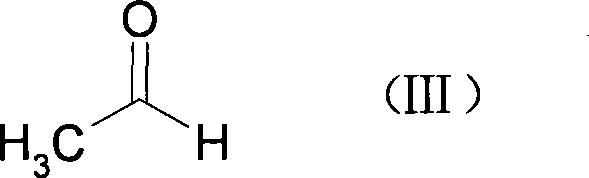Novel method for synthesizing prulifloxacin
A compound, a methyl technology, is applied in the new field of synthesis of prulifloxacin, which can solve the problems of product yield decline, difficulty in purification, and high difficulty, so as to reduce the generation of impurities, simplify the post-processing steps, and improve the utilization rate of raw materials Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 1, the preparation of formula (IV) compound:
[0059] In a 500ml three-necked bottle, N 2 Add 5.3g of magnesium bars, 100ml of anhydrous THF and 5mg of iodine under protection, then slowly add 35.6g of the THF solution of the compound of formula (II) dropwise under heating and reflux, then reflux and stir for 2 hours, and quickly add 9.8g of the formula ( III) acetaldehyde, continue to reflux for 8 hours, heat and concentrate to recover THF, slowly add ice water and ethyl acetate to the residue under cooling and stirring, filter with suction, extract the filtrate, wash the organic phase with water, anhydrous Na 2 SO 4 Dry overnight, concentrate, dissolve the residue in acetone, add Jones reagent (Jones) dropwise while maintaining the reaction temperature at 20°C, continue the reaction for 2 hours, filter with suction, concentrate the filtrate, dissolve the residue in water, extract with chloroform, wash with water, anhydrous Na 2 SO 4 After drying and concentrating, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com