has favorable utilization value in industrial production.
Owner:SHOUGUANG SYNTECH FINE CHEM
Improved method for preparing 4-phenyl-cyanophenyl
ActiveCN103012203AOvercome the conditionsOvercoming pollutionPreparation by carboxylic acid amide dehydrationBulk chemical productionPhenyl groupPhosgene
The invention relates to a method for preparing 4-phenyl-cyanophenyl. The method mainly comprises the steps of: carrying out Friedel-Crafts acylation on biphenyl which is a starting material to obtain 1-chloroacetyl-4-phenyl-benzene; carrying out ammonolysis reaction on the 1-chloroacetyl-4-phenyl-benzene to obtain 4-phenyl-benzamide; and carrying out dehydration reaction on the 4-phenyl-benzamide in the presence of a dehydrating agent to obtain the target product. The method is characterized in that (1) a catalyst used in the Friedel-Crafts acylation reaction is imidazole ionic liquid; and (2) the dehydrating agent used in the dehydration reaction is a mixture of triphosgene and ammonium salt, the molar ratio of the triphosgene to the ammonium salt is 1:(0.05-0.1), and the ammonium salt is organic ammonium salt or / and inorganic ammonium salt. The method for preparing the 4-phenyl-cyanophenyl with better commercial value is provided by the invention.
Owner:LILY GRP CO LTD +1
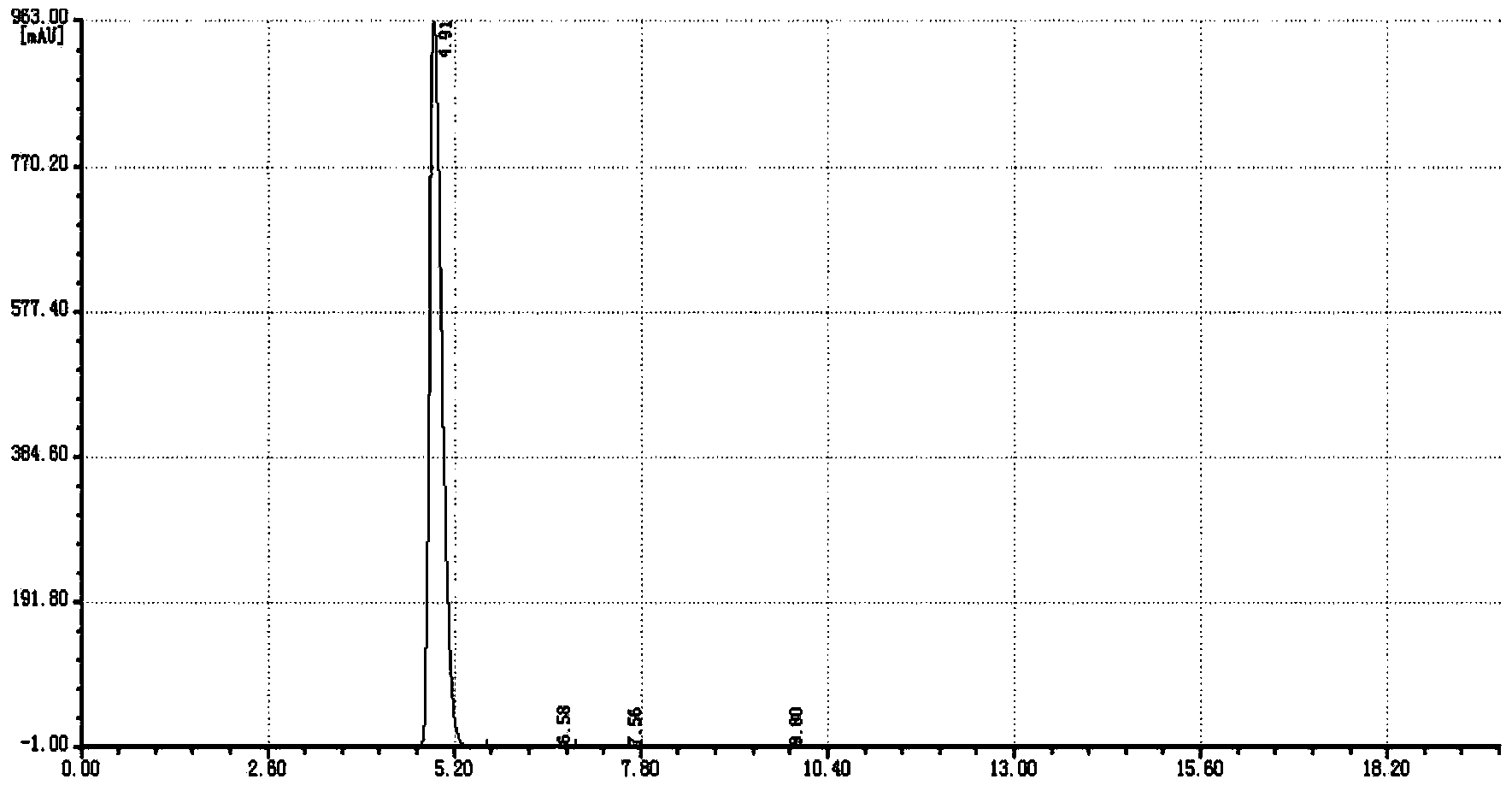
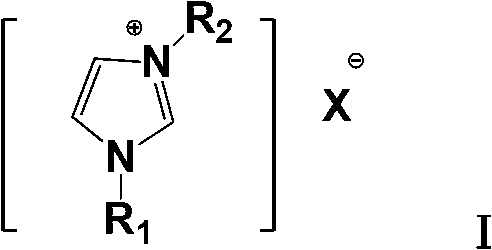
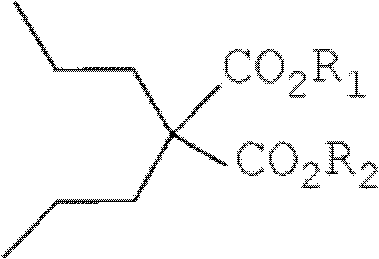
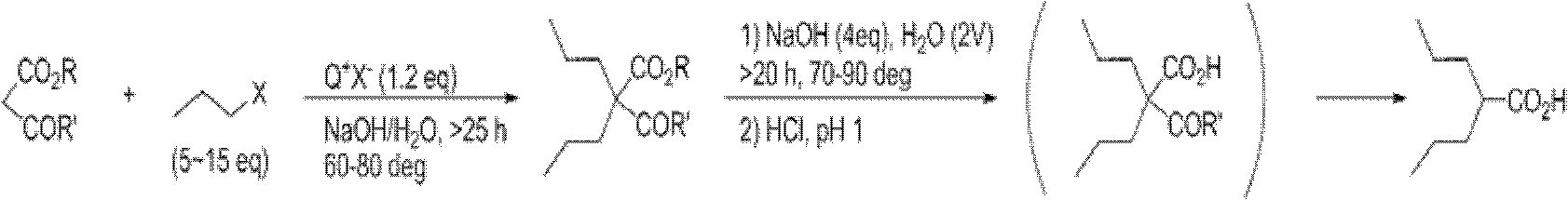
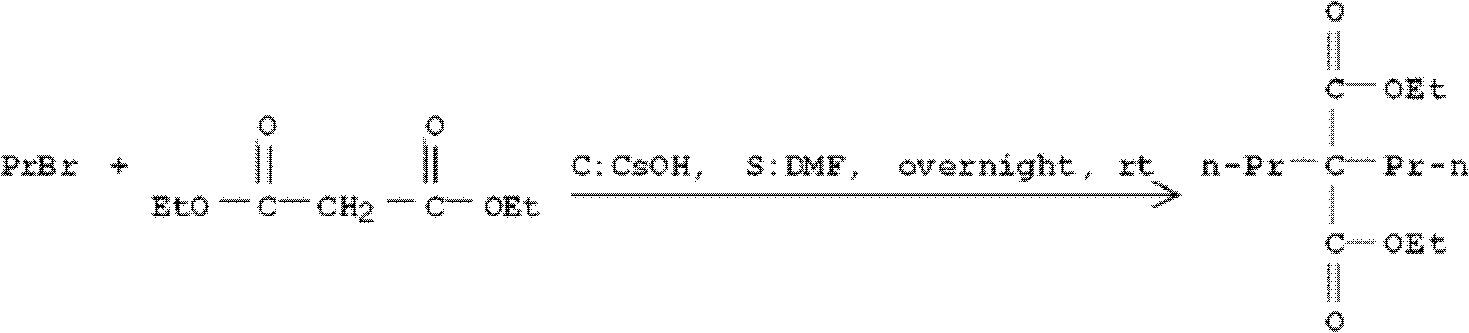
Dipropylmalonic acid diester preparation method
InactiveCN103183612ALess quantitySimplified post-processing stepsOrganic compound preparationCarboxylic acid esters preparationHalogenPotassium
The invention provides a preparation method of dipropylmalonic acid diester. The method comprises the following steps: in an organic reaction solvent, alkali is taken as a catalyst, diester malonate and 1-halogenated n-propane are reacted to prepare dipropylmalonic acid diester, a reaction equation is shown as a reaction equation (I), in the reaction equation, R1 and R2 are respectively and independently selected from straight chain or branched chain alkanes with 1-5 carbon atoms; X is halogen; the alkali is MO-R3, wherein the R3 is straight chain or branched chain alkanes with 1-4 carbon atoms, and M is sodium or potassium. Compared with the prior art, the dipropylmalonic acid diester preparation method has the following advantages and active effects: a phase-transfer catalyst is not added in the reaction, the reaction speed is fast, the postprocessing is simple, dipropylmalonic acid diester can be directly used in a next step, and is suitable for synthesis of pharmaceutical intermediates.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
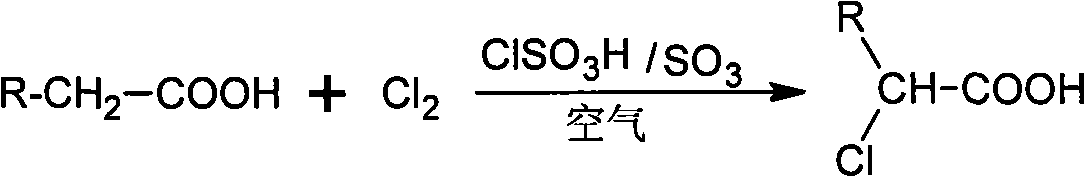
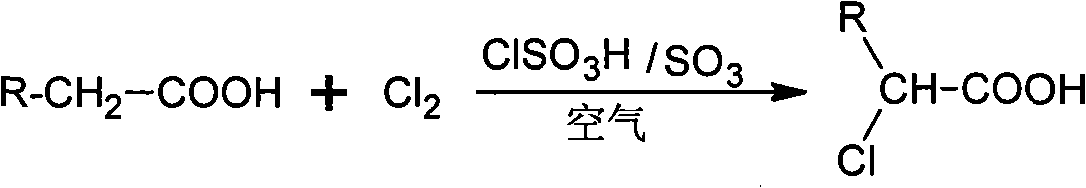
Method for preparing alpha-chloro-fatty acid
ActiveCN101333161AKeep aliveEasy to purifyOrganic compound preparationCarboxylic compound preparationAntioxidantDecomposition
An alpha-chloro-fatty acid preparation method belongs to the organic compound synthesis technical field. The invention takes chlorosulfonic acid as main catalyst, sulfur trioxide as complementary catalyst which is added in a continuous flow way, and uses oxygen in the air to replace pure oxygen to serve as free radical capture agent; the method enables fatty acid and chlorine to be reacted in an atmospheric pressure reactor so as to develop an ideal industrial production method for alpha-chloro-fatty acid production. The alpha-chloro of the invention has high reactivity, good reaction selectivity, high conversion rate, less side reaction products, as well as high purity of products so as to guarantee the catalytic activity in the reaction process and effectively prevent the increasing of the using amount of catalyst caused by decomposition of the main catalyst to result in waste; the method can reduce the insecure factors brought about by pure oxygen in industrial production and can avoid the increase of post-processing steps of products due to the introduction of chemical antioxidants.
Owner:TAIXING LINGFEI CHEM TECH CO LTD +1
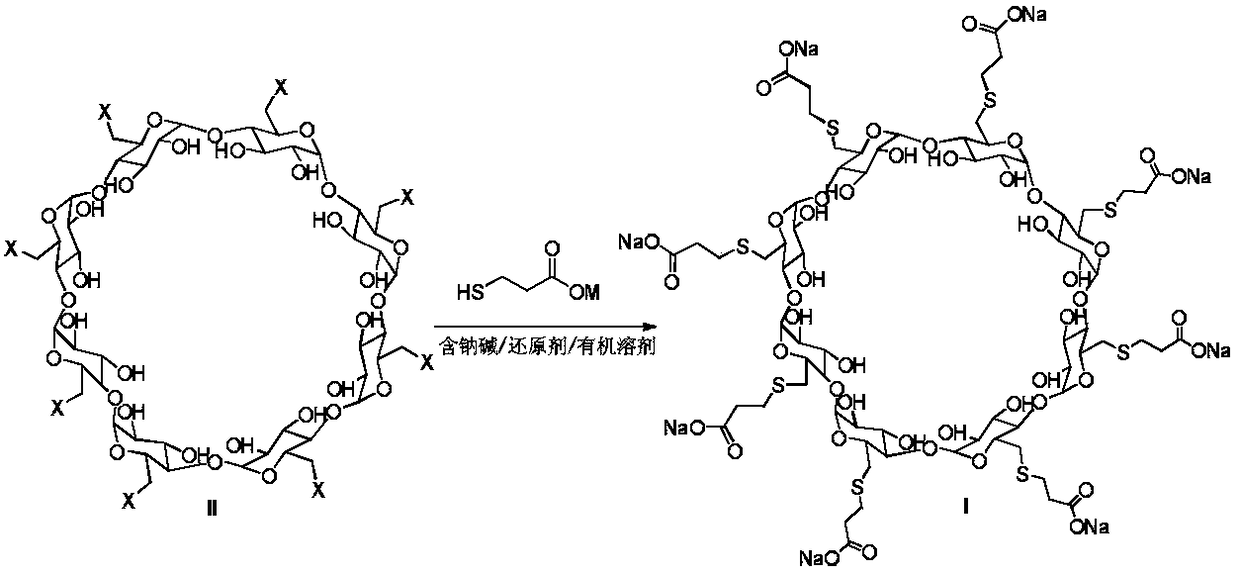
Method for preparing sugammadex sodium
The invention discloses a method for preparing sugammadex sodium. The method is characterized in that perhalogeno gamma-cyclodextrin and 3-mercaptopropionic acid or / and a sodium salt thereof are subjected to a replacement reaction in a sodium base-reducing agent-organic solvent system for preparing sugammadex sodium, the method effectively controls the formation of process impurities with similarstructure of sugammadex sodium from a reaction process, and obtains high-yield and high-purity product after simple post-treatment. The invention further provides a method for purifying of a crude product of the sugammadex sodium and conversion of impurities to the target product in a one-step reaction, which is simple, efficient and wasteful, the method improves the yield and purity from variousaspects, and solves the problems of complex operation, high requirements on instruments and low yield in the prior art method, and is suitable for industrial mass production of the sugammadex sodium.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Novel method for synthesizing prulifloxacin
InactiveCN101418005AImprove shortcomingsSimple reaction conditionsAntibacterial agentsOrganic chemistryQuinolinePrulifloxacin
The invention relates to a method for synthesizing prulifloxacin of formula (I) with a chemical name of 6-fluoro-1-methyl-7-[4-(5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl-1-piperazinyl]-4-oxo-4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylicacid. The method for synthesizing prulifloxacin is suitable for industrial production and has the advantages of simple process, high purity and high yield.
Owner:湖南华纳大药厂手性药物有限公司
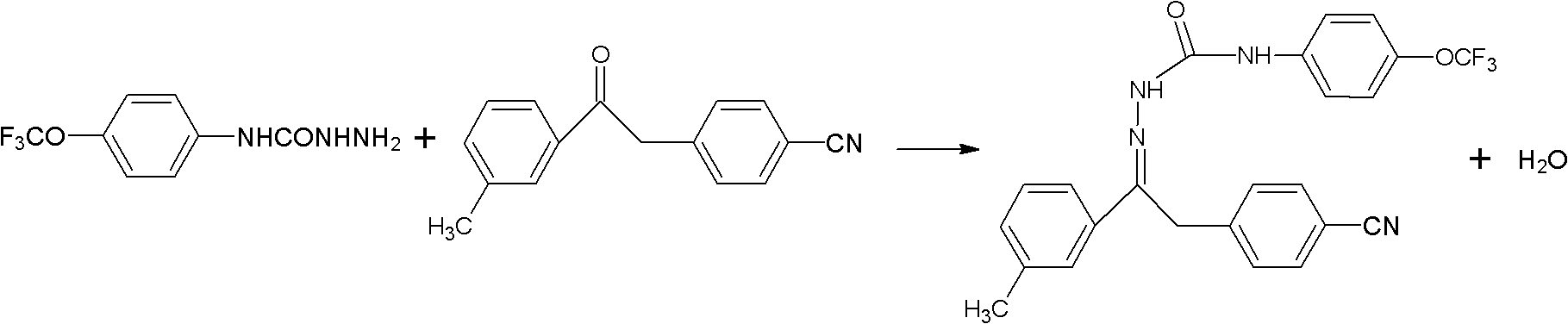
Method for synthesizing metaflumizone
The invention belongs to the technical field of organic synthesis and in particular relates to a method for synthesizing metaflumizone. The method comprises the following steps of: adding m-trifluoromethylphenyl-4-nitrilebenzylketone, p-trifluoromethoxyphenylaminohydrazide, a catalyst and an organic solvent into a reactor, reacting at the temperature of between 80 and 140DEG C for 1 to 3 hours, separating water generated in the reaction when the reaction is carried out, cooling to the temperature of between 0 and 30DEG C after the reaction is finished, and filtering to obtain the product, wherein the organic solvent is a water-insoluble organic solvent. The water generated in the reaction is brought out by the water-insoluble organic solvent, and the reaction is promoted to be carried out rightwards, so that the reaction time is shortened, the yield is improved, and a posttreatment step is simplified. In the synthesis process, the solvent can be recycled, so that the production cost is reduced, the economic benefit is improved, and the solvent has a good application prospect.
Owner:JINGBO AGROCHEM TECH CO LTD
Synthetic method of lipid-lowering drug ciprofibrate
ActiveCN106928047AReduce wasteEmission reductionOrganic compound preparationCarboxylic compound preparationLipid lowering drugPropanoic acid
The invention provides a synthetic method of lipid-lowering drug ciprofibrate. The synthetic method comprises the following steps: catalyzing reaction of p-hydroxy benzaldehyde and propane diacid in a mixed solvent by virtue of alkali, so as to generate p-hydroxystyrene; catalyzing reaction of p-hydroxystyrene, acetone, chloroform and alkali by virtue of a phase transfer catalyst, so as to generate an intermediate 2-methyl-2-(4-vinylphenoxy)propionic acid; and reacting 2-methyl-2-(4-vinylphenoxy)propionic acid with TiCl4, Mg and CCl4, so as to generate ciprofibrate. The synthetic method has the beneficial effects that the reaction route is short, the raw materials are cheap and easily available, reaction conditions are mild, the energy consumption is reduced, and the production cost is lowered; the post-processing step is simple, the emission of three wastes is reduced, and the method is environment-friendly and safe. Compared with routes of predecessors, the synthetic method has the advantages that the reaction selectivity and conversion rate of the route are relatively high, the wasting of the raw materials is reduced, and the economical efficiency is relatively high.
Owner:NANJING UNIV OF TECH
Preparation method of 2-(methyl sulphonyl)-10H-phenothiazine
InactiveCN105837528AEasy to cause lossIncrease contact areaOrganic chemistryEconomic benefitsChloride
The invention relates to a preparation method of 2-(methyl sulphonyl)-10H-phenothiazine and belongs to the technical field of medical intermediate preparation. According to the method, parachlorobenzenesulfonyl chloride and sodium salt of 2-bromothiophenol are taken as starting materials, two novel intermediates M1 and M3 not reported are involved, ideal schemes different from related references are explored in all experimental procedures, and particularly the cyclization process for synthesis of the final product 2-(methyl sulphonyl)-10H-phenothiazine completely breaks through various restrictions in literatures. The method has the advantages that the whole process is simple in step, convenient to operate, mild in reaction condition and easy to control, product yield in all the steps is high and the like, thereby being suitable for industrial production and having high value in use and social and economic benefits.
Owner:DALIAN UNIV OF TECH
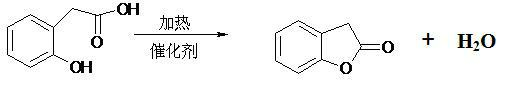
Production method of benzofuranone
ActiveCN102040572AAvoid strong corrosiveReduce investmentOrganic chemistryBenzeneReaction temperature
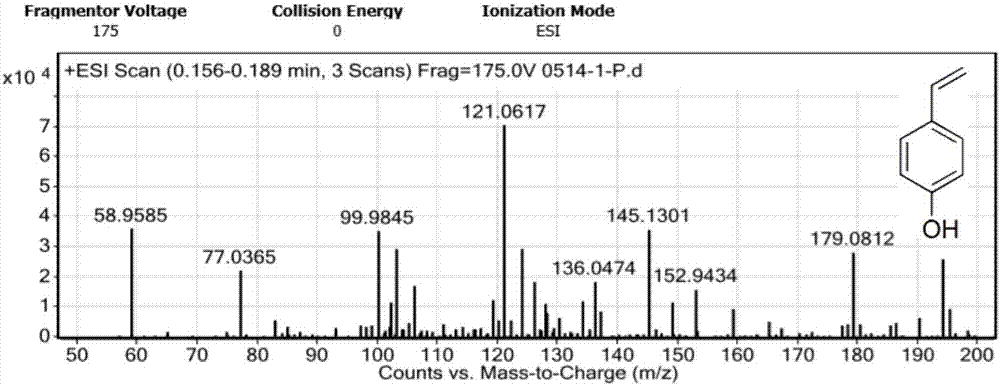
The invention provides a production method of benzofuranone, which is characterized in that o-Hydroxyphenylacetic acid is subject to azeotropic dehydration with a solvent only in the presence of commercial catalyst of cationic resins to generate benzofuranone, wherein the reaction temperature is 60-150 DEG C; the dosage of the catalyst is 0.1-20%; and the solvent accounts for 5-80% of the o-Hydroxyphenylacetic acid. The reacted solvent is no need of special treatment, and the filtered solvent is dehydrated to obtain the benzofuranone with concentration being above 96% in high yield.
Owner:JIANGSU CHANGLONG AGROCHEM CO LTD
Method for synthesizing bisphenol A ethoxy compound
InactiveCN101613261AEasy to separateEasy to collectOrganic-compounds/hydrides/coordination-complexes catalystsEther preparation from oxiranesEthylene oxideReaction temperature
The invention discloses a method for synthesizing a bisphenol A ethoxy compound, which comprises the following steps: adding bisphenol A and a catalyst of dimethyl aminoethanol into a reactor, raising the temperature to a reaction temperature of between 110 and 125 DEG C by stirring and the protection of inert gas; and adding epoxyethane into the mixture to react at a pressure of between 0.1 and 0.35 MPa for 1 to 20 hours to prepare the bisphenol A ethoxy compound. The reaction equation is shown as follows, wherein n is between 1 and 5. The method achieves the solid polymerization of the bisphenol A and the epoxyethane, and selects the novel dimethyl aminoethanol as the catalyst so that the product is free from postprocessing. The product of the bisphenol A ethoxy compound has the advantages of shallow color phase which ranges from 10 to 30, no existence of metallic ions, 0.05 to 0.1 ppm of peroxide and the like.
Owner:YIXING ZHIBO ADVANCED MATERIAL TECH
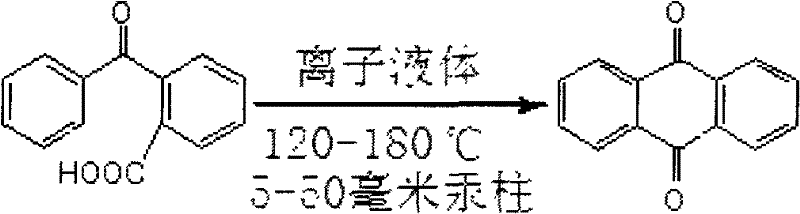
Method for synthesizing anthraquinone
InactiveCN102241579AHigh purityLow yieldOrganic compound preparationQuinone preparationReaction temperatureSolvent
The invention discloses a method for synthesizing anthraquinone, which belongs to the technical field of preparation of organic compounds. In the method, by using acidic ionic liquid a solvent and a catalyst and by heating under a pressure lower than the atmosphere, o-benzoylbenzoic acid is subjected to cyclodehydration, an anthraquinone product is obtained by sublimation and collection, the further purification of the product is not needed, and the ionic liquid can be repeatedly used for many times. In the invention, the used amount of the ionic liquid is 4 to 8 times the mass of o-benzoylbenzoic acid, the reaction temperature is 120 to 180 DEG C, the reaction time is 3 to 10 hours, and the pressure is 5 to 50mmHg. Due to the characteristics of the ionic liquid, three industrial wastes, namely waste gas, waste water and waste residue, are not produced in a reaction process, so the reaction process is a green chemical process; and thus, the pollution to the environment is reduced to the maximum degree. The method has the advantages of simple process, high yield, environment friendliness and low cost, and is suitable for industrial production.
Owner:LIAONING UNIVERSITY OF PETROLEUM AND CHEMICAL TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com