Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
426 results about "Blood test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A blood test is a laboratory analysis performed on a blood sample that is usually extracted from a vein in the arm using a hypodermic needle, or via fingerprick. Multiple tests for specific blood components, such as a glucose test or a cholesterol test, are often grouped together into one test panel called a blood panel or blood work. Blood tests are often used in health care to determine physiological and biochemical states, such as disease, mineral content, pharmaceutical drug effectiveness, and organ function. Typical clinical blood panels include a basic metabolic panel or a complete blood count. Blood tests are also used in drug tests to detect drug abuse. In some of the United States, a blood test is required before marriage.
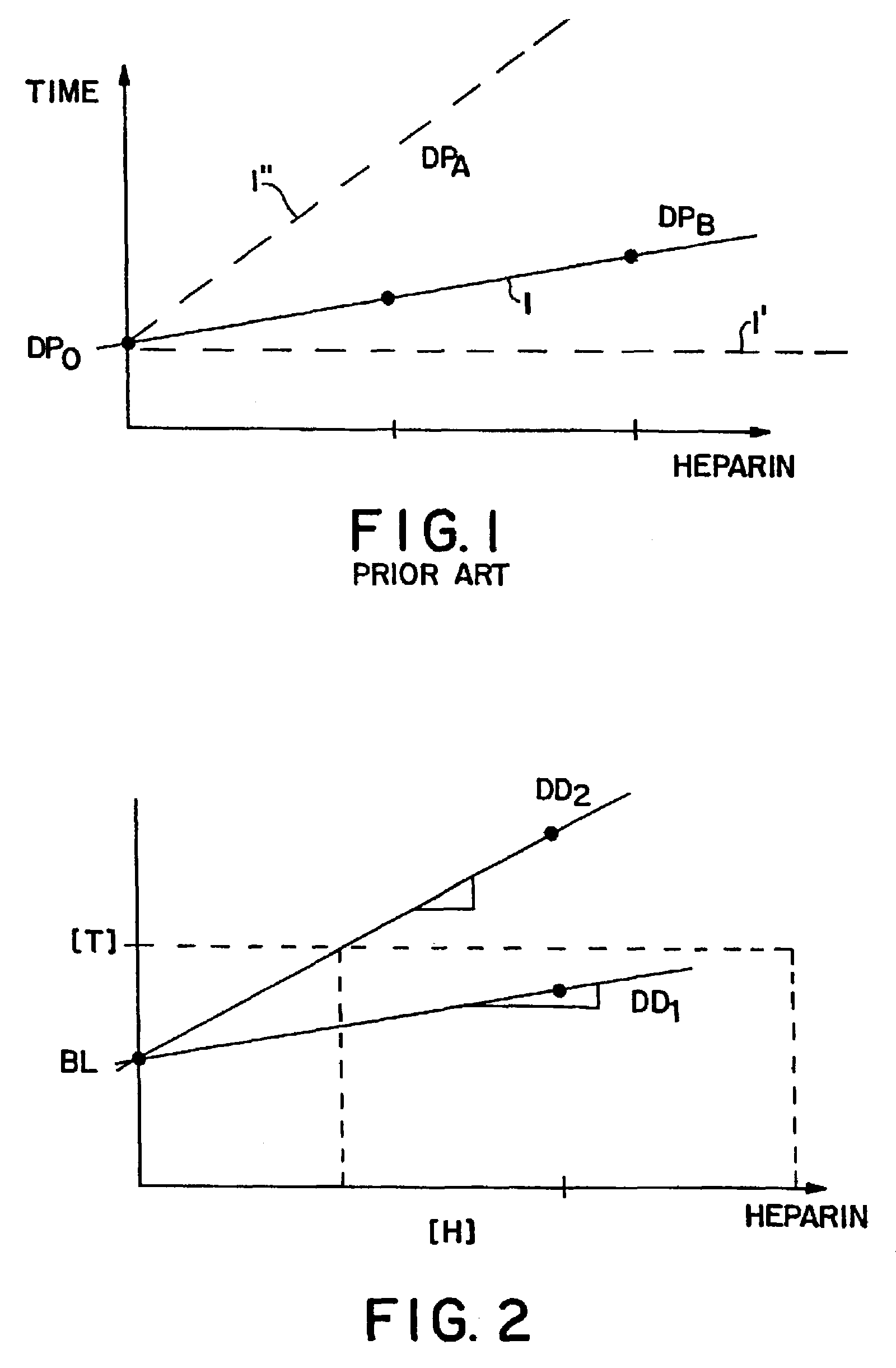
Real time self-adjusting calibration algorithm
Owner:MEDTRONIC MIMIMED INC
Real time self-adjusting calibration algorithm
A method of calibrating glucose monitor data includes collecting the glucose monitor data over a period of time at predetermined intervals. It also includes obtaining at least two reference glucose values from a reference source that temporally correspond with the glucose monitor data obtained at the predetermined intervals. Also included is calculating the calibration characteristics using the reference glucose values and corresponding glucose monitor data to regress the obtained glucose monitor data. And, calibrating the obtained glucose monitor data using the calibration characteristics is included. In preferred embodiments, the reference source is a blood glucose meter, and the at least two reference glucose values are obtained from blood tests. In additional embodiments, calculation of the calibration characteristics includes linear regression and, in particular embodiments, least squares linear regression. Alternatively, calculation of the calibration characteristics includes non-linear regression. Data integrity may be verified and the data may be filtered.
Owner:MEDTRONIC MIMIMED INC
Real time self-adjusting calibration algorithm
A method of calibrating glucose monitor data includes collecting the glucose monitor data over a period of time at predetermined intervals. It also includes obtaining at least two reference glucose values from a reference source that temporally correspond with the glucose monitor data obtained at the predetermined intervals. Also included is calculating the calibration characteristics using the reference glucose values and corresponding glucose monitor data to regress the obtained glucose monitor data. And, calibrating the obtained glucose monitor data using the calibration characteristics is included. In preferred embodiments, the reference source is a blood glucose meter, and the at least two reference glucose values are obtained from blood tests. In additional embodiments, calculation of the calibration characteristics includes linear regression and, in particular embodiments, least squares linear regression. Alternatively, calculation of the calibration characteristics includes non-linear regression. Data integrity may be verified and the data may be filtered.
Owner:MEDTRONIC MIMIMED INC
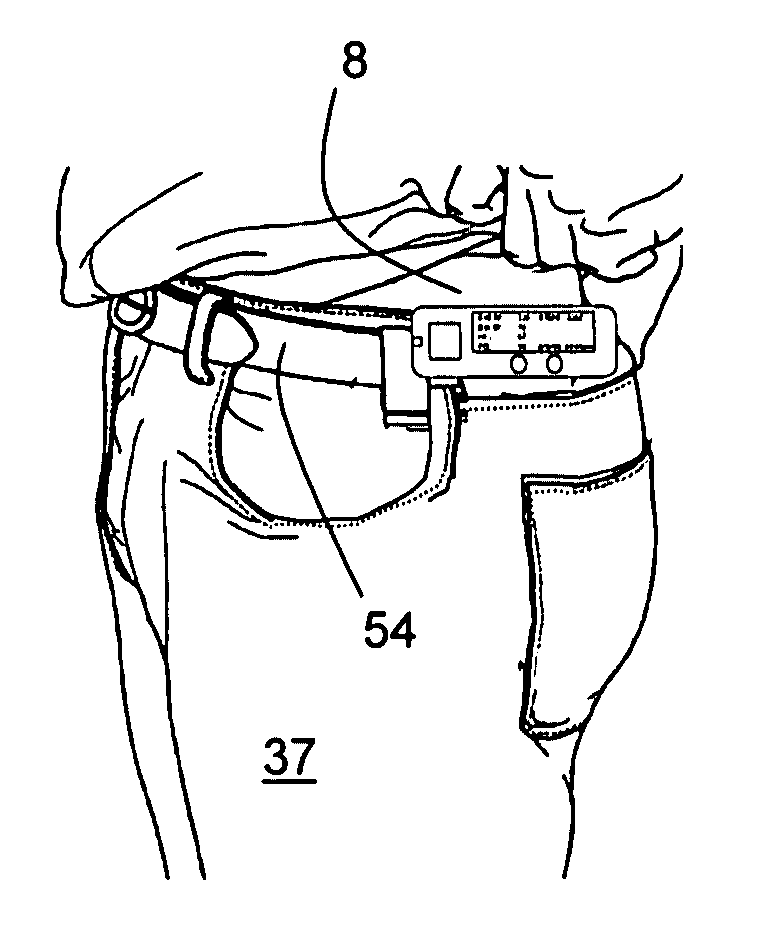
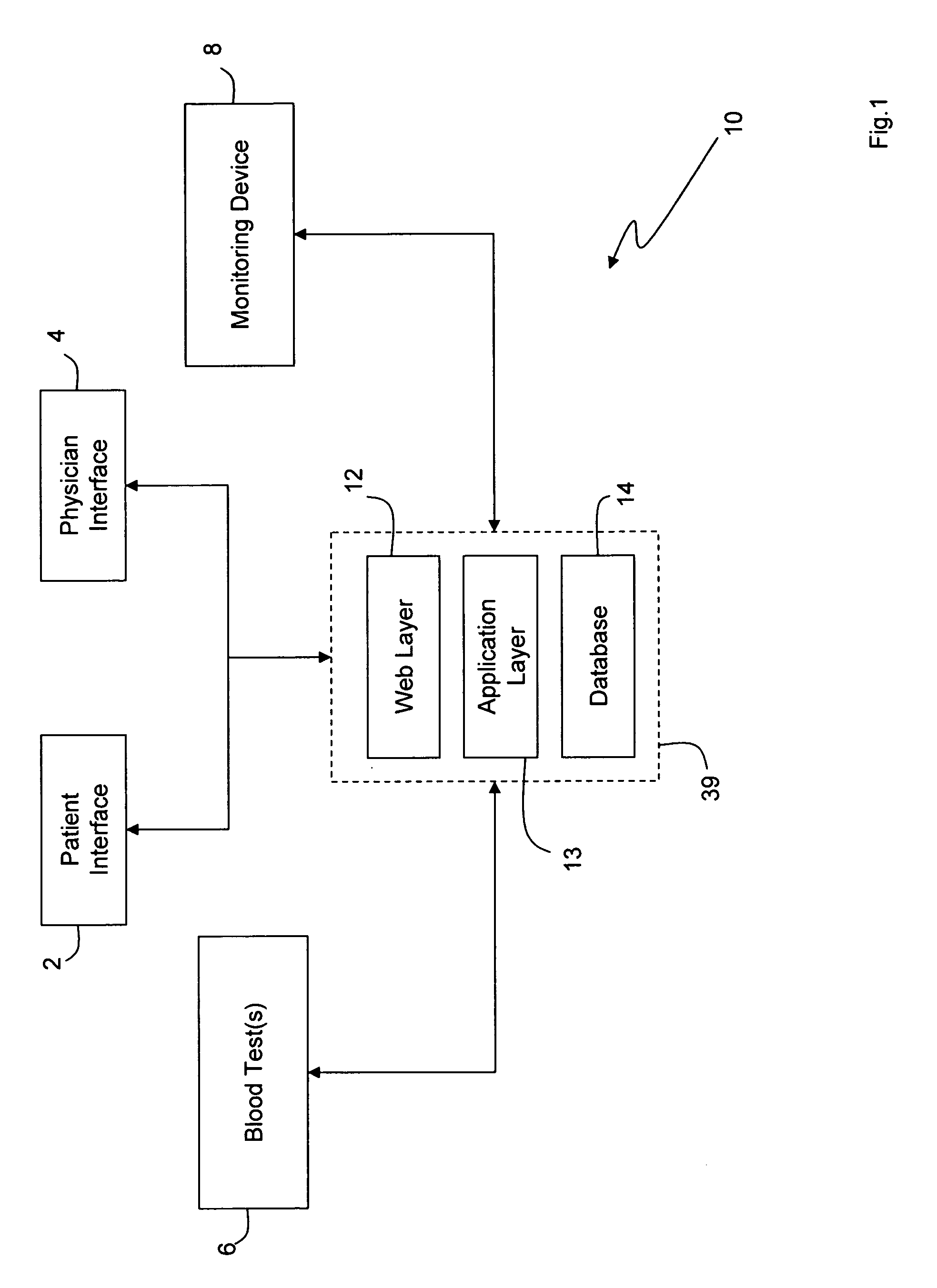
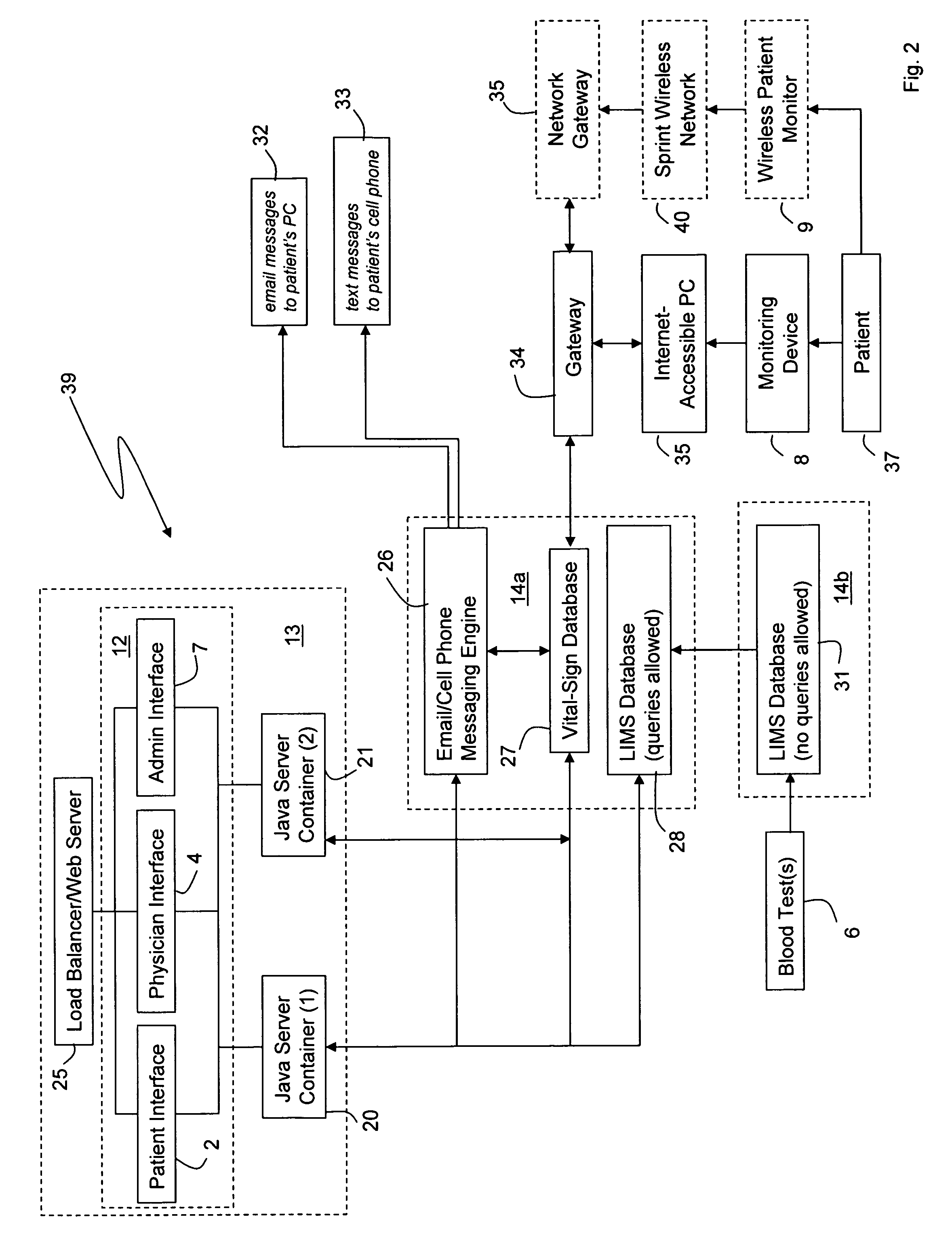
Internet based system for monitoring blood test, vital sign and exercise information from a patient
InactiveUS20070071643A1Quick modificationFast updatePhysical therapies and activitiesMaterial analysis by optical meansBlood testVital signs
The invention provides a system for monitoring a patient that includes: 1) a first database that stores the patient's blood test information; 2) a monitoring device that collects the patient's cardiovascular and exercise information; 3) a second database that receives cardiovascular and exercise information from the monitoring device; and 4) an Internet-based system that displays the blood test, cardiovascular, and exercise information.
Owner:BERKELEY HEARTLAB
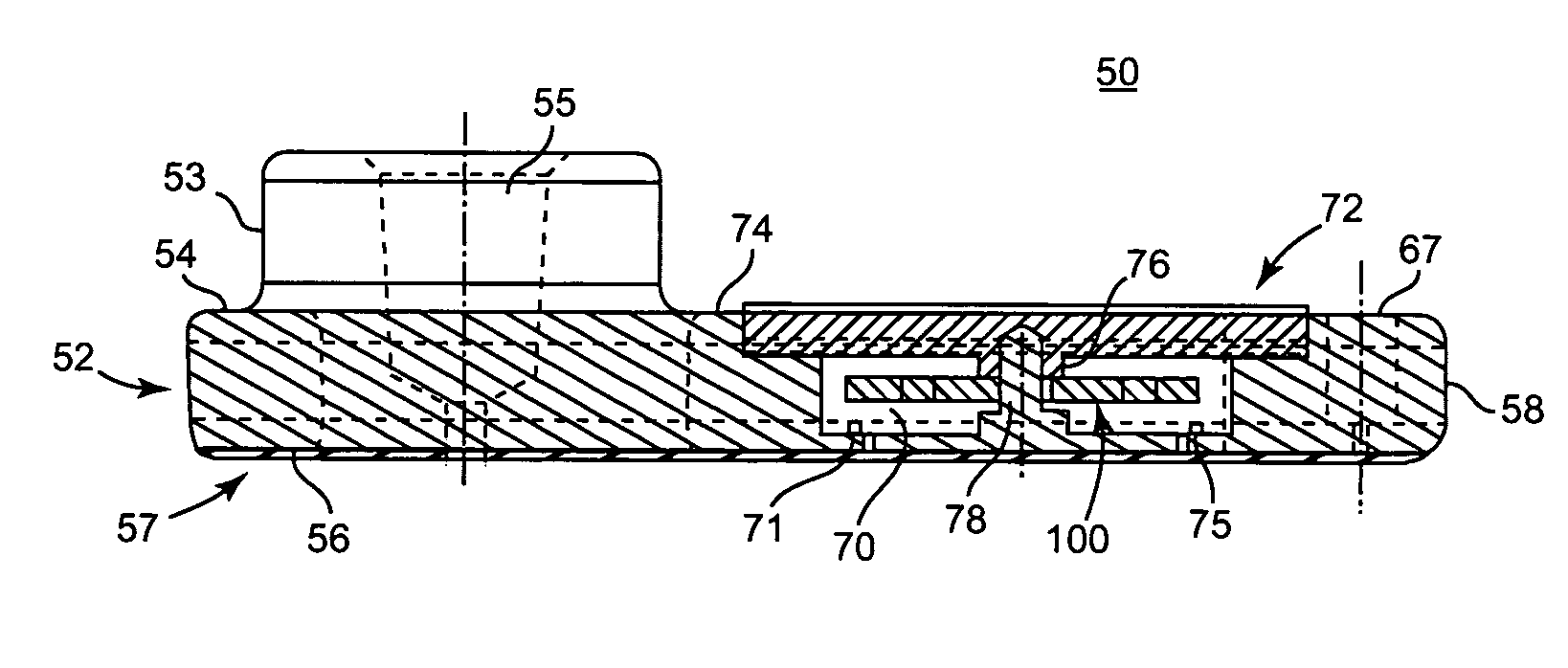
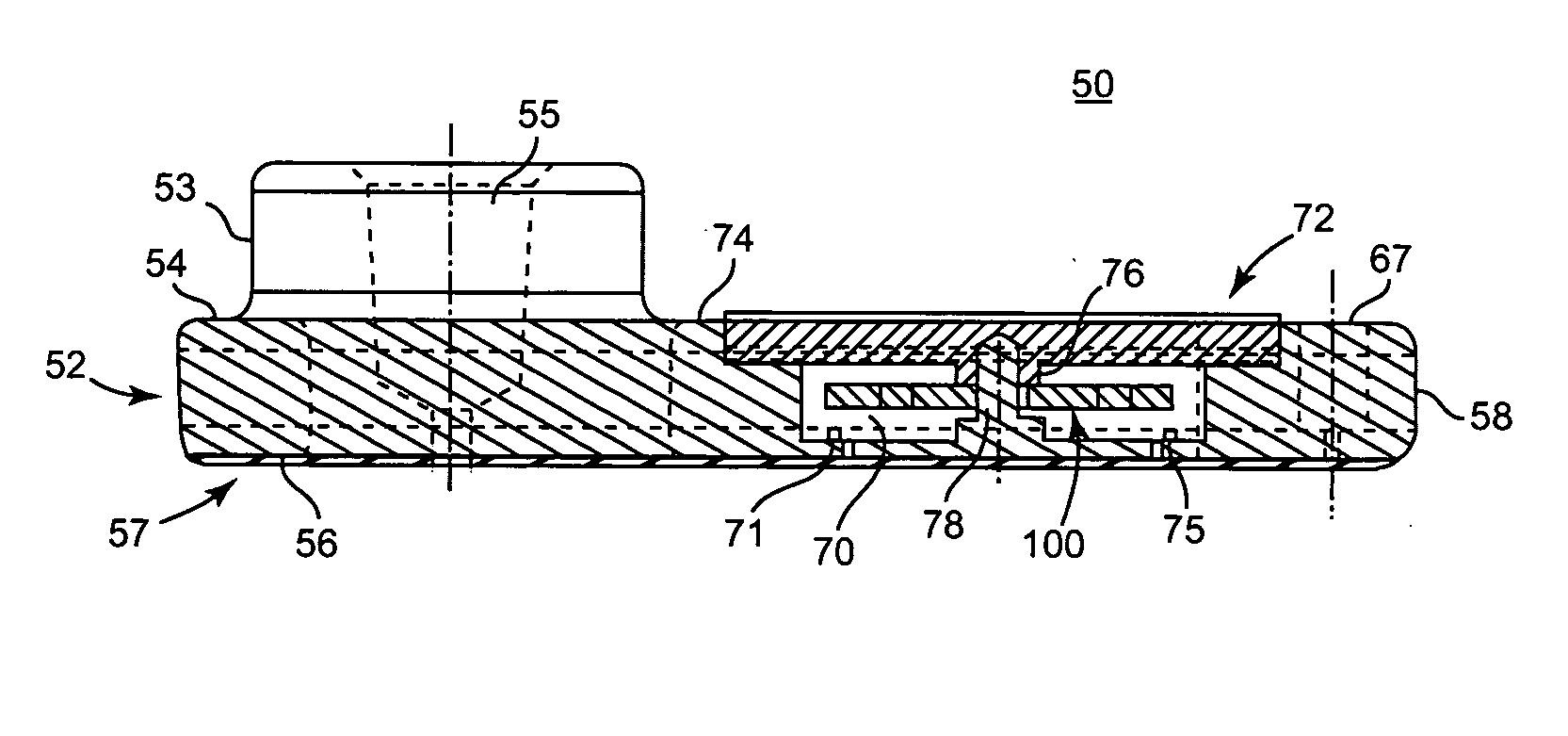
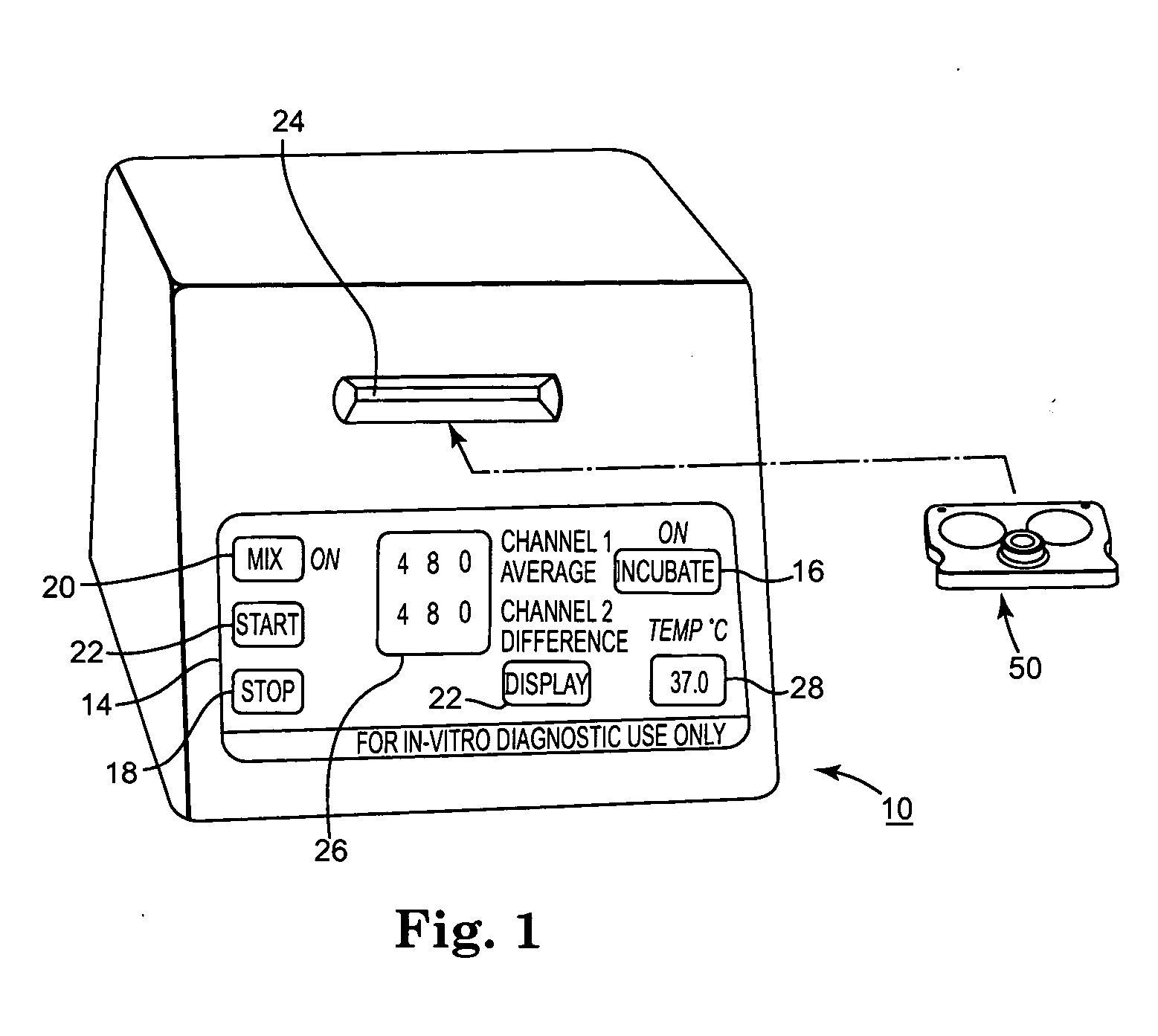
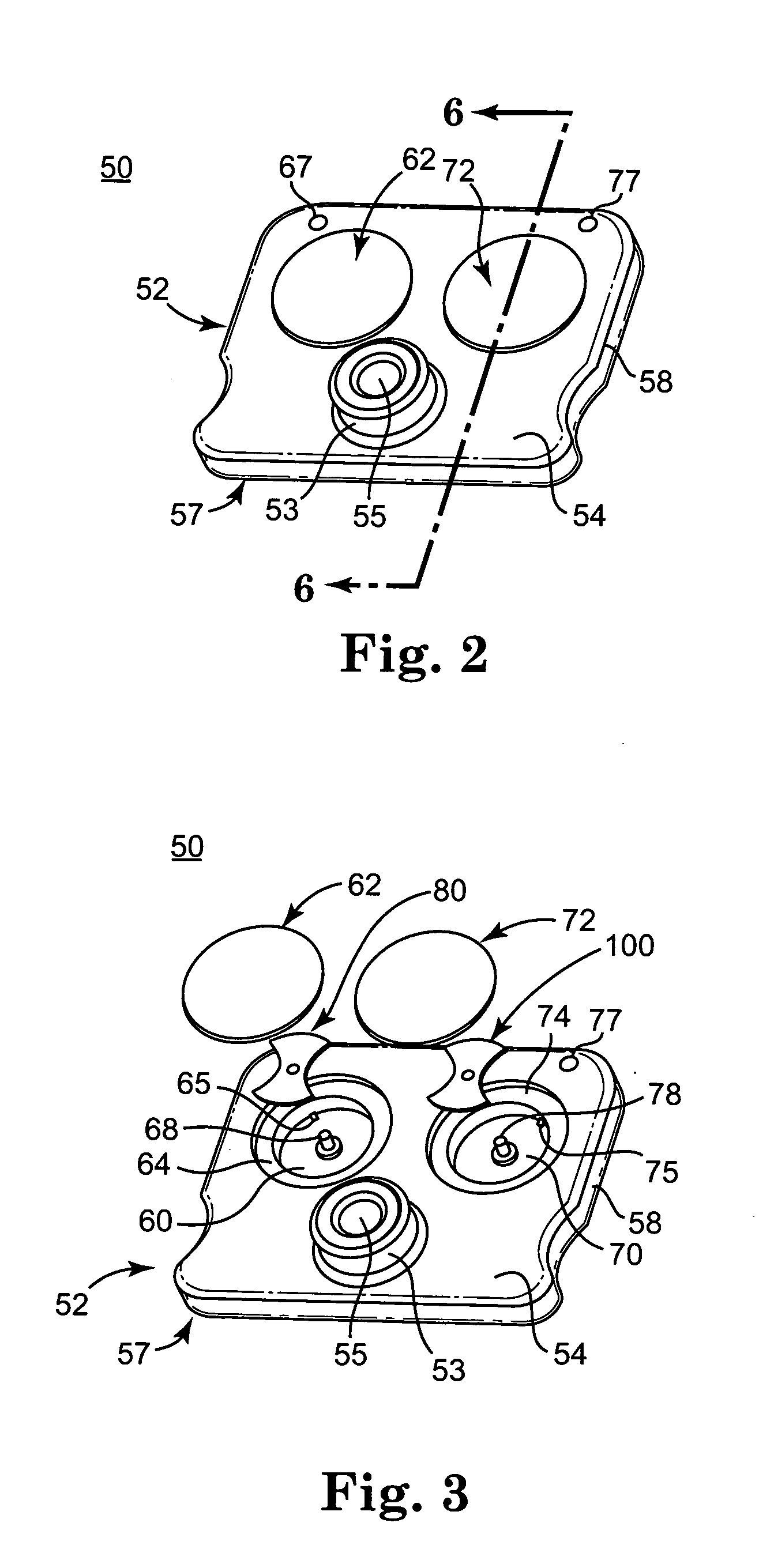
Blood coagulation test cartridge, system, and method
ActiveUS7399637B2Practical and convenientRapid and reliableAnalysis using chemical indicatorsMicrobiological testing/measurementBlood testTest sample
A system and method for determining a coagulation time, e.g., TT, PT, aPTT, and ACT, of a blood test sample deposited in a test cartridge is disclosed. A cartridge housing having upper and lower major sides and a minor sidewall encloses a test chamber having a test chamber pivot element and is provided with a cartridge port for introducing a test sample into the test chamber,. Ferromagnetic agitator vane leaflets extend from an agitator pivot element supported by the test chamber pivot element intermediate the upper and lower major sides for rotational motion. The agitator vane leaflets can be swept, in response to an external magnetic field, through the test sample in the absence of coagulation. A timer is started when the agitator movement is commenced whereupon the agitator moves freely. Resistance to agitator movement due to coagulation is detected, and the coagulation time is measured.
Owner:MEDTRONIC INC
Device for performing a blood, cell, and/or pathogen count and methods for use thereof
ActiveUS20130273524A1Rapid and accurate and affordable laboratory-quality blood testingEliminate replacementBioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careBlood test
Devices and methods for performing a point of care blood, cell, and / or pathogen count or a similar blood test. Disclosed herein are systems that can be used to provide rapid, accurate, affordable laboratory-quality testing at the point of care. The systems described herein are capable of imaging and counting individual cells in a prepared cell sample (e.g., a peripheral blood smear or a blood sample prepared in a microfluidic device) or another prepared cell-containing sample without the need for a microscope or other expensive and cumbersome optics. The systems described herein are designed to eliminate or replace expensive, centralized clinical testing equipment and technical personnel. Such systems may include automated data reporting and decision support.
Owner:I CALQ
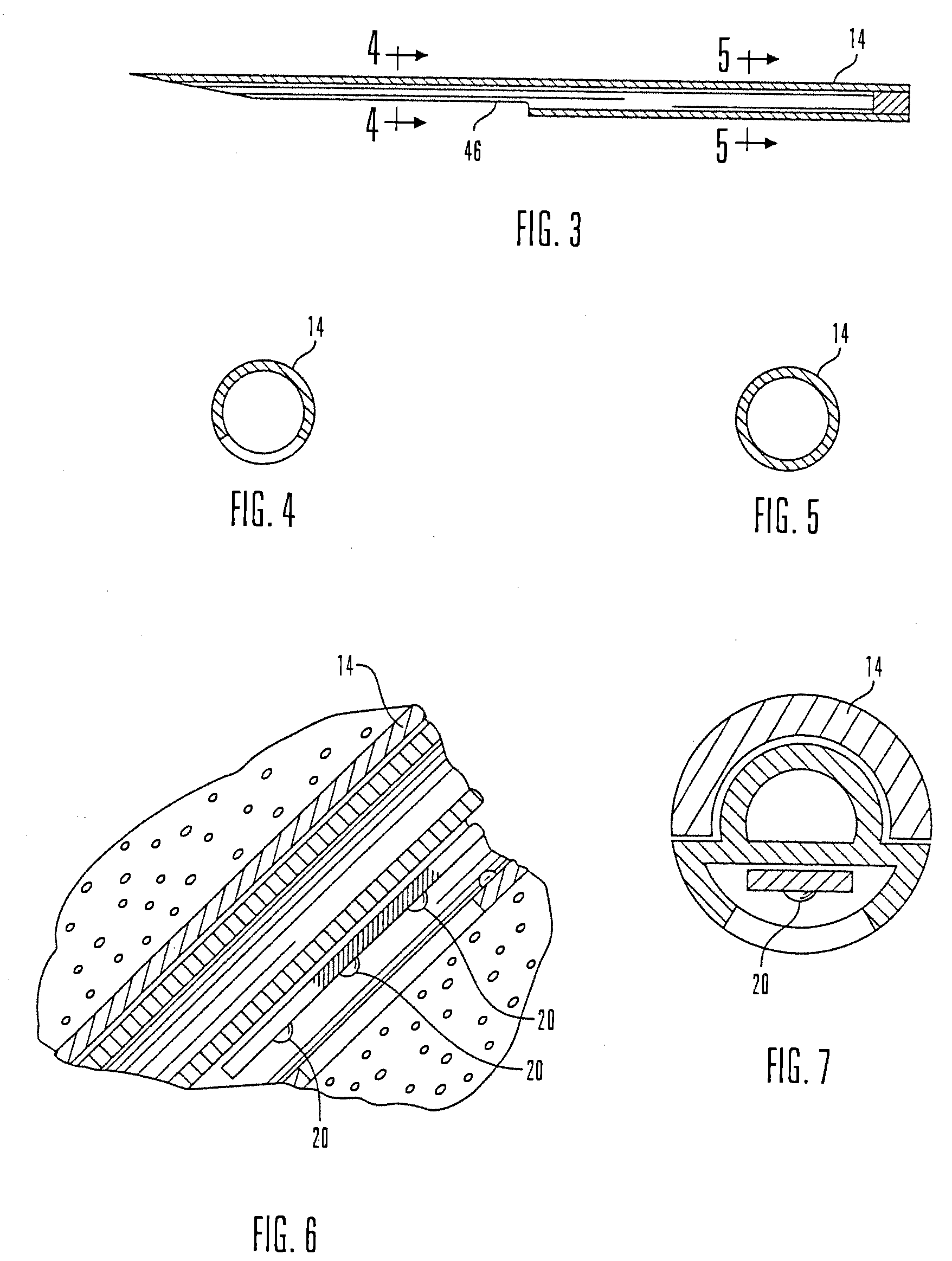
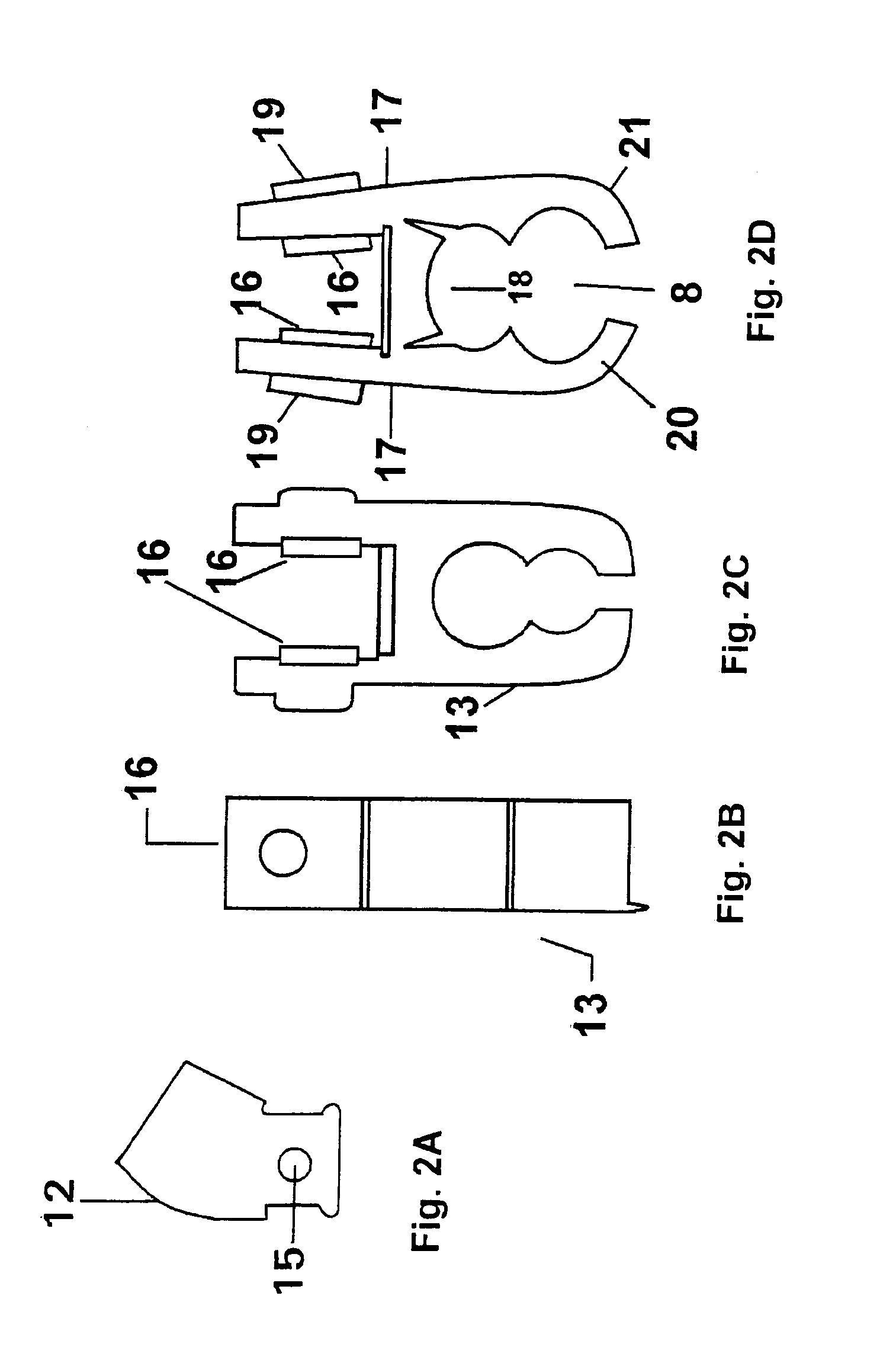
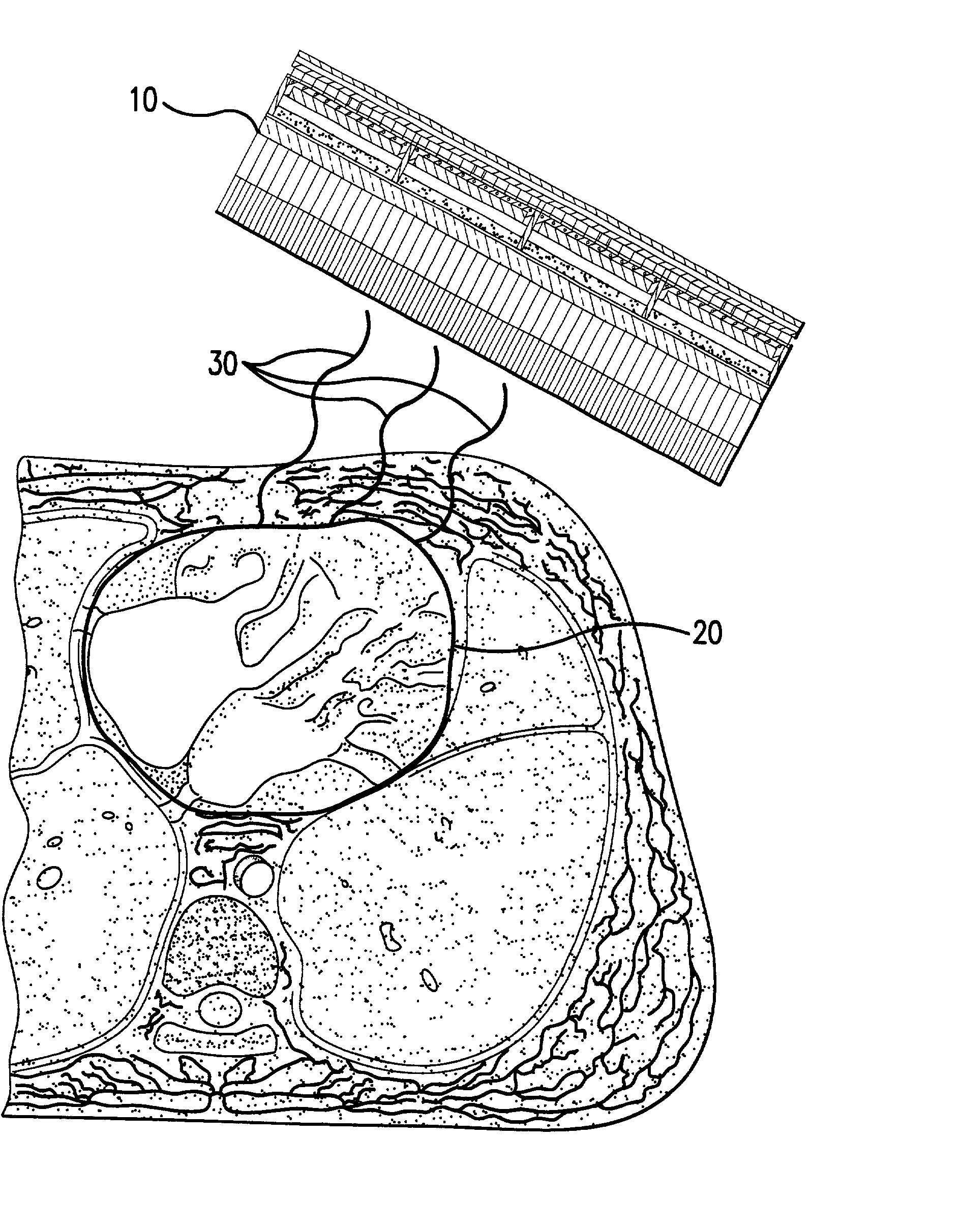
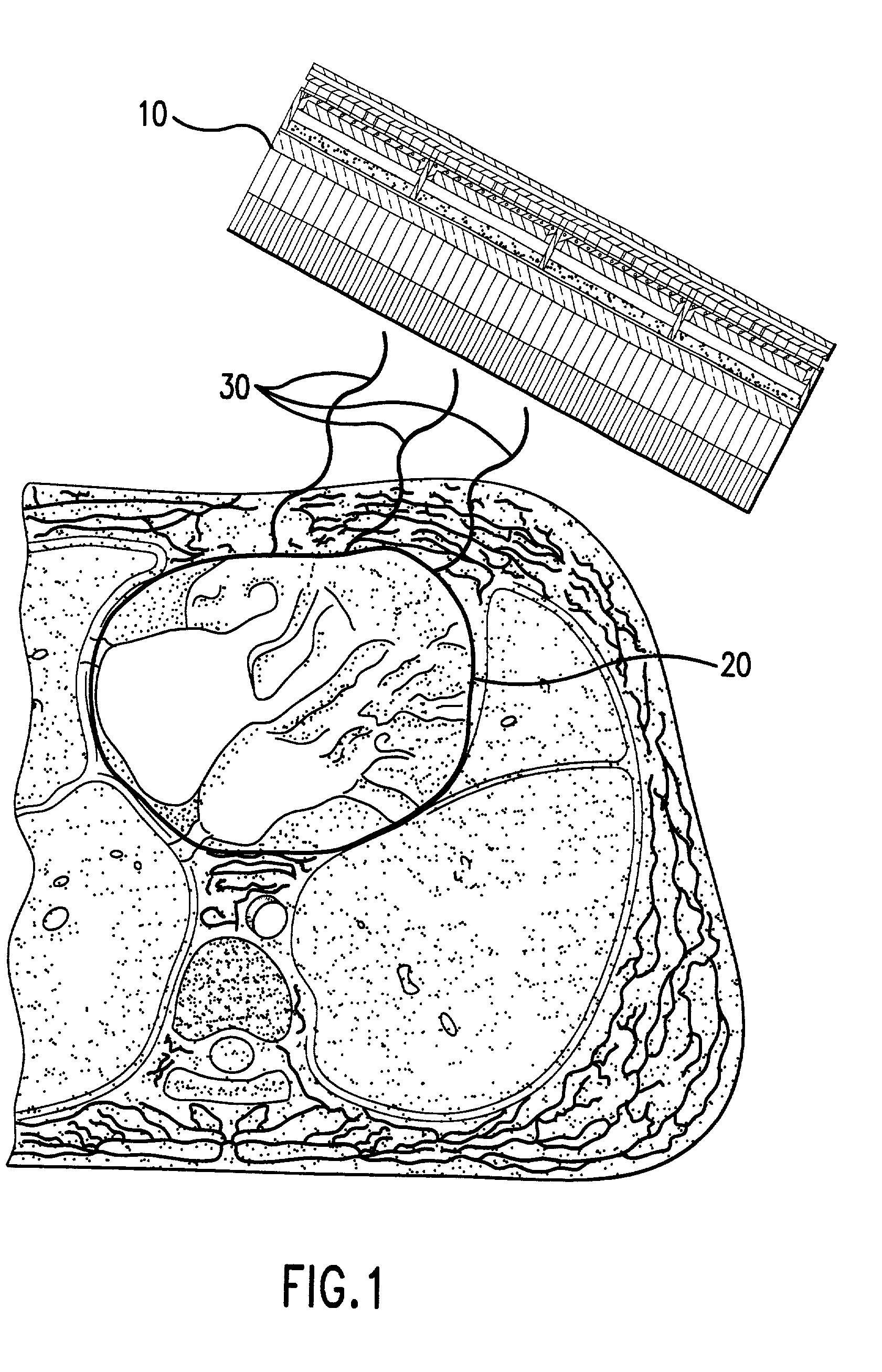
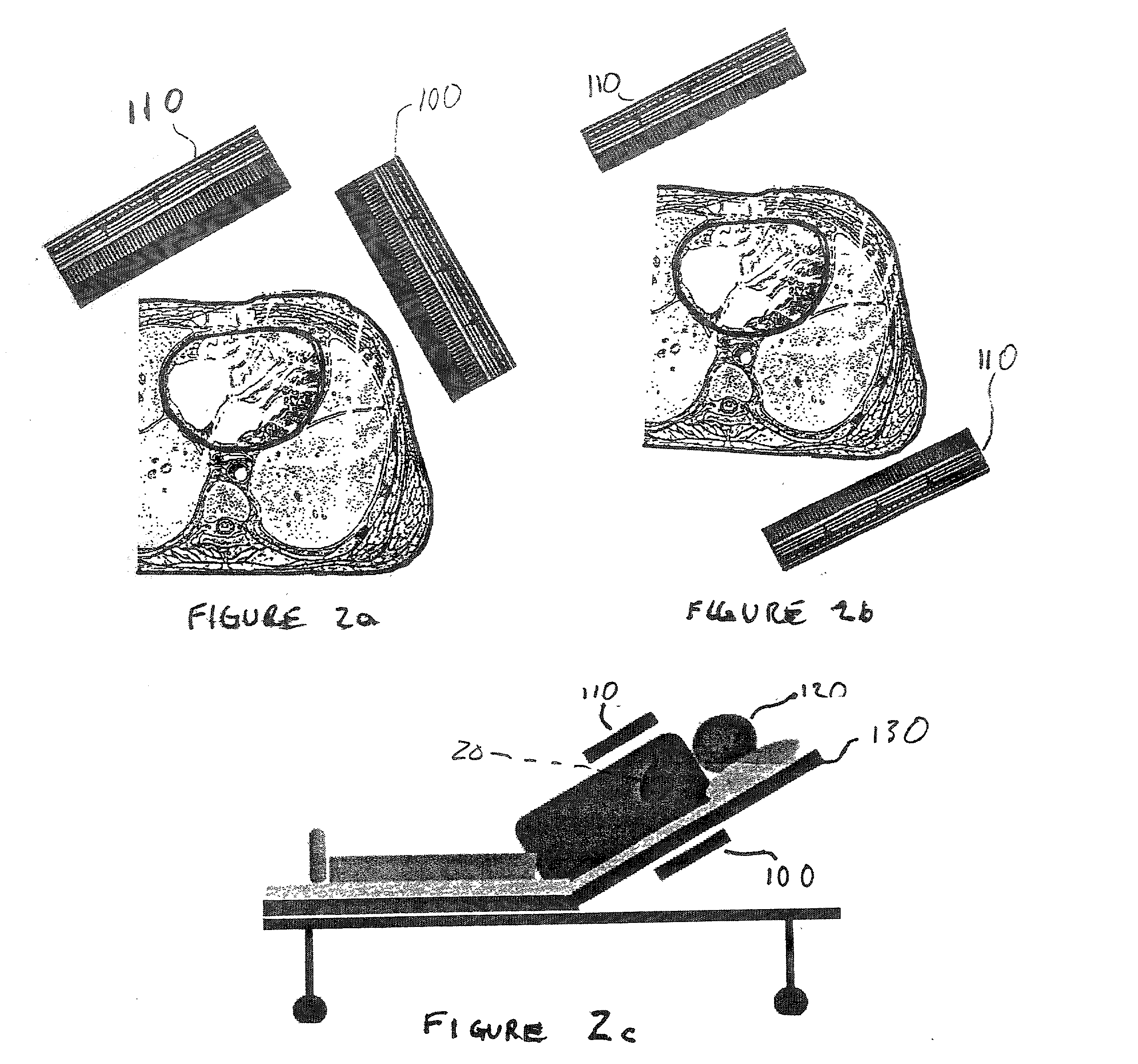
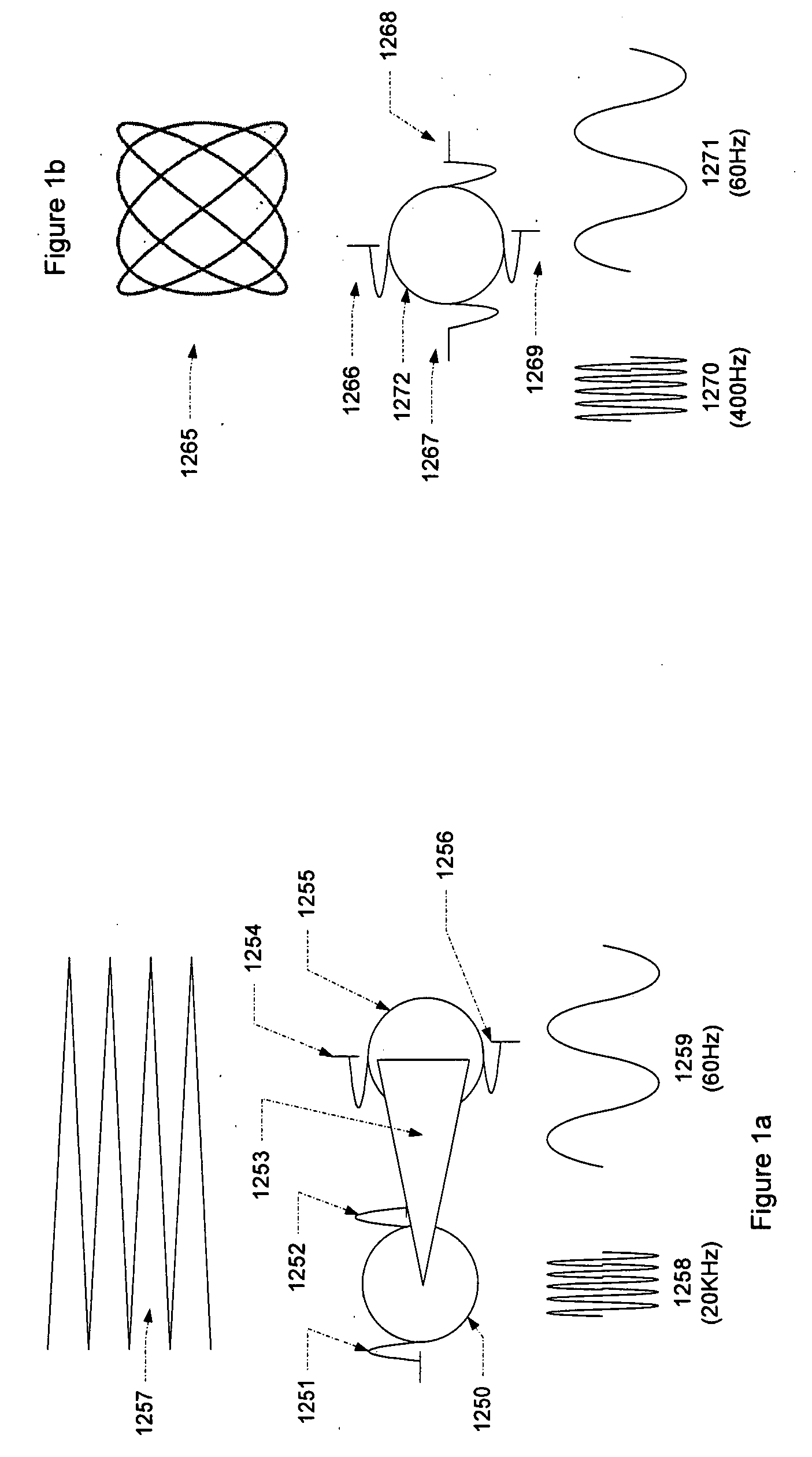
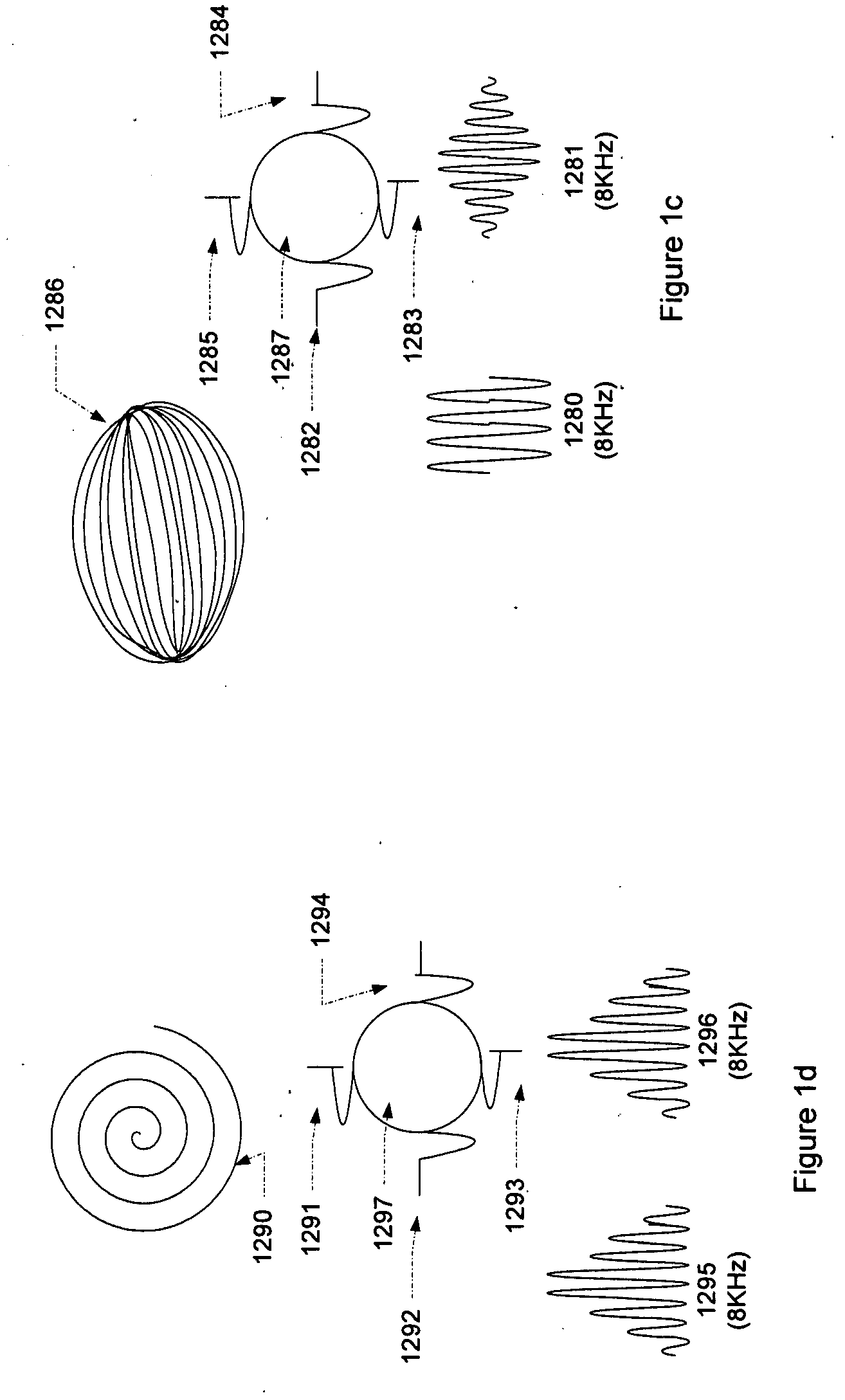
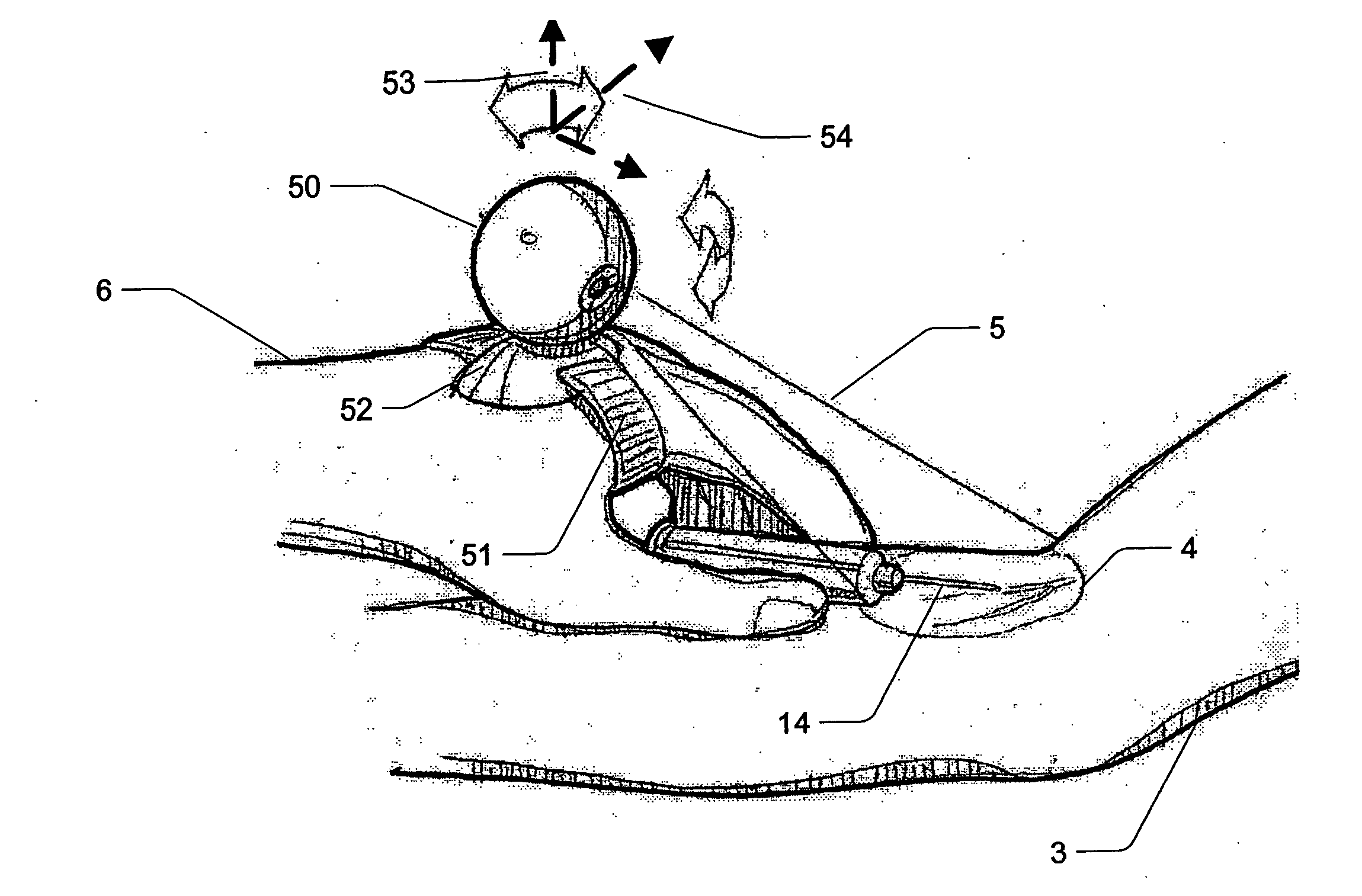
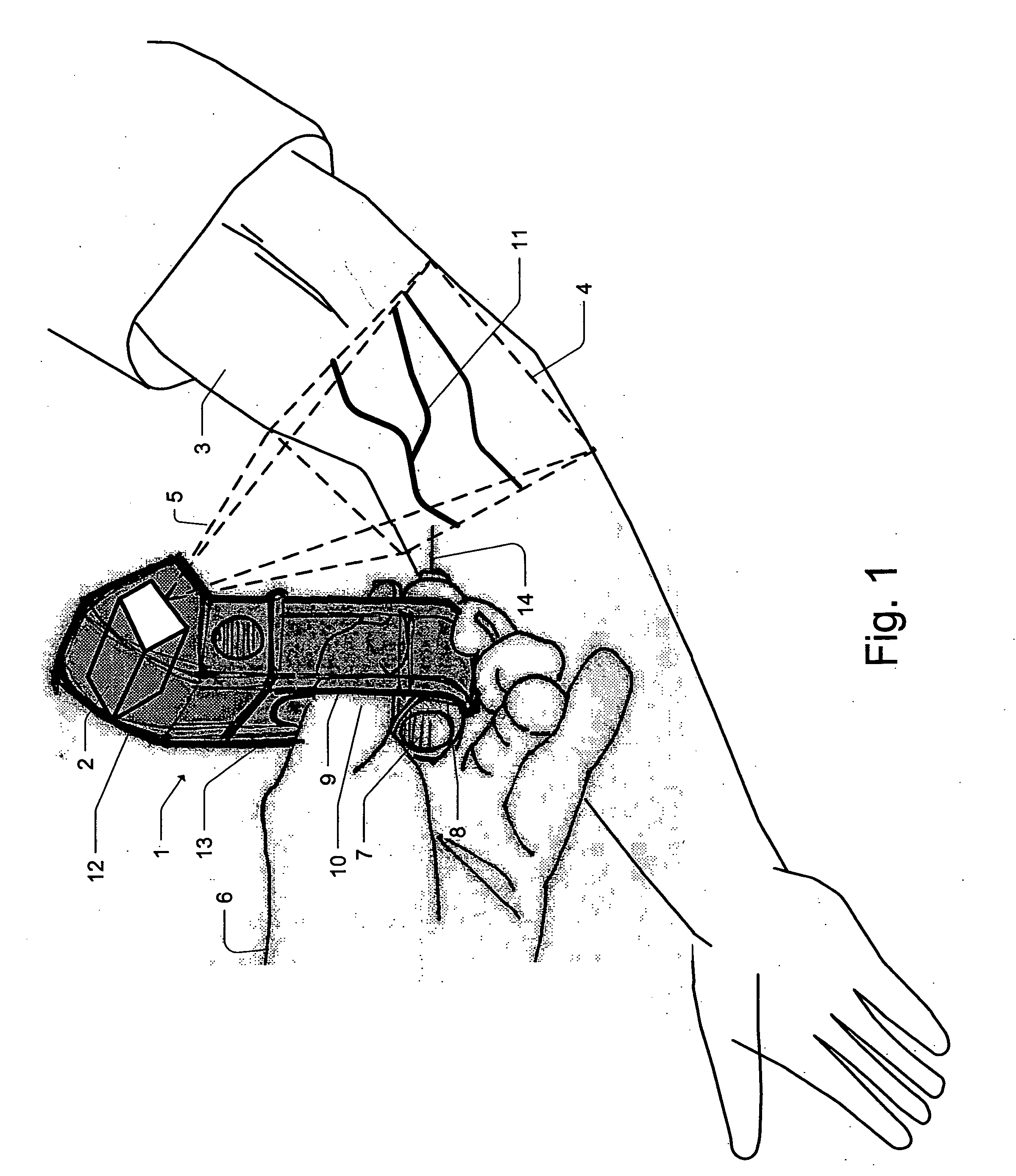
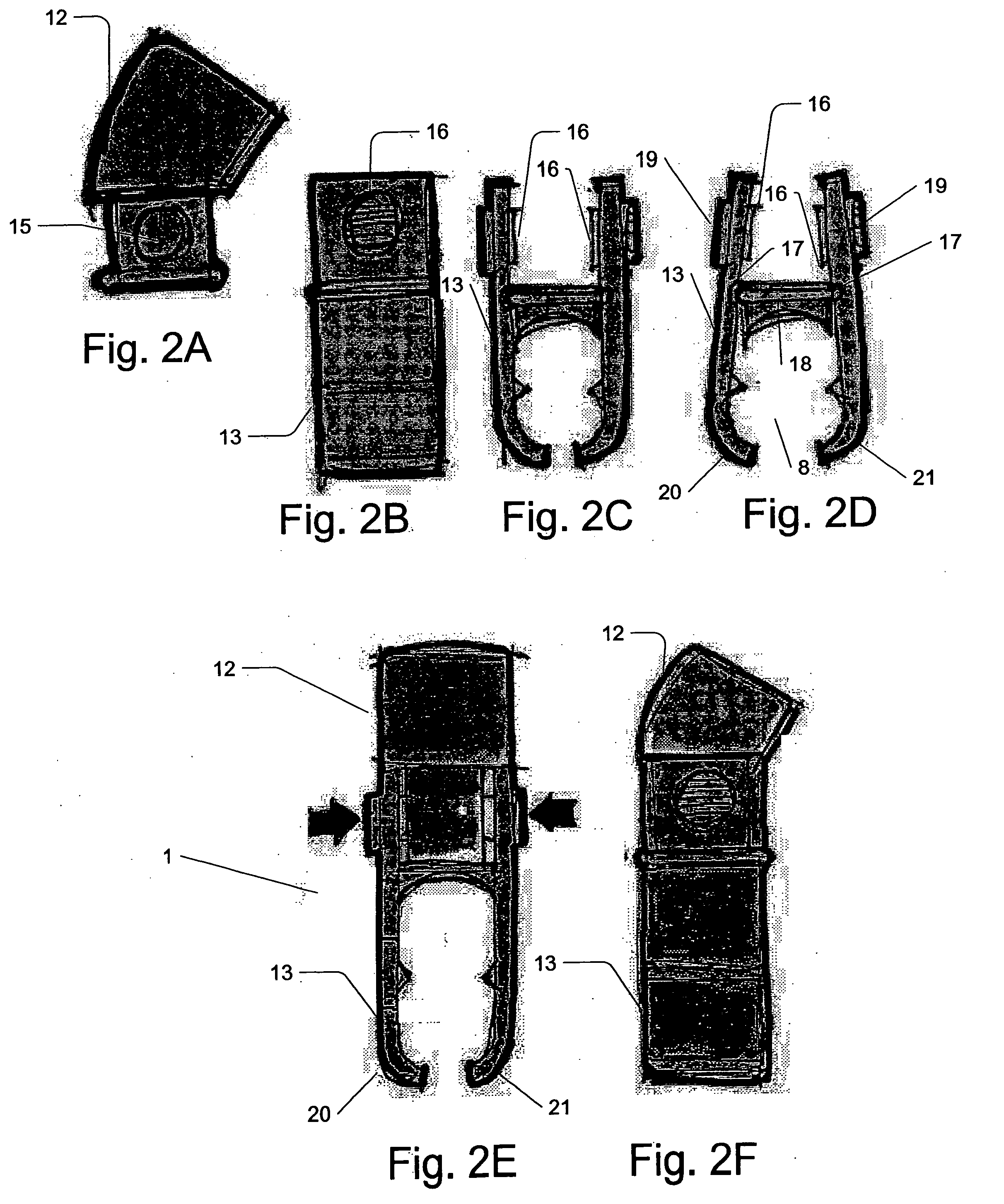
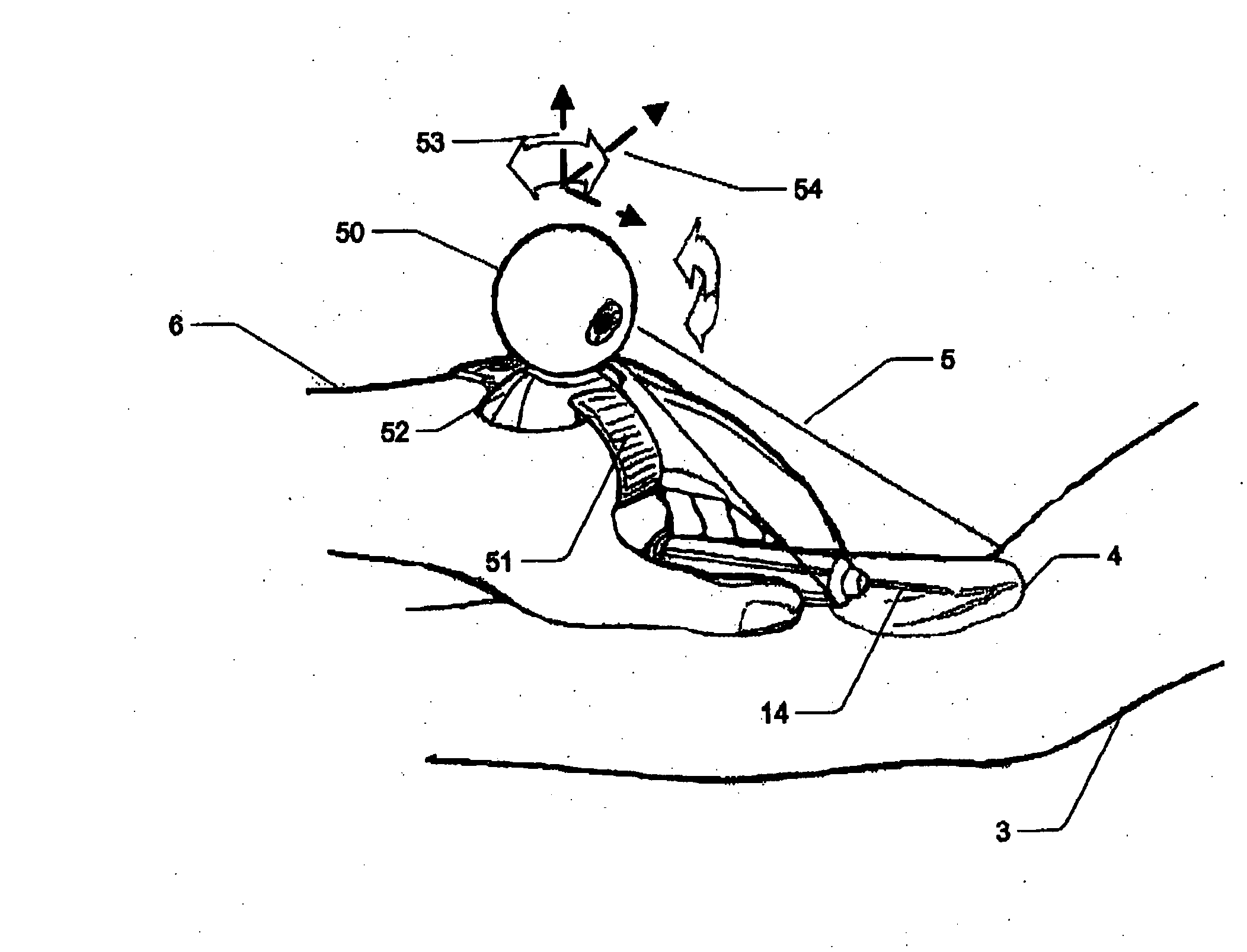
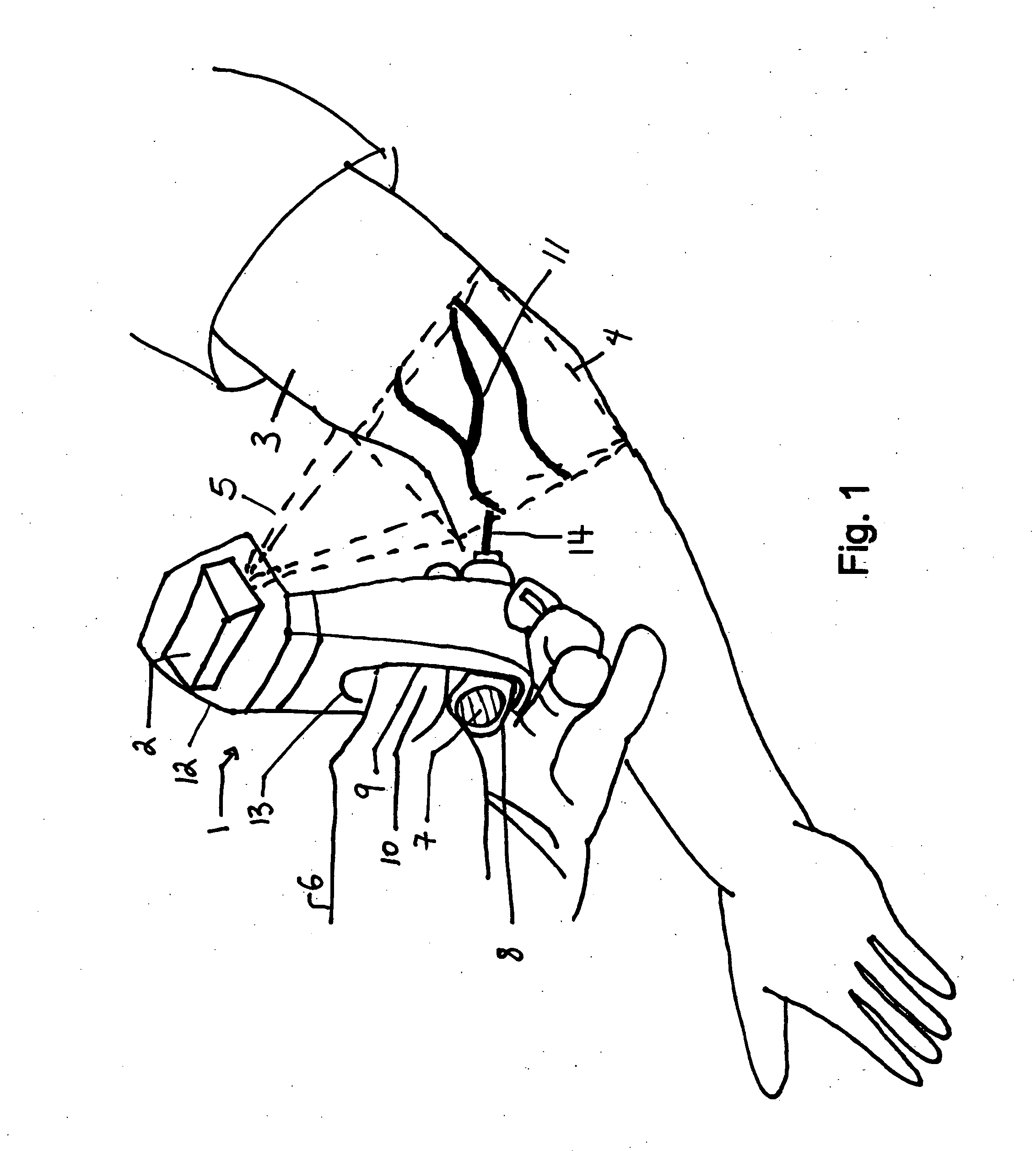
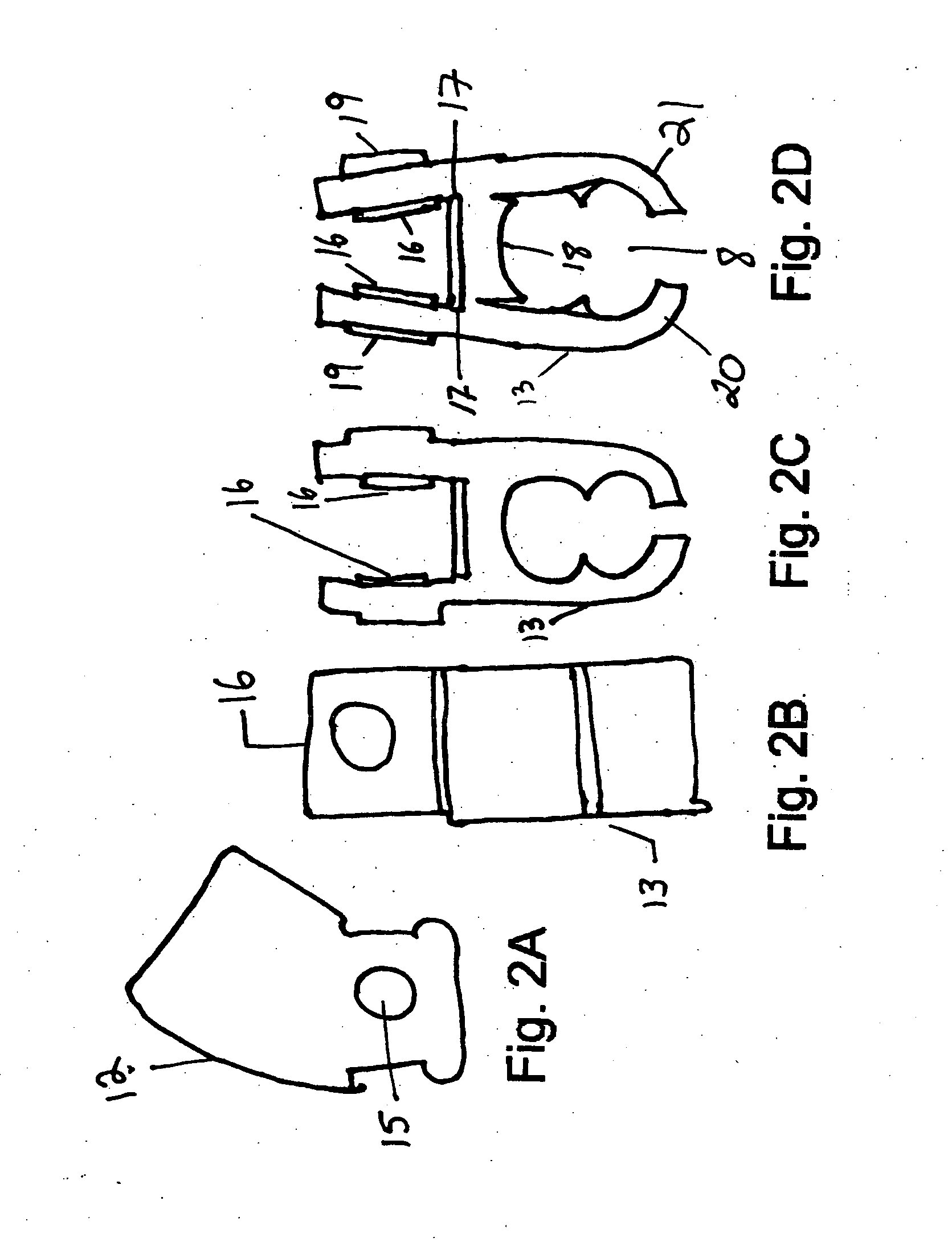
Micro vein enhancer
ActiveUS7904138B2Reduce impactExpand the scope of workImage analysisDiagnostics using lightVeinBlood test
The present invention is a Miniature Vein Enhancer that includes a Miniature Projection Head. The Miniature Projection Head may be operated in one of three modes, AFM, DBM, and RTM. The Miniature Projection Head of the present invention projects an image of the veins of a patient, which aids the practitioner in pinpointing a vein for an intravenous drip, blood test, and the like. The Miniature projection head may have a cavity for a power source or it may have a power source located in a body portion of the Miniature Vein Enhancer. The Miniature Vein Enhancer may be attached to one of several improved needle protectors, or the Miniature Vein Enhancer may be attached to a body similar to a flashlight for hand held use. The Miniature Vein Enhancer of the present invention may also be attached to a magnifying glass, a flat panel display, and the like.
Owner:ACCUVEIN
Cardiovascular imaging and functional analysis system
InactiveUS20020188197A1Handling using diaphragms/collimetersMaterial analysis by optical meansRadioactive tracerNon invasive
A Cardiovascular imaging and functional analysis system and method employing a dedicated fast, sensitive, compact and economical imaging gamma camera system that is especially suited for heart imaging and functional analysis. The system uses a dedicated nuclear cardiology small field of view imaging camera, allowing image physiology, while offering inexpensive and portable hardware. In some variations, a basic modular design suitable for cardiac imaging with one of several radionucleide tracers is used. The detector is positioned in close proximity to the chest and heart from several different projections, allowing rapid accumulation of data for first-pass analysis, positron imaging, quantitative stress perfusion, and multi-gated equilibrium pooled blood tests. In one variation, a Cardiovascular Non-Invasive Screening Probe system provides rapid, inexpensive preliminary indication of coronary occlusive disease by measuring the activity of emitted particles from an injected bolus of radioactive tracer.
Owner:NORTH COAST IND INC
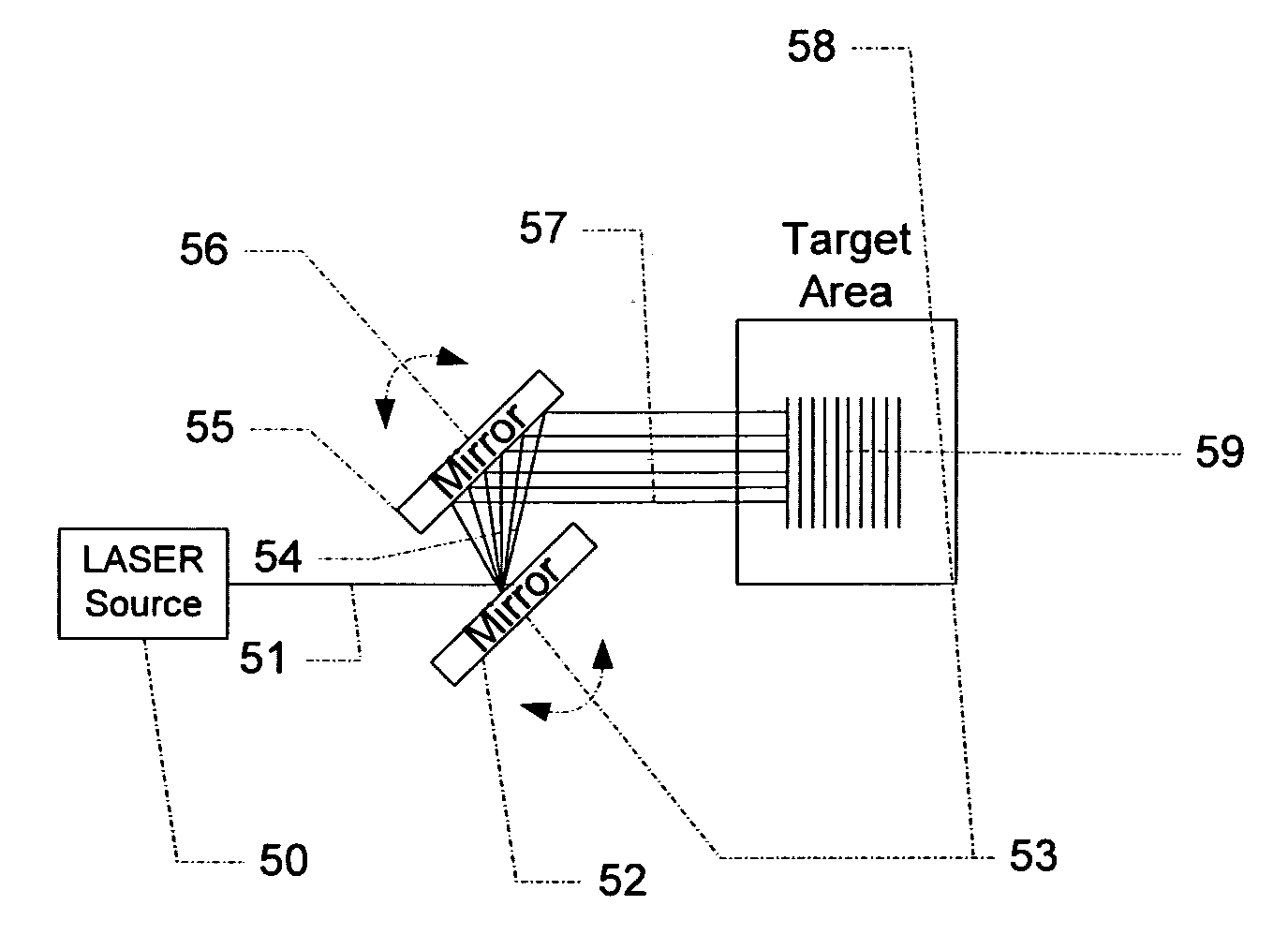
Scanned laser vein contrast enhancer
Owner:ACCUVEIN
Blood test prototypes and methods for the detection of circulating tumor and endothelial cells
InactiveUS20050244843A1Enhance anti-tumor immune responseDramatic utilityBioreactor/fermenter combinationsBiological substance pretreatmentsAbnormal tissue growthProgenitor
Methods and devices for isolating and diagnosing disease with a cell adhesion matrix system, mimicking a metastatic, cardiovascular or placental environment, are disclosed. The cell adhesion matrix facilitates the enrichment of target cells such as metastatic tumor cells, fetal cells and endothelial progenitor cells from a fluid sample such as blood for diagnostic and therapeutic applications in treating patients afflicted with disease, such as cancerous, cardiovascular and fetal diseases, as well as for research applications in molecular analysis of metastatic, and cardiovascular and fetal diseases. Blood test prototypes and methods for the cell enrichment and detection of circulating tumor and endothelial cells using multiplex molecular analysis are described herein. In addition, methods and compositions for determining host immunity to tumor in subjects with risk of cancer progression and methods for isolating an enriched fraction of fetal cells from pregnant females for prenatal diagnosis are also described herein.
Owner:CHEN WEN TIEN +2
Blood test to monitor the genetic changes of progressive cancer using immunomagnetic enrichment and fluorescence in situ hybridization (FISH)
InactiveUS20080113350A1Accurate measurementEasy accessMicrobiological testing/measurementLymphatic SpreadGenetic Change
Amplification and overexpression of theHER-2 oncogene in breast cancer is felt to be stable over the course of disease and concordant between the primary tumor and metastases. Therefore, patients with HER-2 negative primary tumors will rarely receive anti-HER-2 antibody therapy. A very sensitive blood test is used to capture circulating tumor cells (CTC's) and evaluate their HER-2 gene status by FISH evaluation. The HER-2 status of the primary tumor and corresponding CTC's is used to assess the ratio of CTC's as a reliable surrogate marker. HER-2 expression of 10 CTC's is sufficient to make a definitive diagnosis of the HER-2 gene status for the whole population of CTC's in patients with recurrent breast cancer.
Owner:JANSSEN DIAGNOSTICS LLC
Blood coagulation test cartridge, system, and method
ActiveUS20050233466A1Practical and convenientRapid and reliableAnalysis using chemical indicatorsFlow propertiesBlood testTest sample
A system and method for determining a coagulation time, e.g., TT, PT, aPTT, and ACT, of a blood test sample deposited in a test cartridge is disclosed. A cartridge housing having upper and lower major sides and a minor sidewall encloses a test chamber having a test chamber pivot element and is provided with a cartridge port for introducing a test sample into the test chamber,. Ferromagnetic agitator vane leaflets extend from an agitator pivot element supported by the test chamber pivot element intermediate the upper and lower major sides for rotational motion. The agitator vane leaflets can be swept, in response to an external magnetic field, through the test sample in the absence of coagulation. A timer is started when the agitator movement is commenced whereupon the agitator moves freely. Resistance to agitator movement due to coagulation is detected, and the coagulation time is measured.
Owner:MEDTRONIC INC
Scanned laser vein contrast enhancer
The present invention is a Miniature Vein Enhancer that includes a Miniature Projection Head. The Miniature Projection Head may be operated in one of three modes, AFM, DBM, and RTM. The Miniature Projection Head of the present invention projects an image of the veins of a patient, which aids the practitioner in pinpointing a vein for an intravenous drip, blood test, and the like. The Miniature projection head may have a cavity for a power source or it may have a power source located in a body portion of the Miniature Vein Enhancer. The Miniature Vein Enhancer may be attached to one of several improved needle protectors, or the Miniature Vein Enhancer may be attached to a body similar to a flashlight for hand held use. The Miniature Vein Enhancer of the present invention may also be attached to a magnifying glass, a flat panel display, and the like.
Owner:ACCUVEIN
Scanned laser vein contrast enhancer
Owner:ACCUVEIN
Method of accelerating blood coagulation using an antimicrobial metal
InactiveUS6495367B1Prolong lifeExtended shelf lifeMicrobiological testing/measurementBiological testingBlood componentBlood test
Owner:SEKISUI CHEM CO LTD
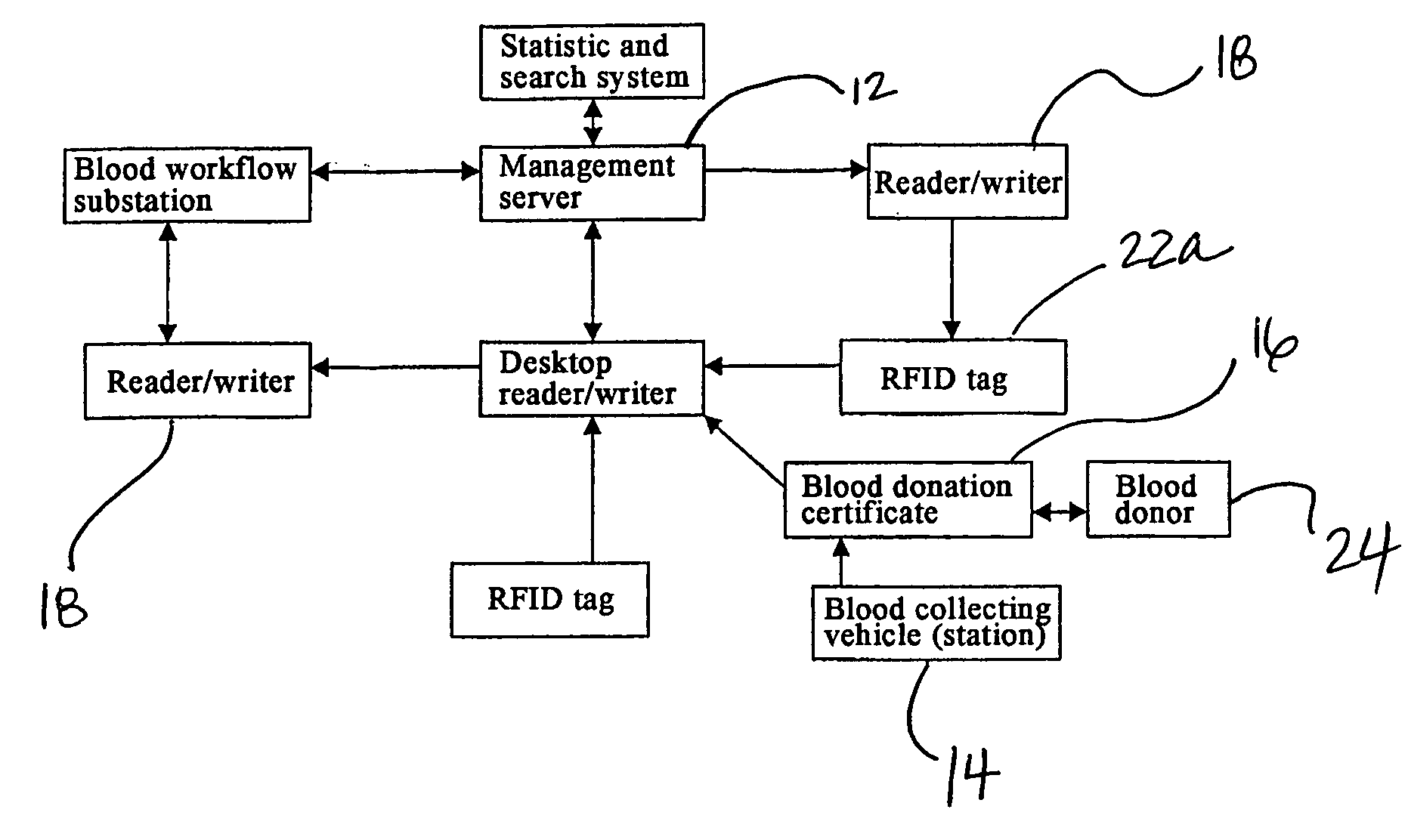
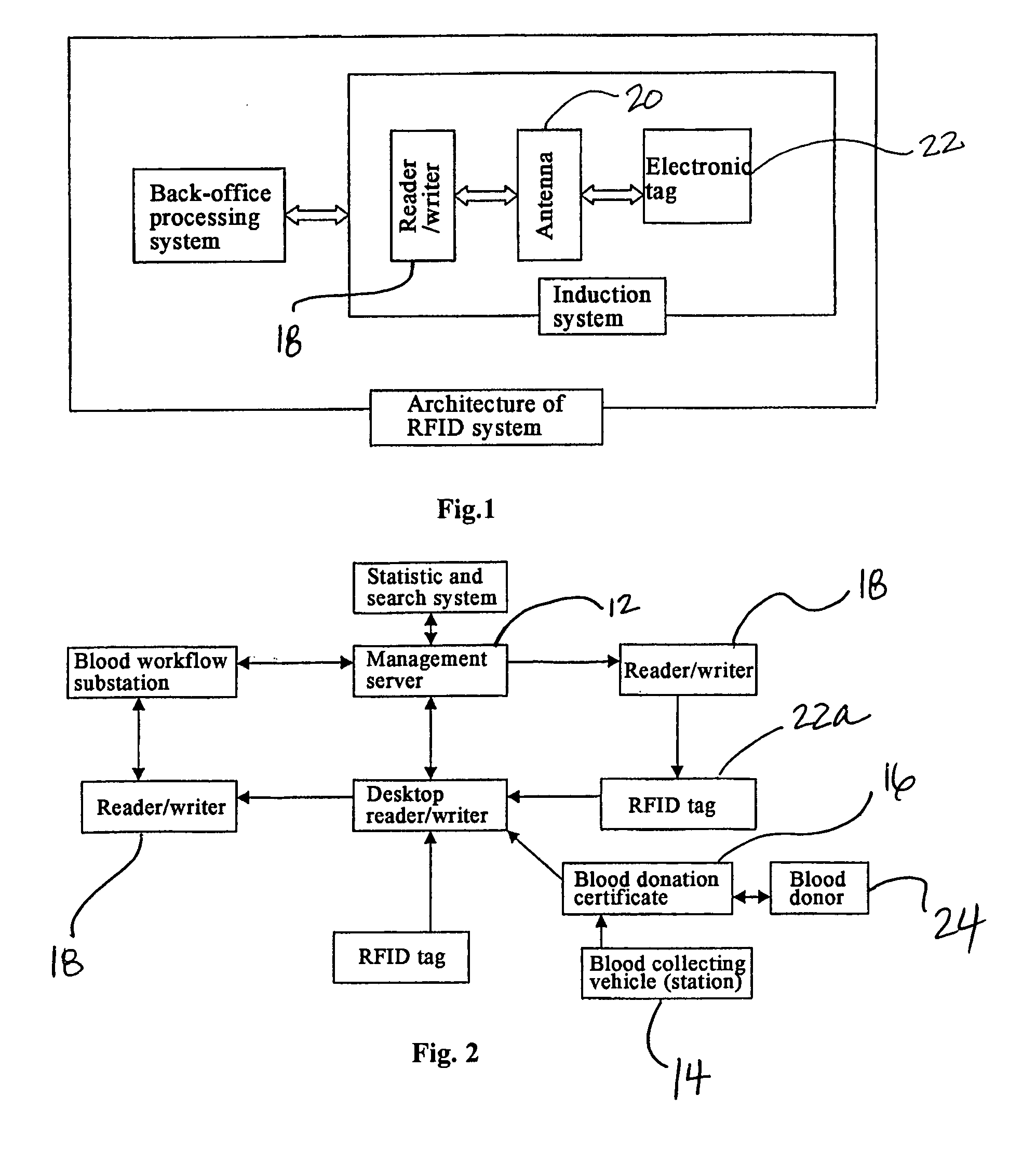
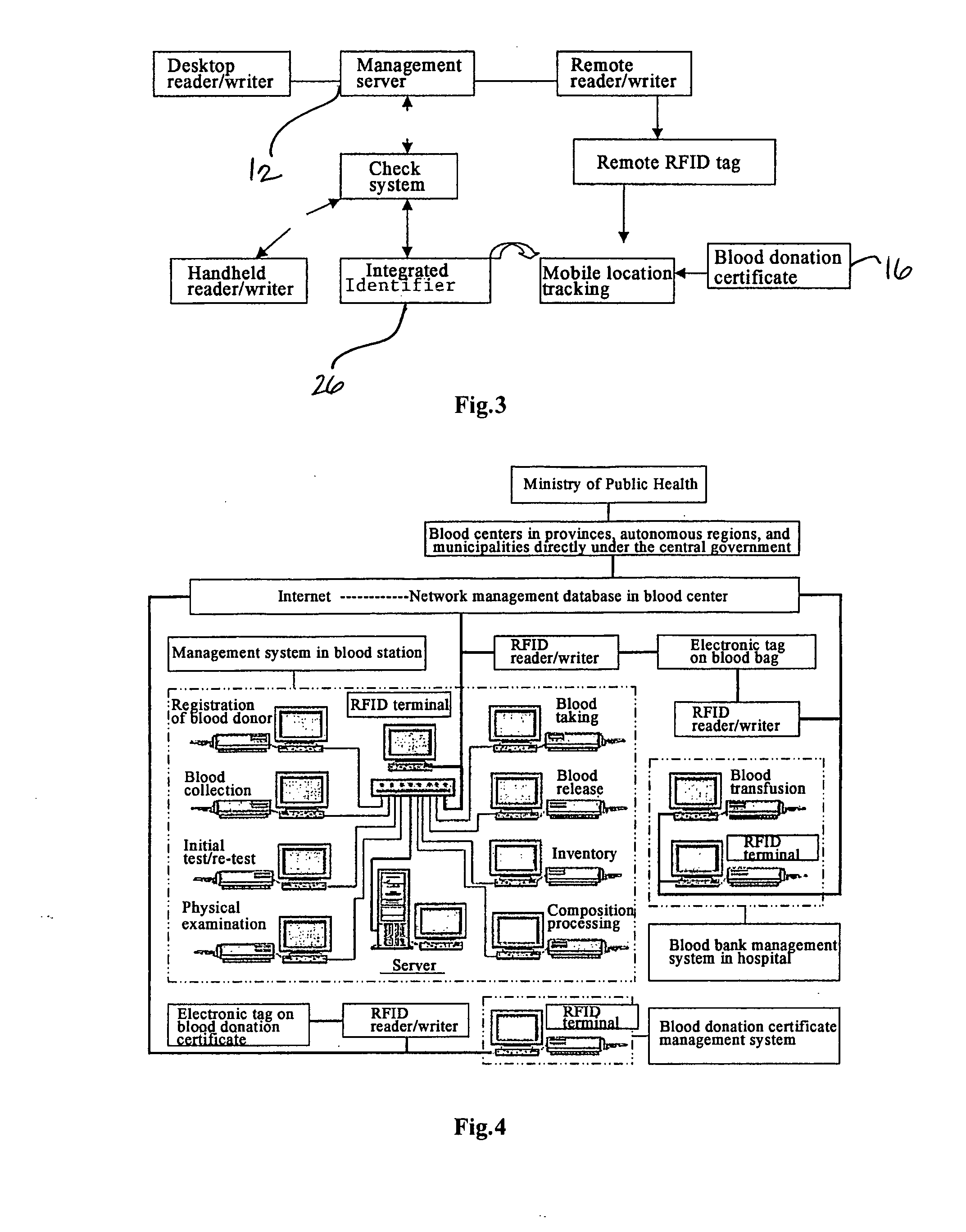
Method and System of Using Rfid in the Workflow of Blood Center
InactiveUS20080208750A1Improve abilitiesReduce probabilityData processing applicationsComputer-assisted medical data acquisitionCustomer relationship managementBlood center
A method and a system for using RFID (Radio Frequency Identification) in the workflow of a blood center and a medical institution from a network Customer Relationship Management (CRM) information system. The CRM comprises an RFID technique system, a computer database system, and a computer information network. The information transferred between an integrated circuit and a reader-writer occurs through the radio waves in the workflow of, for example, a blood center or other medical institution. The related information ranges from identity information to biological product information. In each procedure of the blood collecting and supply workflow, the information is read / written by the computer into electronic tag and through the computer information network into the service management information system. The work flow of blood centers (stations), such as blood test reports before and after blood transfusions and the desired information of people and objects involved in blood transfusions, can thereby be recorded completely, integrally, precisely, and comprehensively by the RFID system.
Owner:CHEN YAN
Micro vein enhancer
The present invention is a Miniature Vein Enhancer that includes a Miniature Projection Head. The Miniature Projection Head may be operated in one of three modes, AFM, DBM, and RTM. The Miniature Projection Head of the present invention projects an image of the veins of a patient, which aids the practitioner in pinpointing a vein for an intravenous drip, blood test, and the like. The Miniature projection head may have a cavity for a power source or it may have a power source located in a body portion of the Miniature Vein Enhancer. The Miniature Vein Enhancer may be attached to one of several improved needle protectors, or the Miniature Vein Enhancer may be attached to a body similar to a flashlight for hand held use. The Miniature Vein Enhancer of the present invention may also be attached to a magnifying glass, a flat panel display, and the like.
Owner:ACCUVEIN
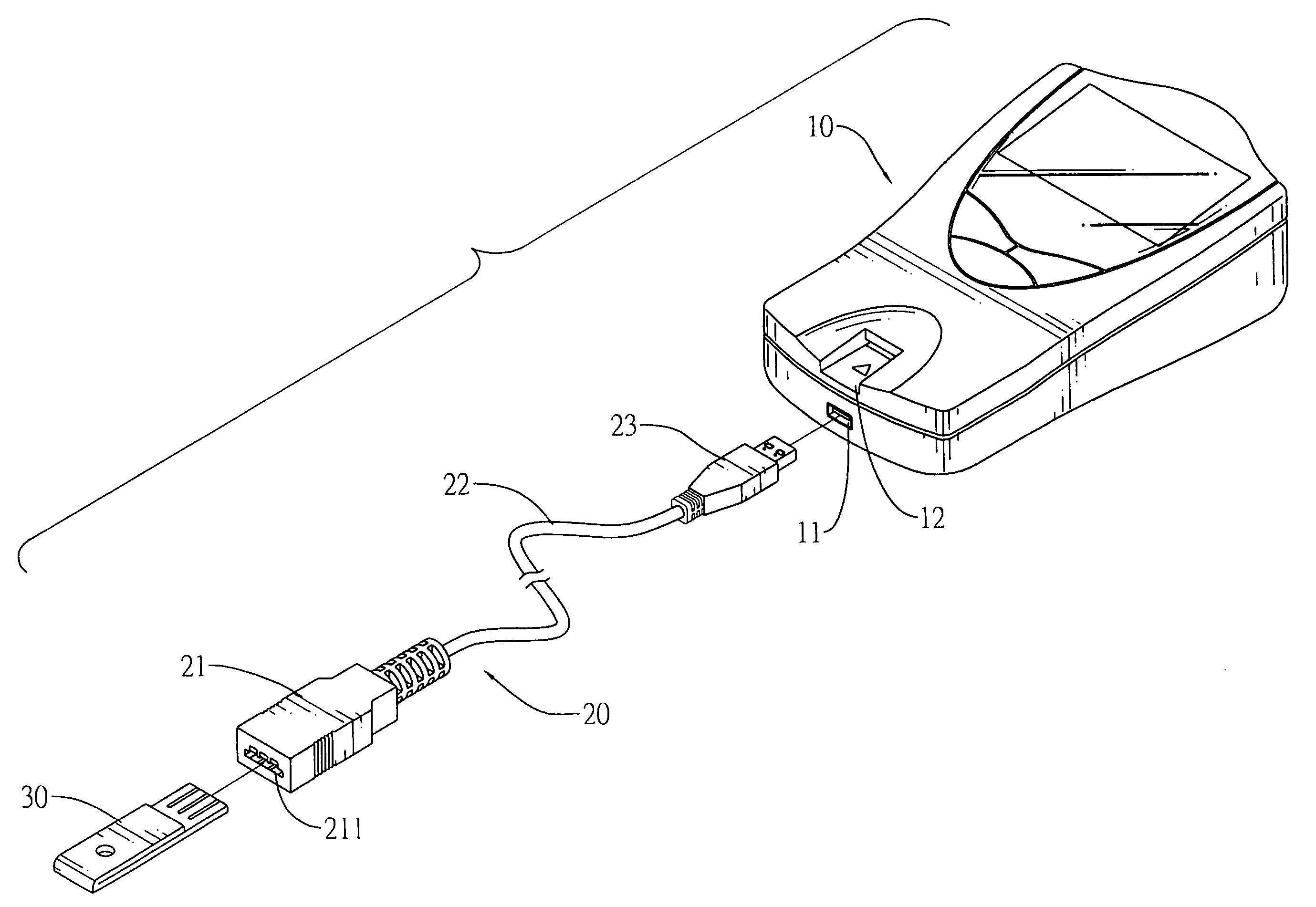
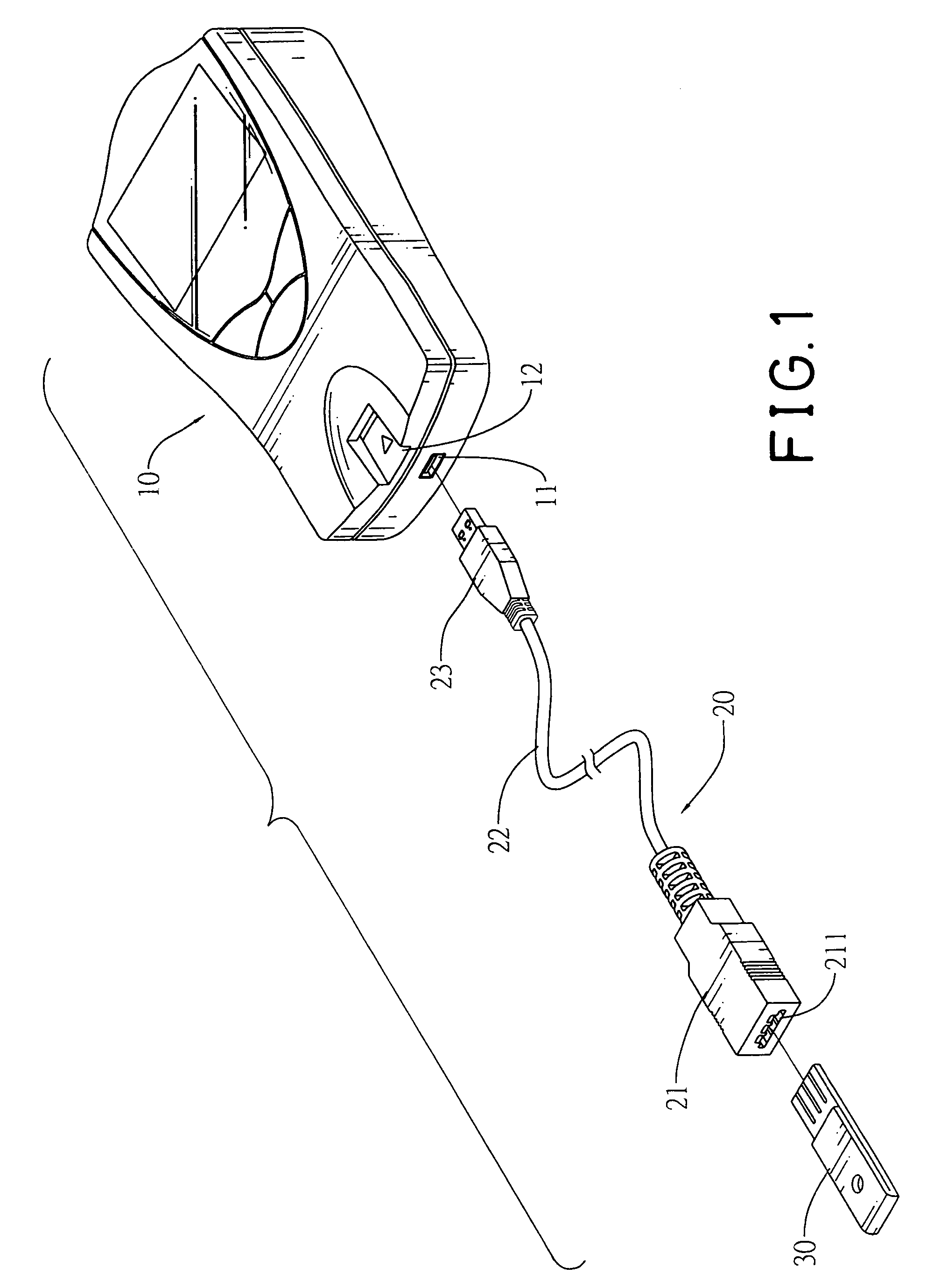
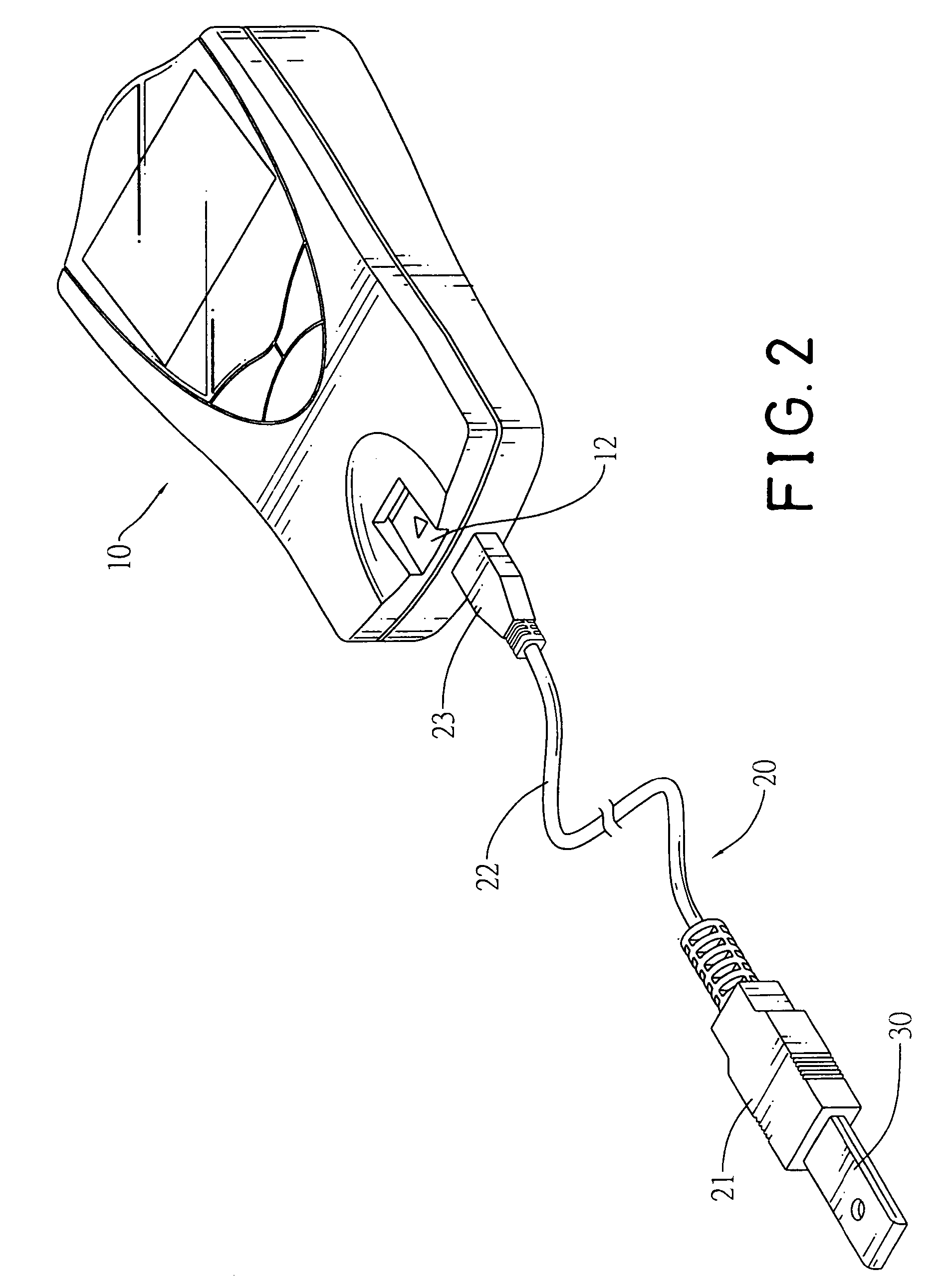
Connector to receive blood test chips for use with a blood-substance measuring device
InactiveUS7488216B2Efficient use ofCoupling device connectionsAnalysis using chemical indicatorsBlood testEngineering
A connector to receive blood test chips for use with a blood-substance measuring device includes a receptacle, a plug and a wire lead. The receptacle has two ends and a slot. The slot is formed on one end of the receptacle and receives a test chip. The wire lead connects to the other end of the receptacle and the plug. The plug is adapted to be inserted into a corresponding socket in the blood-substance measuring device to electrically connect the test chip to the device.
Owner:BIOMEDIX TAIWAN
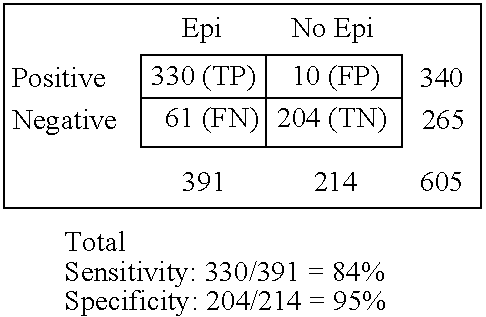
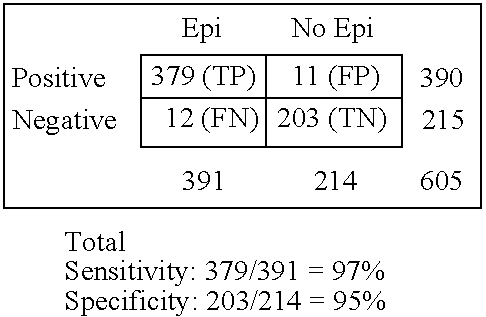
Rapid multiple panel of biomarkers in laboratory blood tests for TIA/stroke
InactiveUS6896872B2Diagnosing the progression of TIA orMonitor effectivenessImmunoglobulins against cell receptors/antigens/surface-determinantsPeptide preparation methodsNR1 NMDA receptorNMDA receptor
A methods, kits and compositions for diagnosing a central nervous system disorder, particularly transient ischemic attack or stroke, comprising measuring the level of NR2A and / or NR2B NMDA receptor or fragment thereof, in a biological sample from a human subject, and optionally measuring other biomarkers such as homocysteine and glutamate. The method is particularly useful for identifying individuals that are at risk for stroke, and for diagnosing stroke in an emergency room setting.
Owner:CIS BIOTECH
Device for performing a blood, cell, and/or pathogen count and methods for use thereof
ActiveUS9322767B2Rapid and accurate and affordable laboratory-quality blood testingEliminate equipmentBioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careDiagnosis laboratory
Devices and methods for performing a point of care blood, cell, and / or pathogen count or a similar blood test. Disclosed herein are systems that can be used to provide rapid, accurate, affordable laboratory-quality testing at the point of care. The systems described herein are capable of imaging and counting individual cells in a prepared cell sample (e.g., a peripheral blood smear or a blood sample prepared in a microfluidic device) or another prepared cell-containing sample without the need for a microscope or other expensive and cumbersome optics. The systems described herein are designed to eliminate or replace expensive, centralized clinical testing equipment and technical personnel. Such systems may include automated data reporting and decision support.
Owner:I CALQ
Analysis element for use in method of testing specimen
InactiveUS20070178009A1Sufficient amountEasy to operateAnalysis using chemical indicatorsMaterial analysis by observing effect on chemical indicatorBlood testEngineering
An analysis element for use in a blood test method enabling easy and simple operations that are performed quickly up to measurement, an analysis element for use in a blood test method that operations up to the measurement for many components can be quickly performed, and that is safe and has a sufficient measurement accuracy thereof, and an analysis element for use in a test method using body fluids and urines of humans and animals, plain water, seawater, soil extract, agricultural products, marine products, processed-food extracts, and liquid for use in scientific research as specimens, are provided, the present invention relates to a multi-component measurement dry analysis element for use in a method for testing a specimen, the method using an area sensor as a detector to obtain a result of measurement according to information represented by 1000 pixels or more per one component, and to perform simultaneous measurements of plural components.
Owner:FUJIFILM CORP
DNA methylation biomarkers in lymphoid and hematopoietic malignancies
InactiveUS20090264306A1Guaranteed maximum utilizationImprove the detection rateMicrobiological testing/measurementLibrary screeningDNA methylationLymphocytic cell
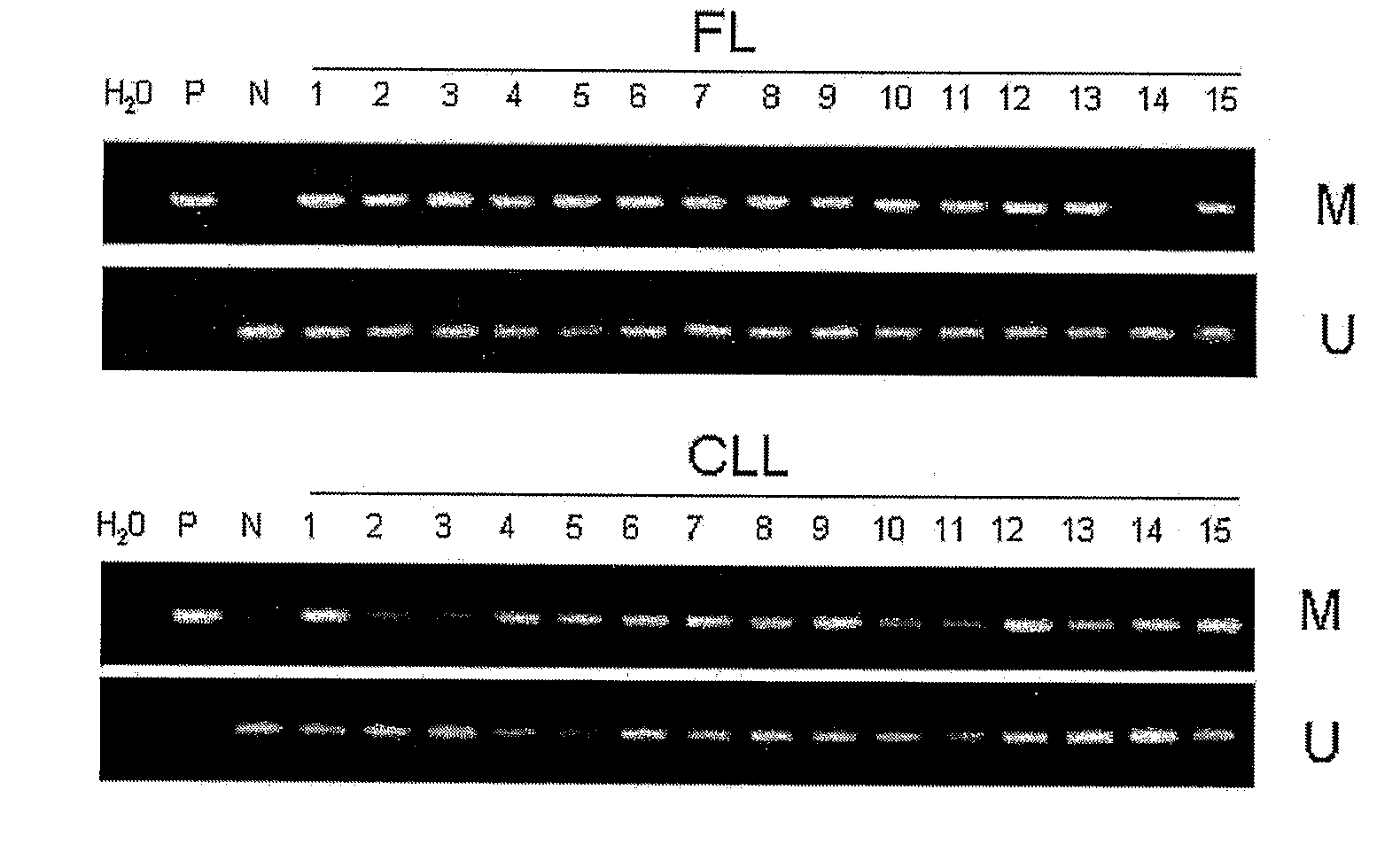
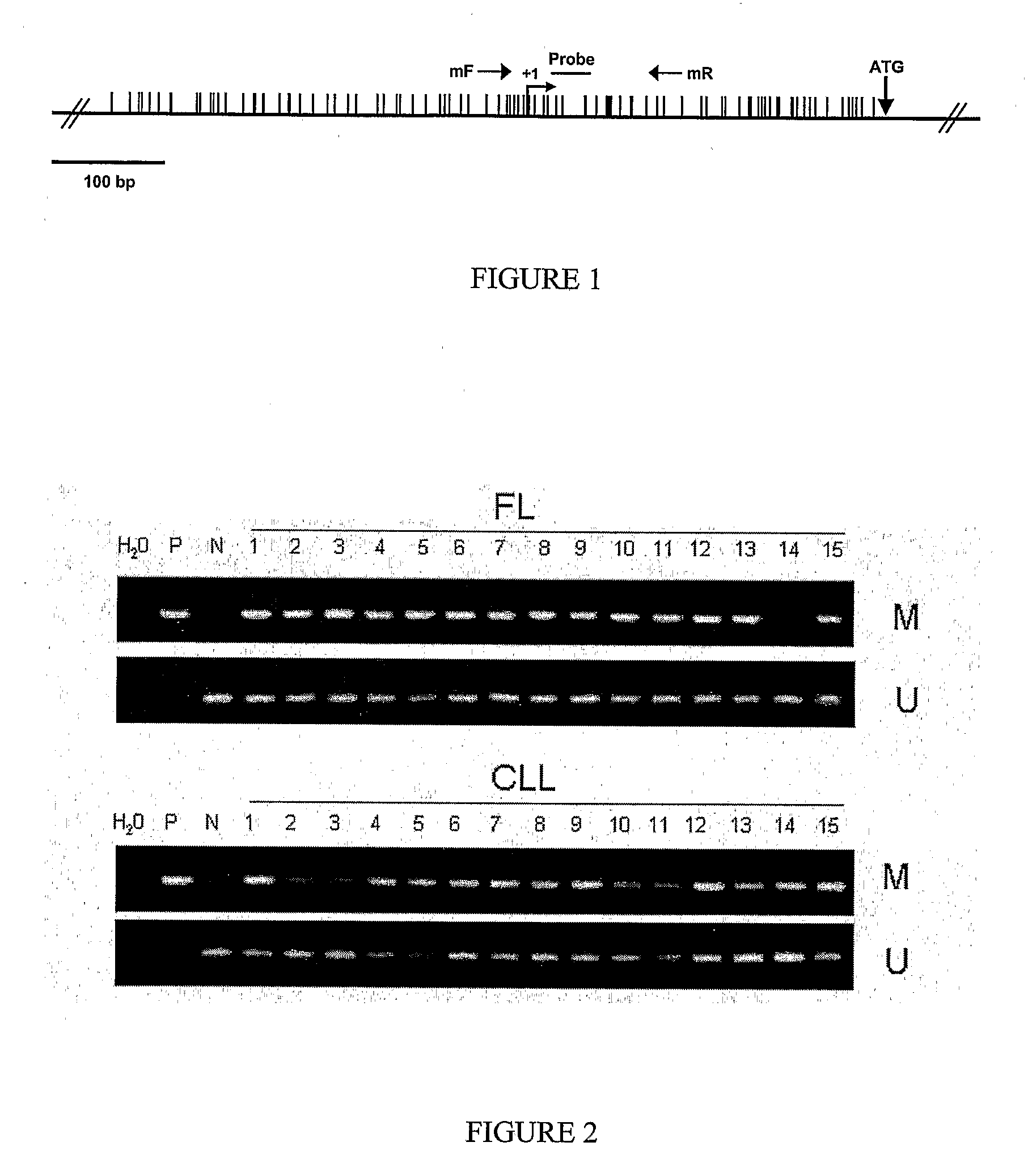
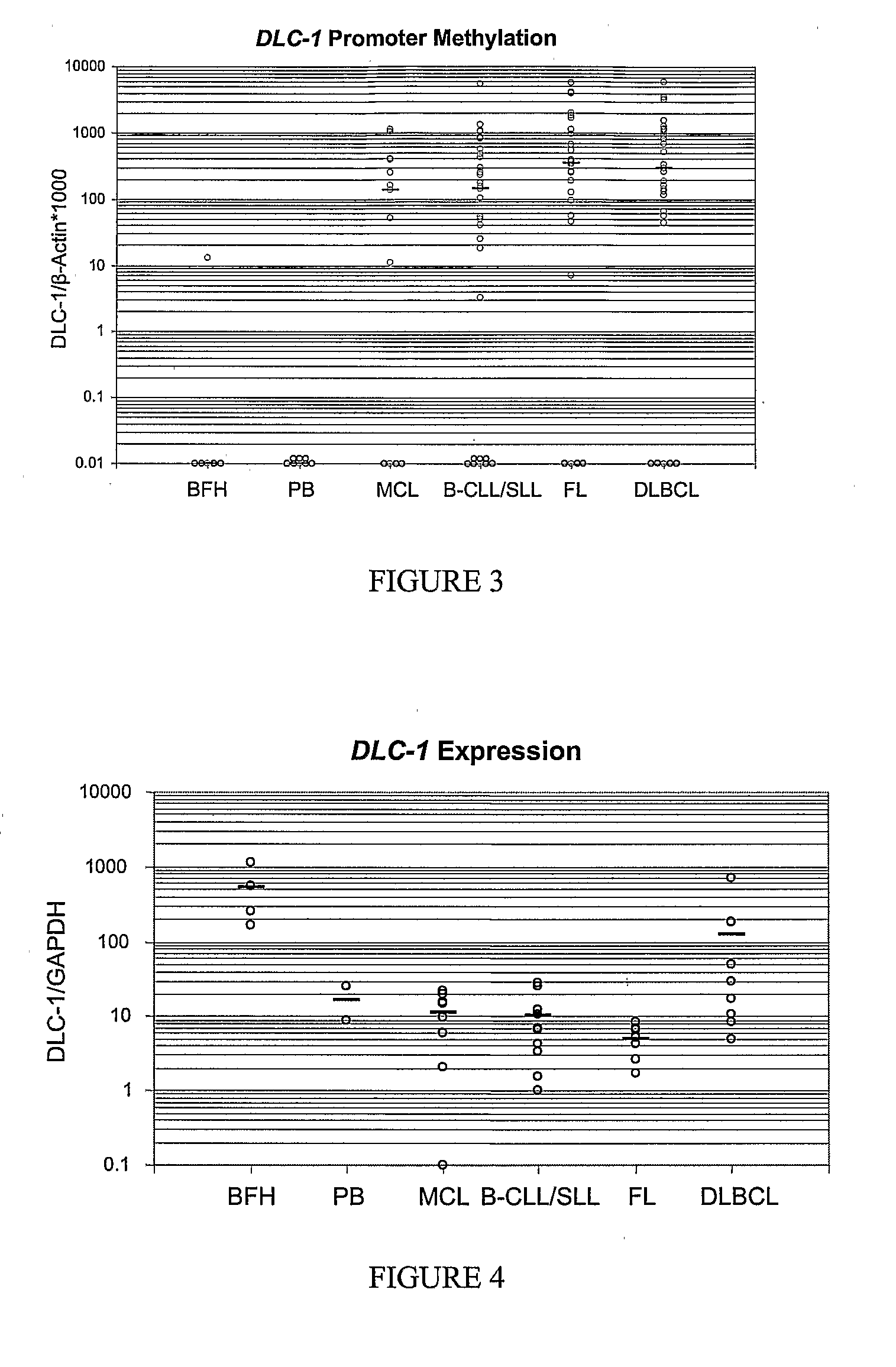
Differential Methylation Hybridization (DMH) was used to identify novel methylation markers and methylation profiles for hematopoieetic malignancies, leukemia, lymphomas, etc. (e.g., non-Hodgkin's lymphomas (NHL), small B-cell lymphomas (SBCL), diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), B-cell chronic lymphocytic leukemia / small lymphocytic lymphoma (B-CLL / SLL), chronic lymphocytic leukemia (CLL), multiple myeloma (MM), acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), etc.). Particular aspects provide novel biomarkers for NHL and subtypes thereof (e.g., MCL, B-CLL / SLL, FL, DLBCL, etc.), AML, ALL and MM, and further provide non-invasive tests (e.g. blood tests) for lymphomas and leukemias. Additional aspects provide markers for diagnosis, prognosis, monitoring responses to therapies, relapse, etc., and further provide targets and methods for therapeutic demethylating treatments. Further aspects provide cancer staging markers, and expression assays and approaches comprising idealized methylation and / or patterns” (IMP and / or IEP) and fusion of gene rankings.
Owner:UNIVERSITY OF MISSOURI
Blood test reagent and method
ActiveCN101726579AExcellent and fast dyeing actionAvoid fluorescent background interferenceIndividual particle analysisBiological testingBlood testFluorescence
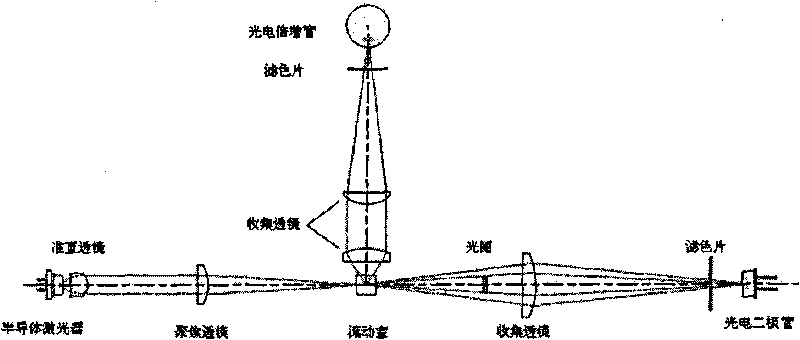
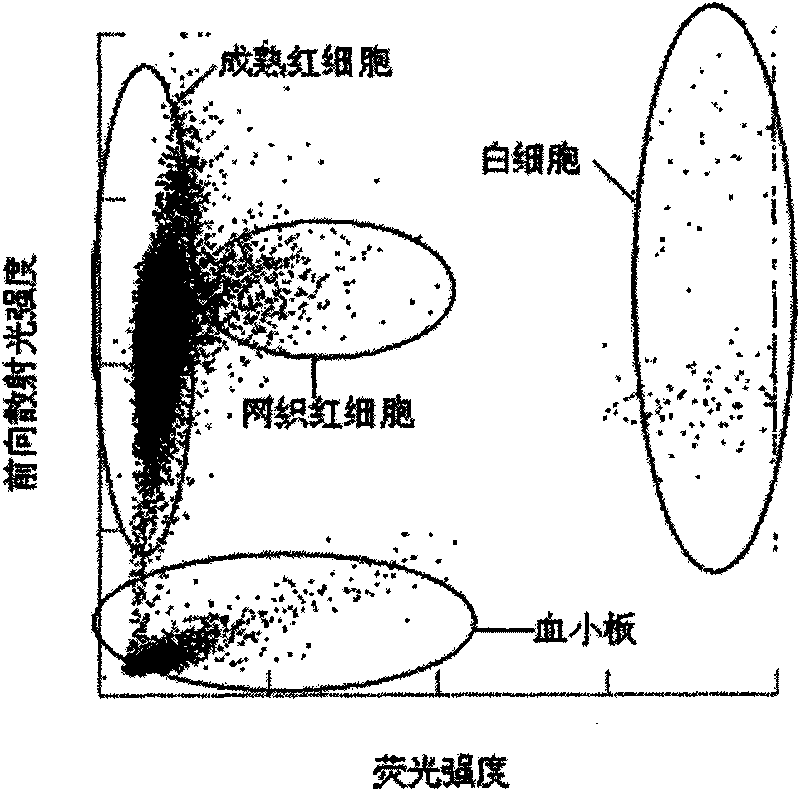
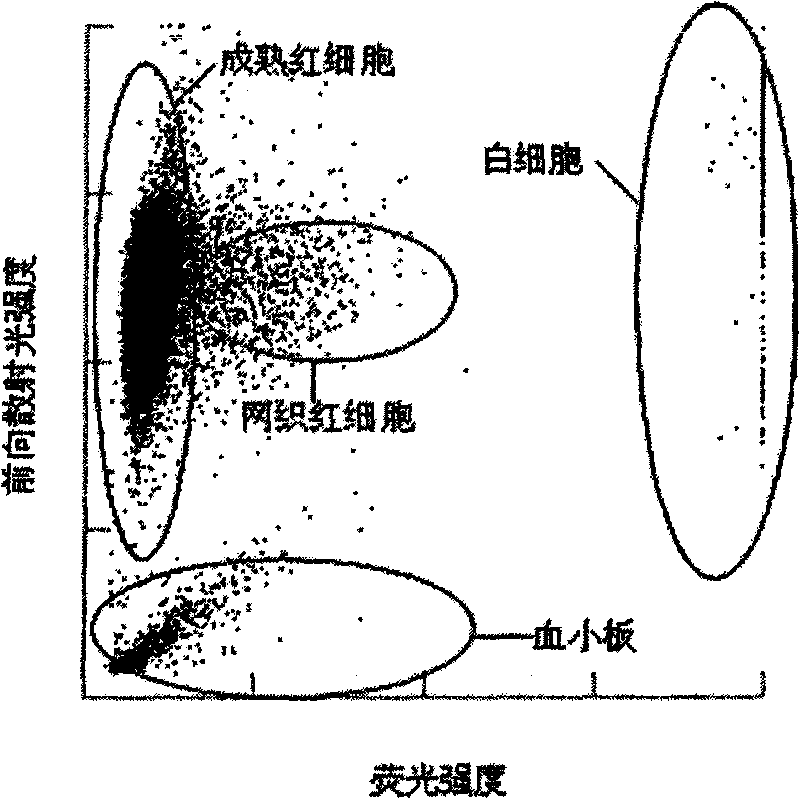
The invention provides a blood test reagent. The blood test reagent comprises: (1) a compound which is used as fluorochrome and has the structure represented by a general formula I, wherein n, X, R1, R2, R3, R4, R5 and Y are defined in the description; and (2) a surfactant which may be a cationic surfactant, an amphoteric surfactant or an anionic surfactant. The invention also provides a method for testing the blood, which comprises the following steps: (a) mixing a blood sample and the blood test reagent of the invention to form cell suspension; (b) detecting the scattered light and the fluorescent signal of cells; and (c) classifying and counting the cells in the blood according to the scattered light and the fluorescent signal.
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD
Immunosorbent blood tests for assessing paroxysmal cerebral discharges
InactiveUS20050181466A1Effective therapeutic interventionRapid inexpensiveNervous disorderDisease diagnosisDiseaseParoxysmal AF
Immunosorbents, kits and compositions for diagnosing a central nervous system disorder, particularly paroxysmal cerebral discharges and epilepsy, comprising measuring the concentration of GluR1 or fragment thereof and / or GluR1 antibodies in a biological sample from a human subject. The method is particularly useful for identifying individuals that are at risk for brain related seizures and epilepsy, for distinguishing epilepsy from pseudo-epilepsy and epilepsy-like disorders, for following up after anticonvulsive treatment, and for the adjustment of adequate therapy and doses.
Owner:GRACE LAB
Biological Fluid Collection Device and Biological Fluid Collection and Testing System
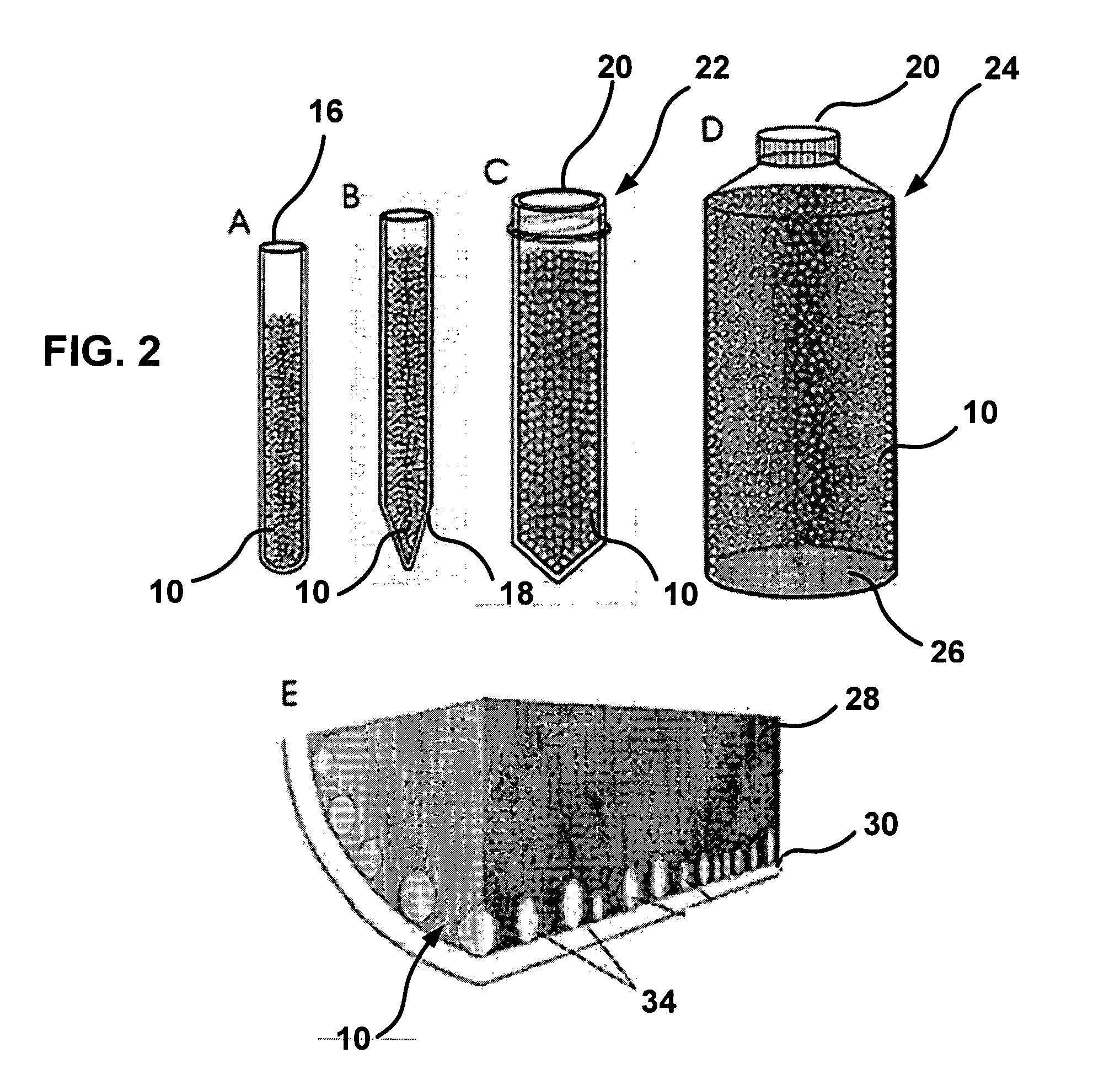
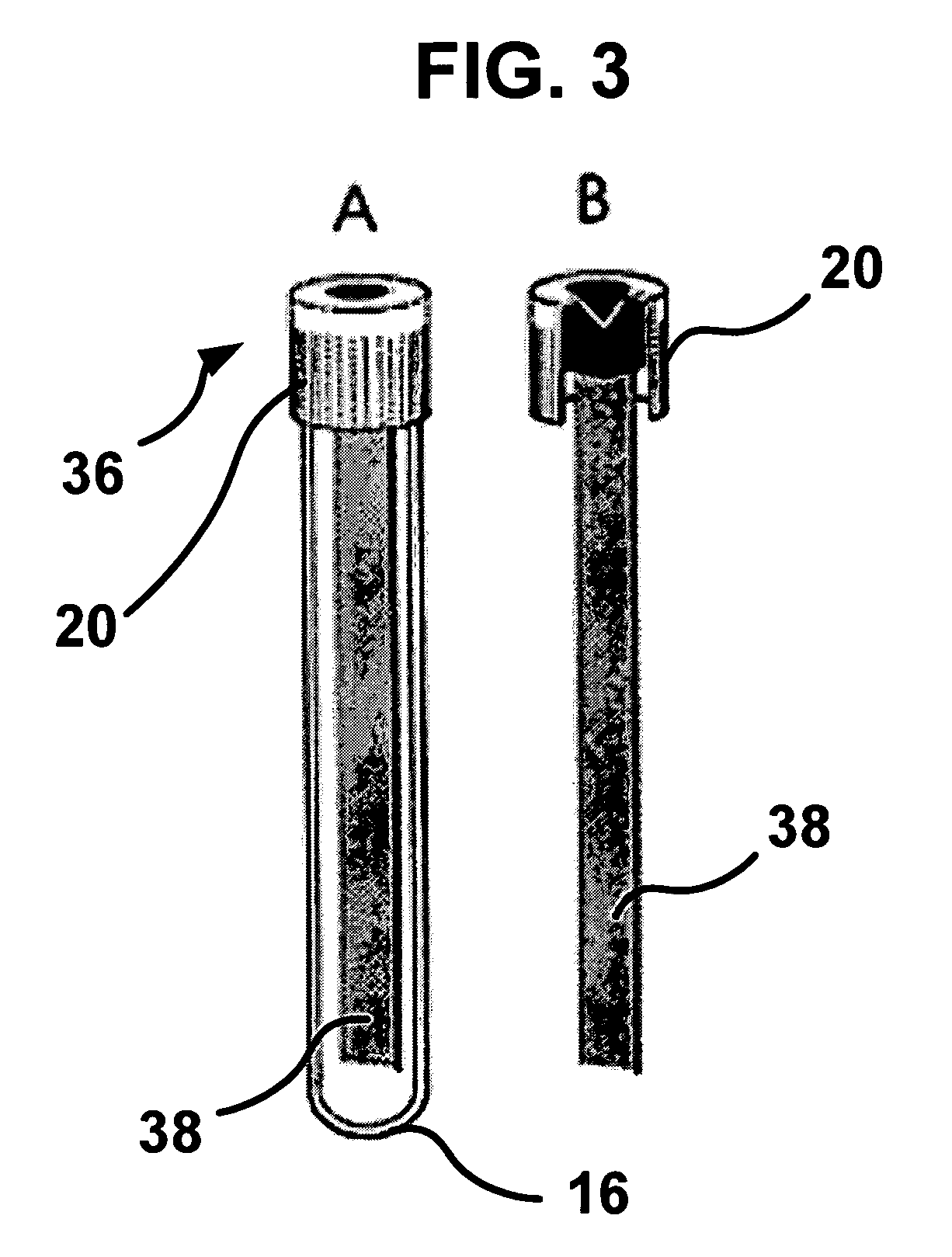
ActiveUS20140309556A1Reduce blood exposureQuick mixAnalysis material containersOther blood circulation devicesBlood Collection TubeBlood test
A blood collection device adapted to receive a multi-component blood sample is disclosed. After collecting the blood sample, the blood collection device separates a plasma portion from a cellular portion. After separation, the blood collection device is able to transfer the plasma portion of the blood sample to a point-of-care testing device. The blood collection device of the present disclosure also provides a closed collection and transfer system that reduces the exposure of a blood sample and provides fast mixing of a blood sample with a sample stabilizer. The blood collection device is engageable with a blood testing device for closed transfer of a portion of the plasma portion from the blood collection device to the blood testing device. The blood testing device is adapted to receive the plasma portion to analyze the blood sample and obtain test results.
Owner:BECTON DICKINSON & CO
Method for testing peritoneal function
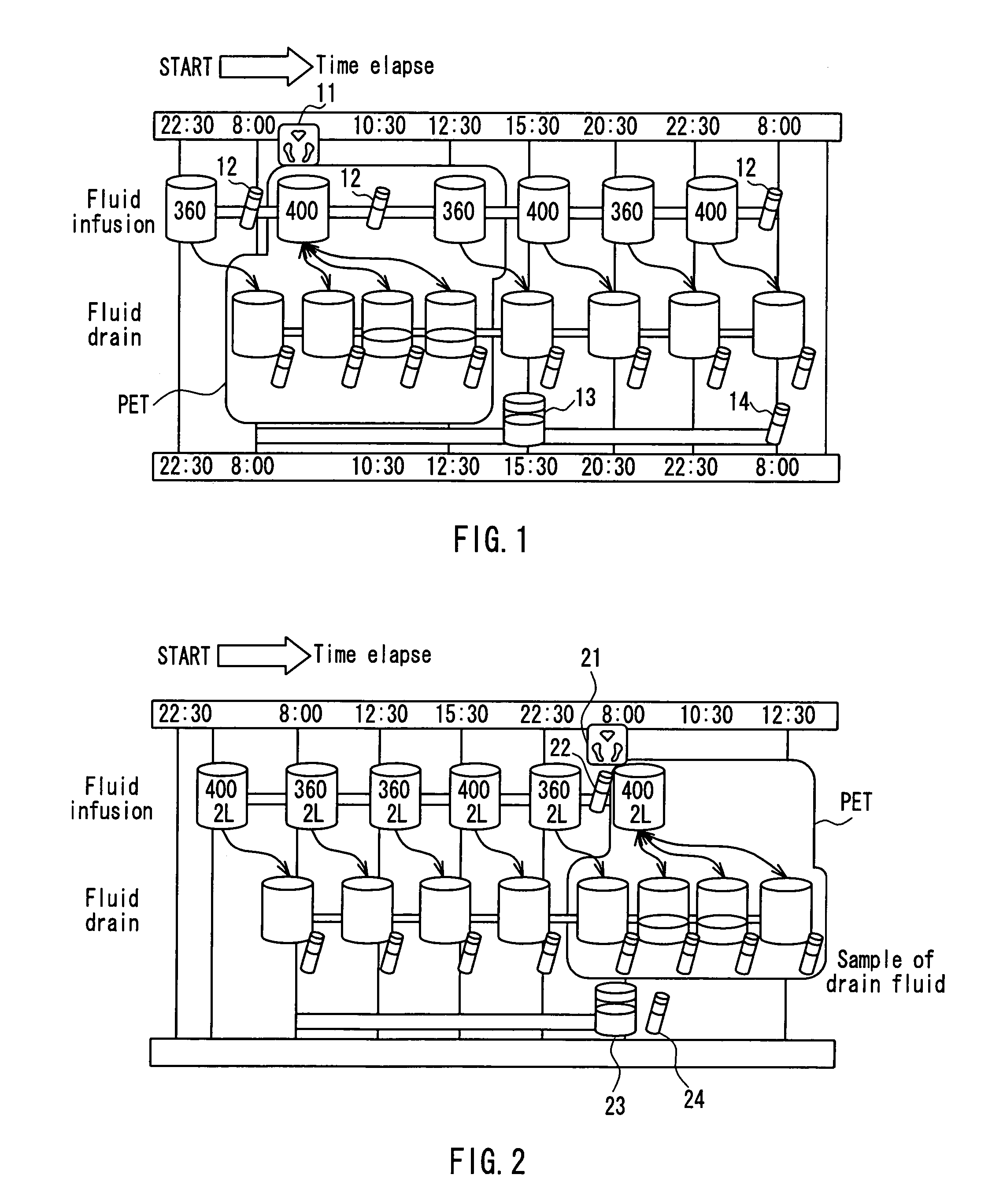
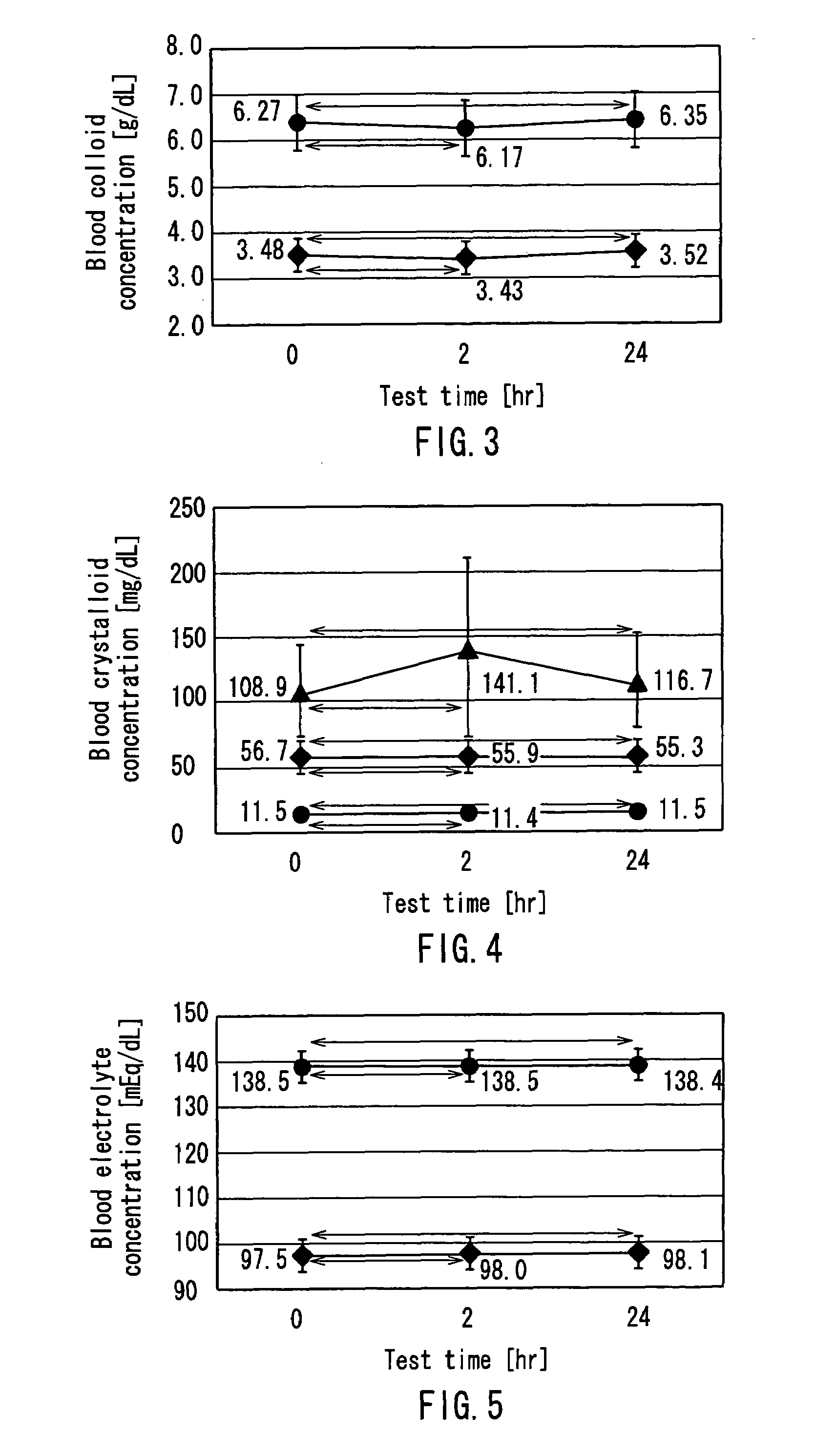
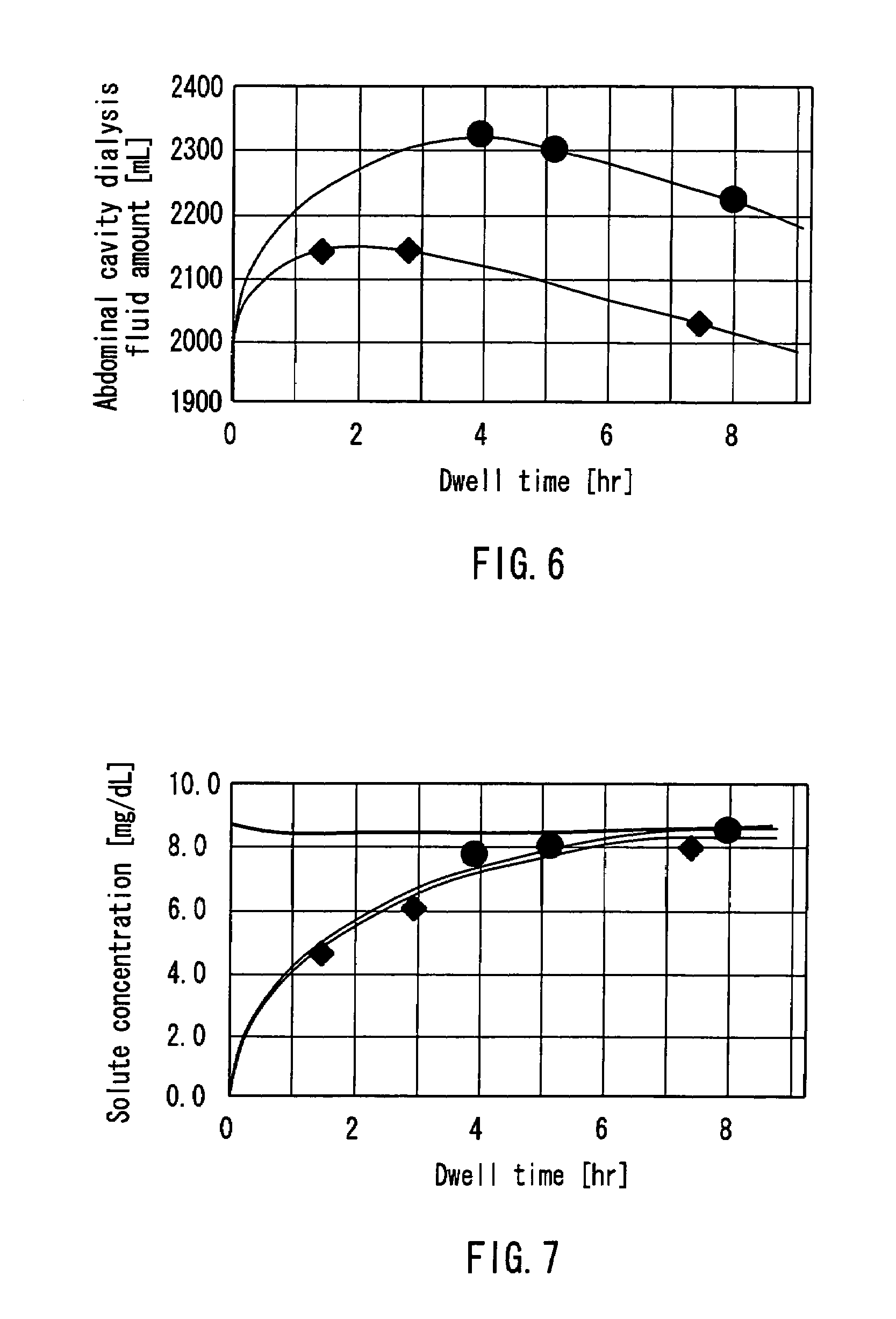
InactiveUS7303541B2Efficiently and accurately evaluate the current conditionVaccination/ovulation diagnosticsMedical devicesPeritoneal equilibration testAbdominal cavity
Owner:JMS CO LTD
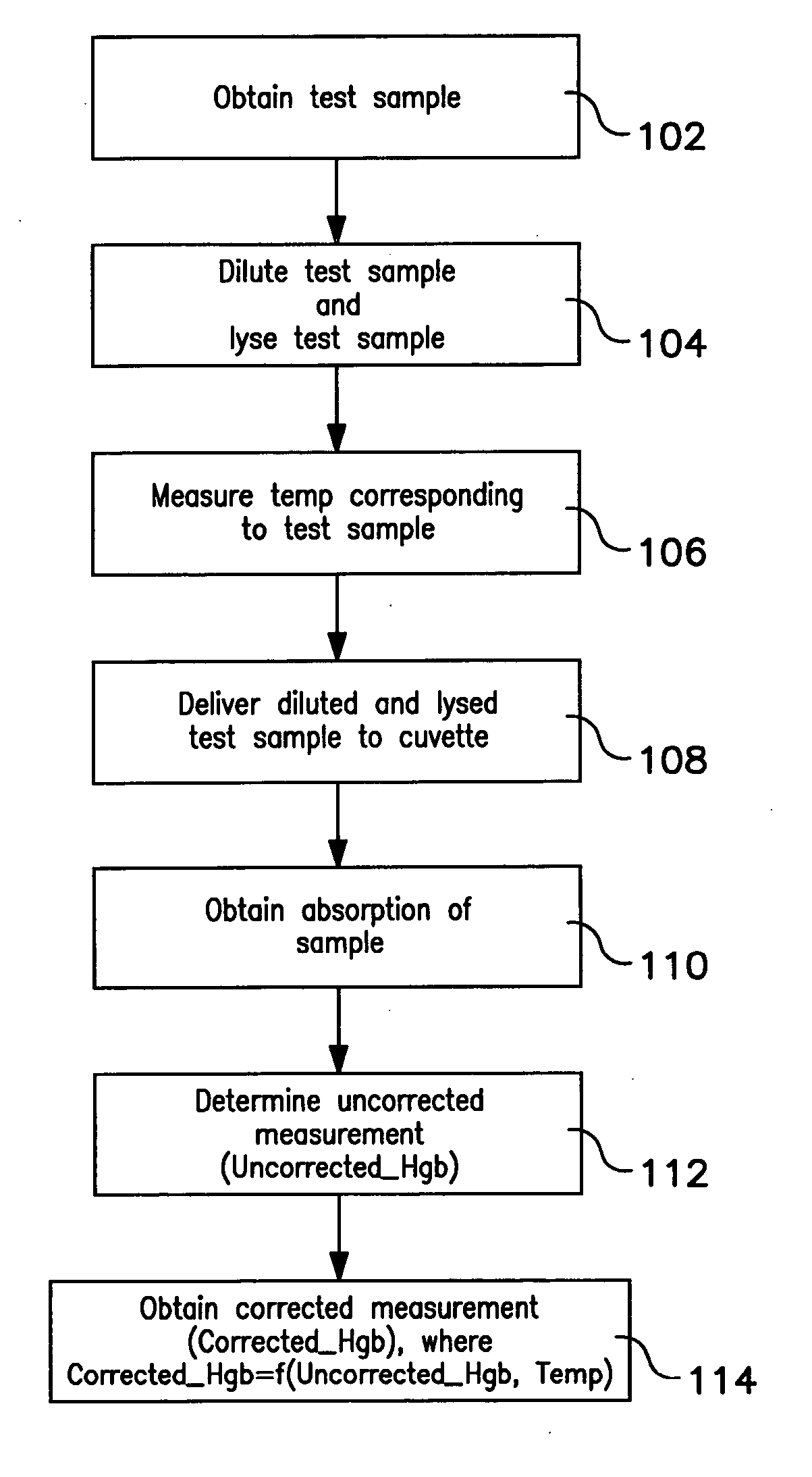
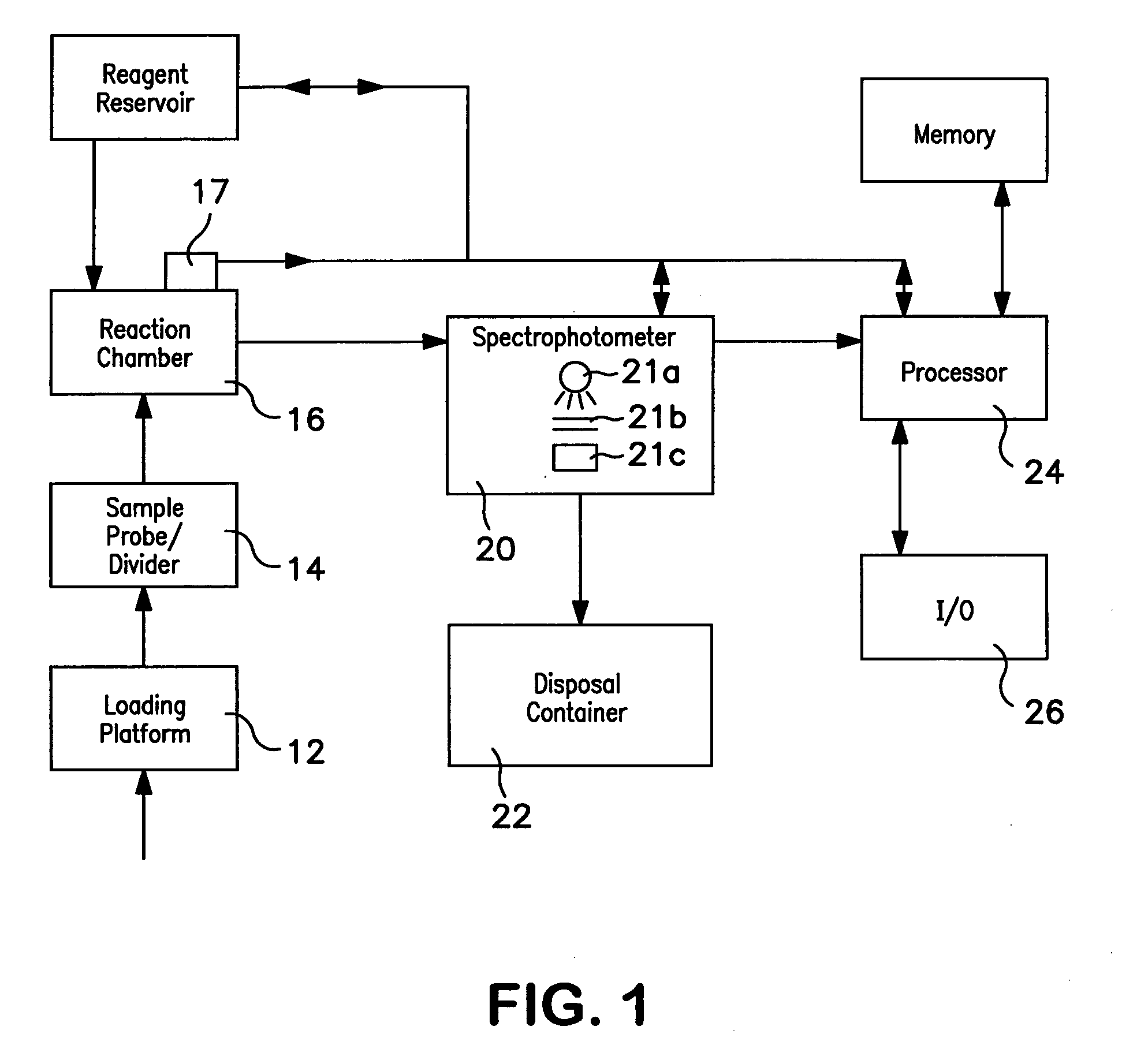
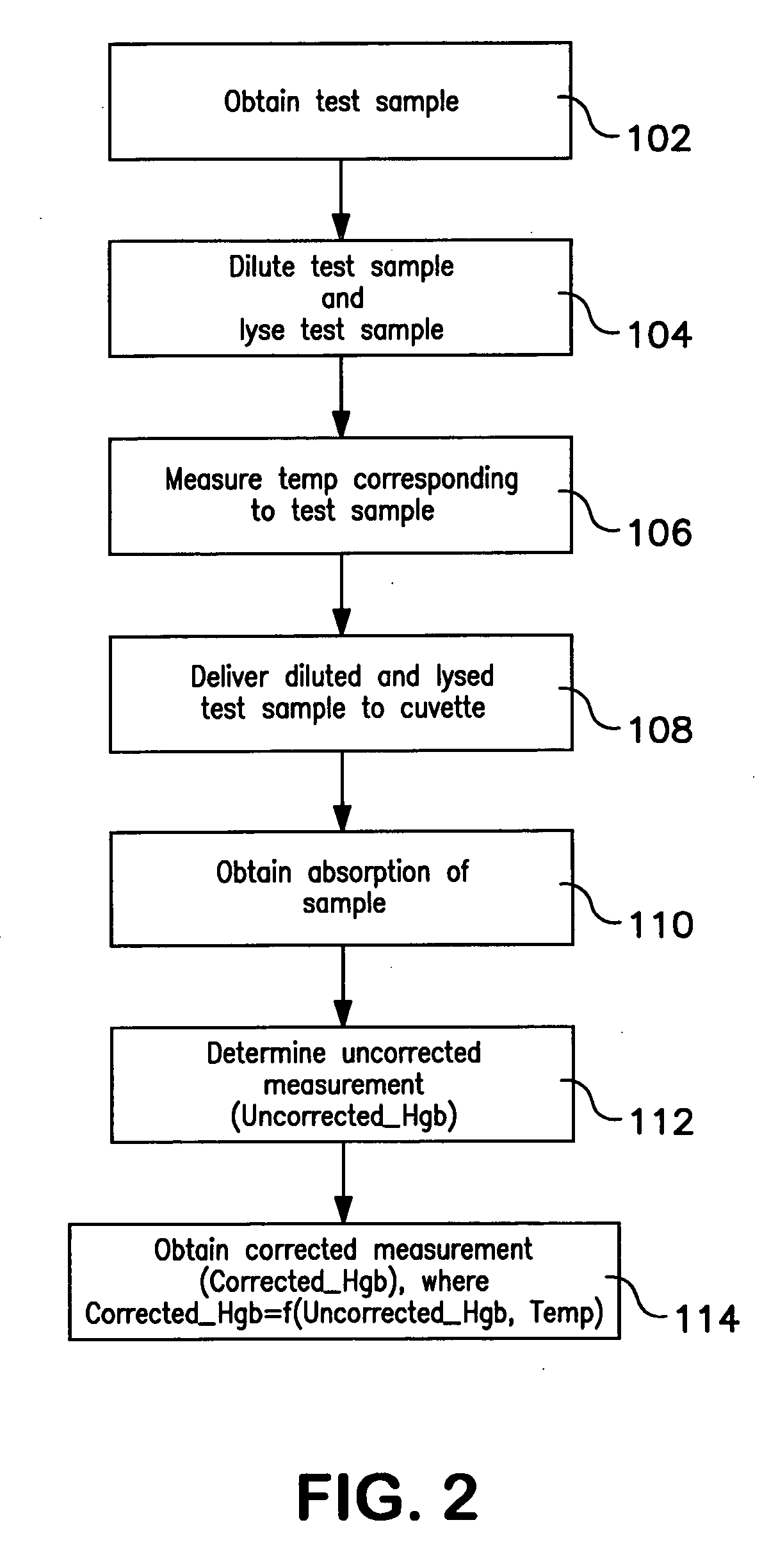
Method of hemoglobin correction due to temperature variation
A method of measuring a hemoglobin parameter of a test sample of blood comprises diluting and lysing a test sample. Then, a temperature corresponding to the test sample is obtained. The diluted and lysed test sample is then delivered to a cuvette, and a spectrophotometer determines the absorbance and / or transmittance of the sample in the cuvette. With the absorbance and / or transmittance of the test sample, a first measurement of the hemoglobin parameter of the test sample is obtained. After a first measurement of the hemoglobin parameter is obtained, a processor determines a corrected measurement of the hemoglobin parameter of the test sample. The corrected measurement is a function of the measured temperature that corresponds to the test sample and the first measurement of the hemoglobin parameter. The method of measuring a hemoglobin parameter is valid over a range of test sample temperatures.
Owner:BECKMAN COULTER INC
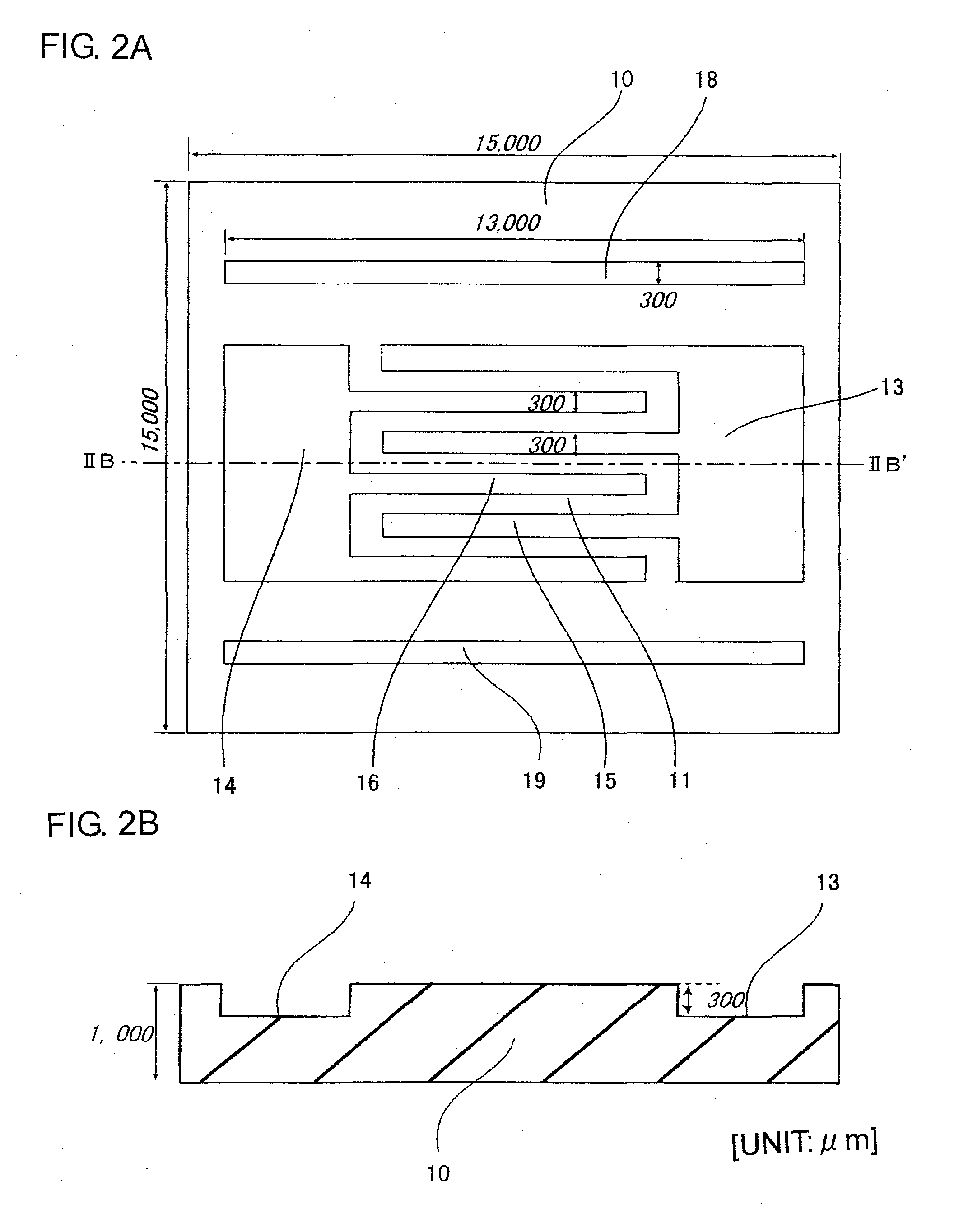
Microchannel array and method for producing the same, and blood measuring method employing it
InactiveUS20090149345A1More flow channelLibrary screeningMaterial analysis by electric/magnetic meansBlood testEngineering
A microchannel array, a method of manufacturing the same, and a blood test method. The microchannel array is formed by joining first and second substrates, each including a fluid inlet and outlet on their surfaces. An internal space structure connects the fluid inlet and outlet, and includes an upstream flow channel connected with the fluid inlet, a downstream flow channel connected with the fluid outlet with a gap therebetween, and a micro flow channel connecting the upstream and downstream flow channels. A minimum distance from a center of a sectional surface of the micro flow channel to a side wall of the micro flow channel is smaller than that of the upstream and downstream flow channels. Each surface of the first and second substrates includes grooves for creating the upstream and downstream flow channels, and the surface of the second substrate has grooves for creating the micro flow channel.
Owner:KURARAY CO LTD
Micro Vein Enhancer
ActiveUS20070161909A1Reduce in quantityEasy to operateImage analysisDiagnostics using lightBlood testFlat panel display
The present invention is a Miniature Vein Enhancer that includes a Miniature Projection Head. The Miniature Projection Head may be operated in one of three modes, AFM, DBM, and RTM. The Miniature Projection Head of the present invention projects an image of the veins of a patient, which aids the practitioner in pinpointing a vein for an intravenous drip, blood test, and the like. The Miniature projection head may have a cavity for a power source or it may have a power source located in a body portion of the Miniature Vein Enhancer. The Miniature Vein Enhancer may be attached to one of several improved needle protectors, or the Miniature Vein Enhancer may be attached to a body similar to a flashlight for hand held use. The Miniature Vein Enhancer of the present invention may also be attached to a magnifying glass, a flat panel display, and the like.
Owner:ACCUVEIN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com