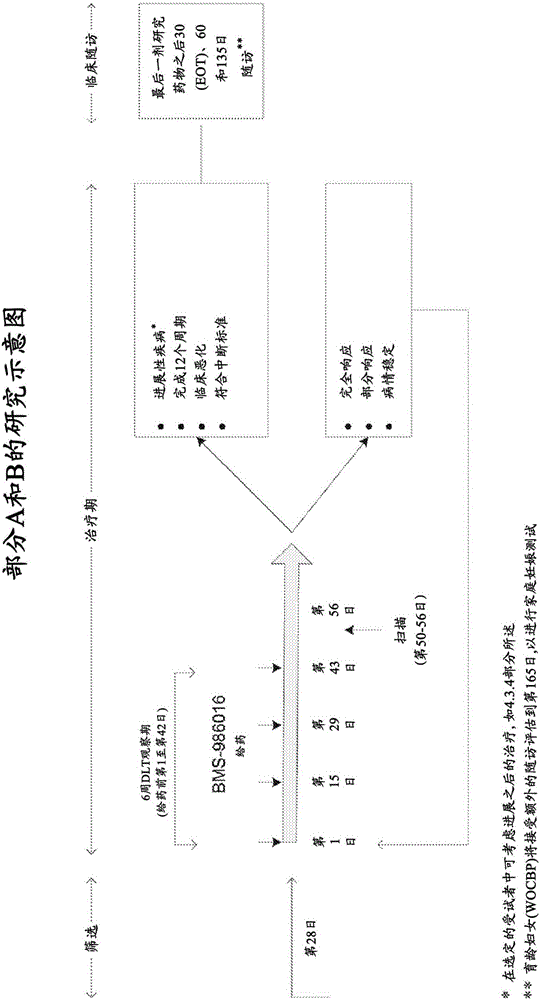
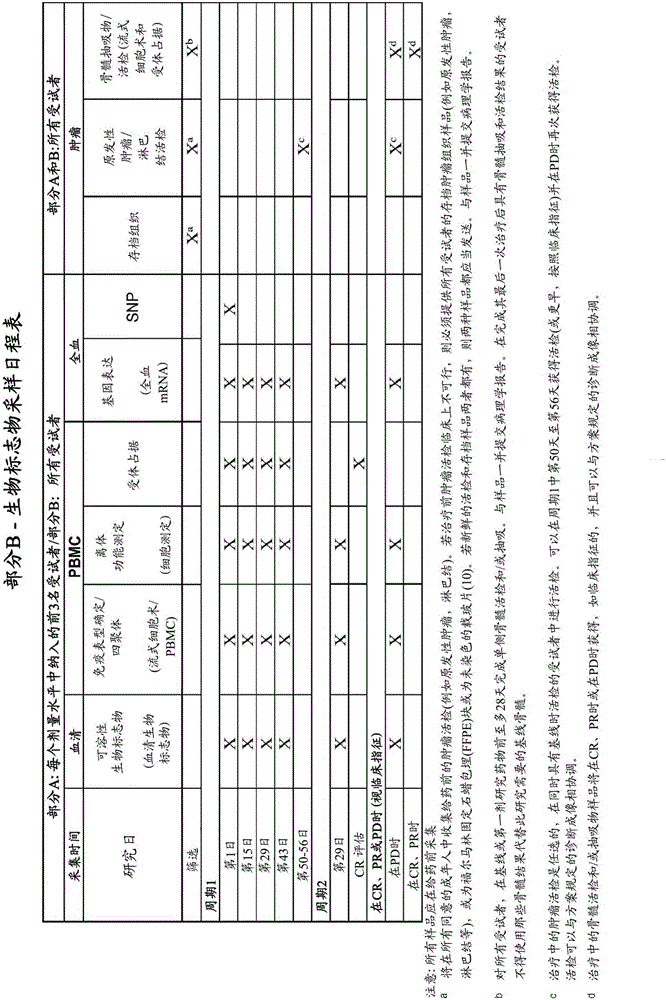
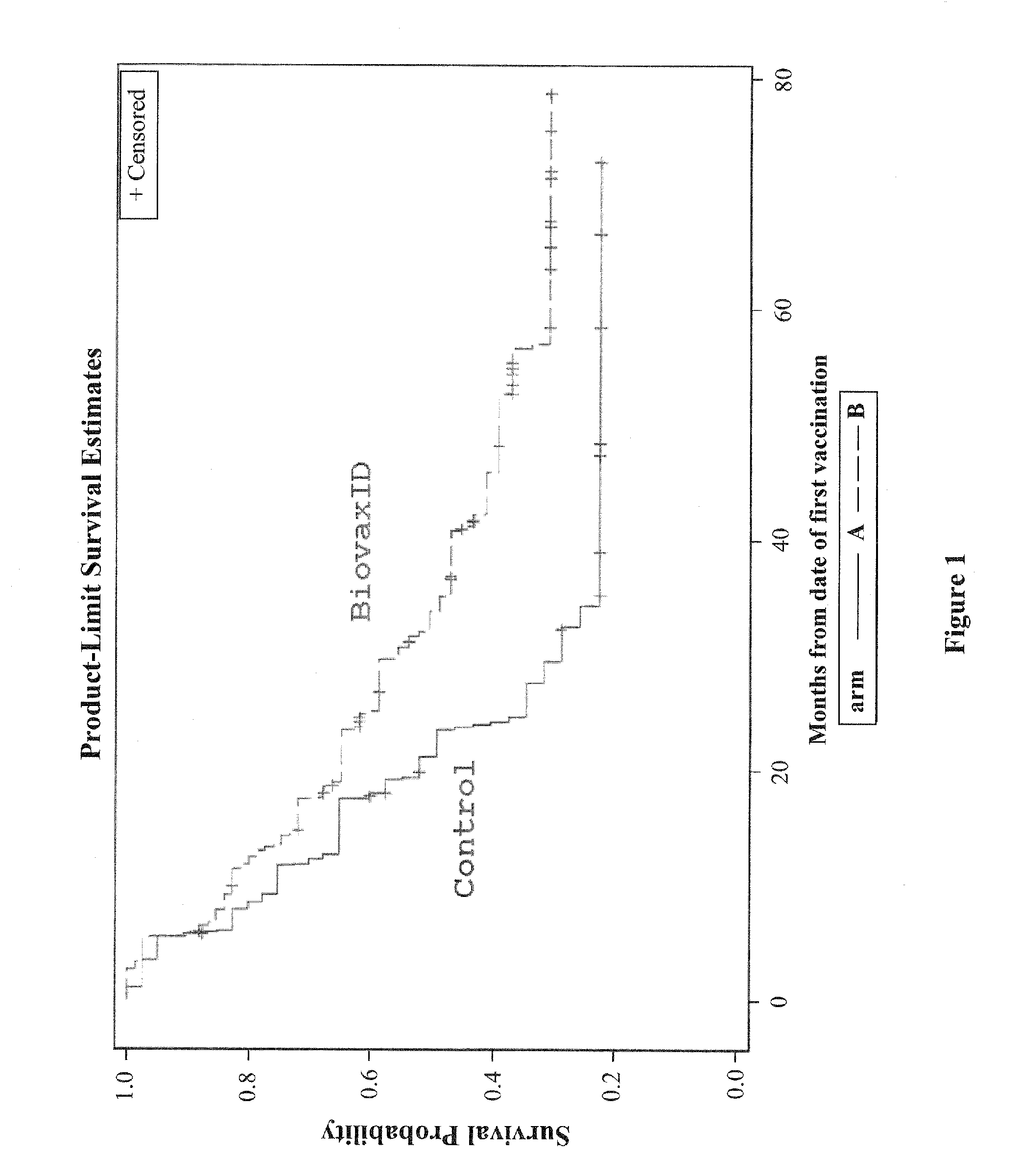
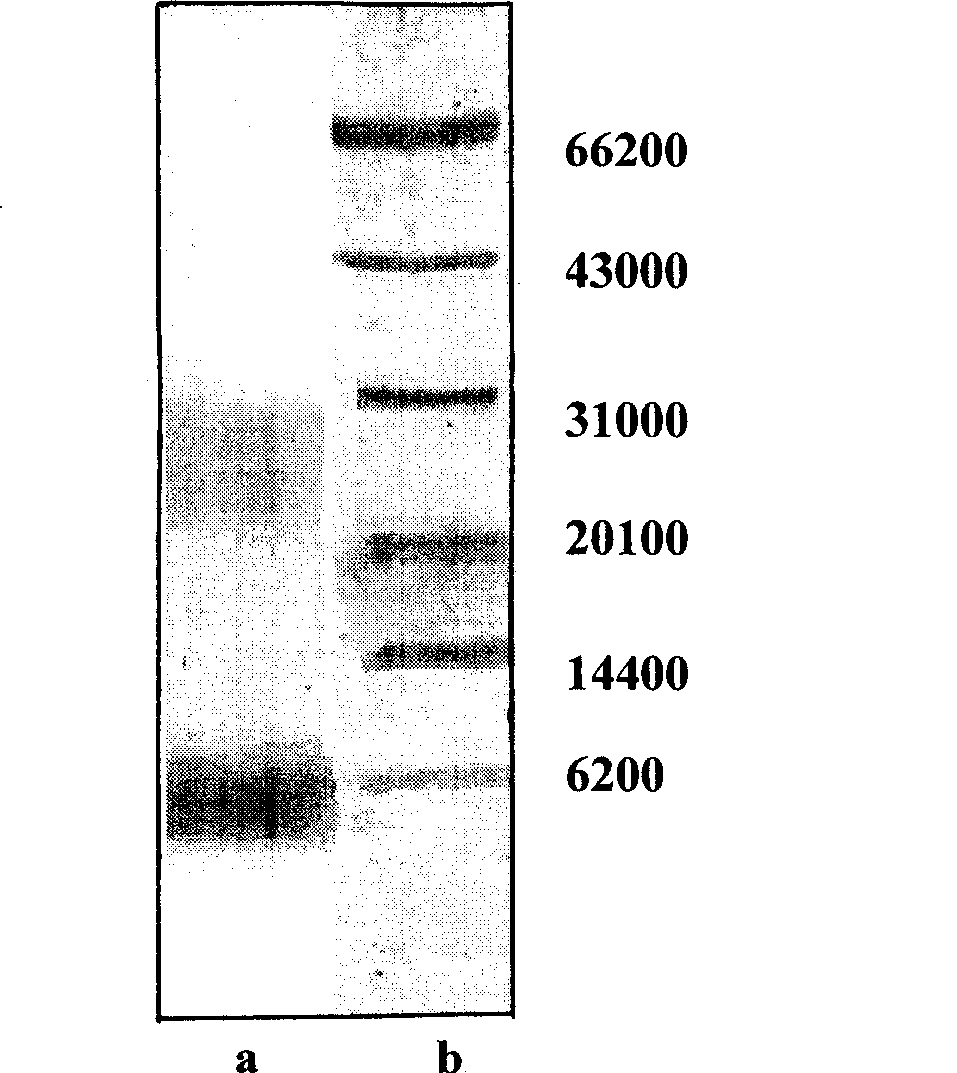
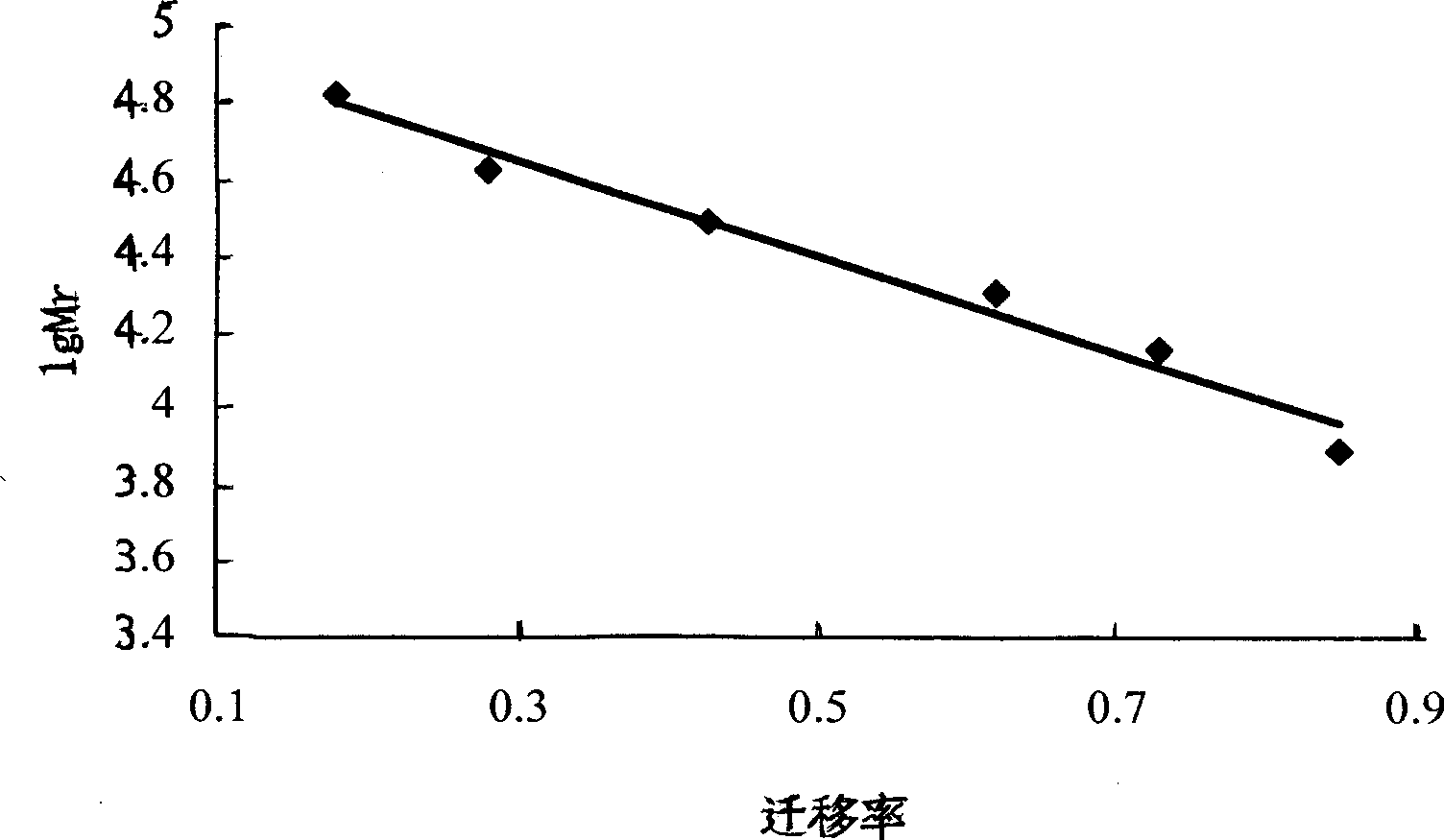
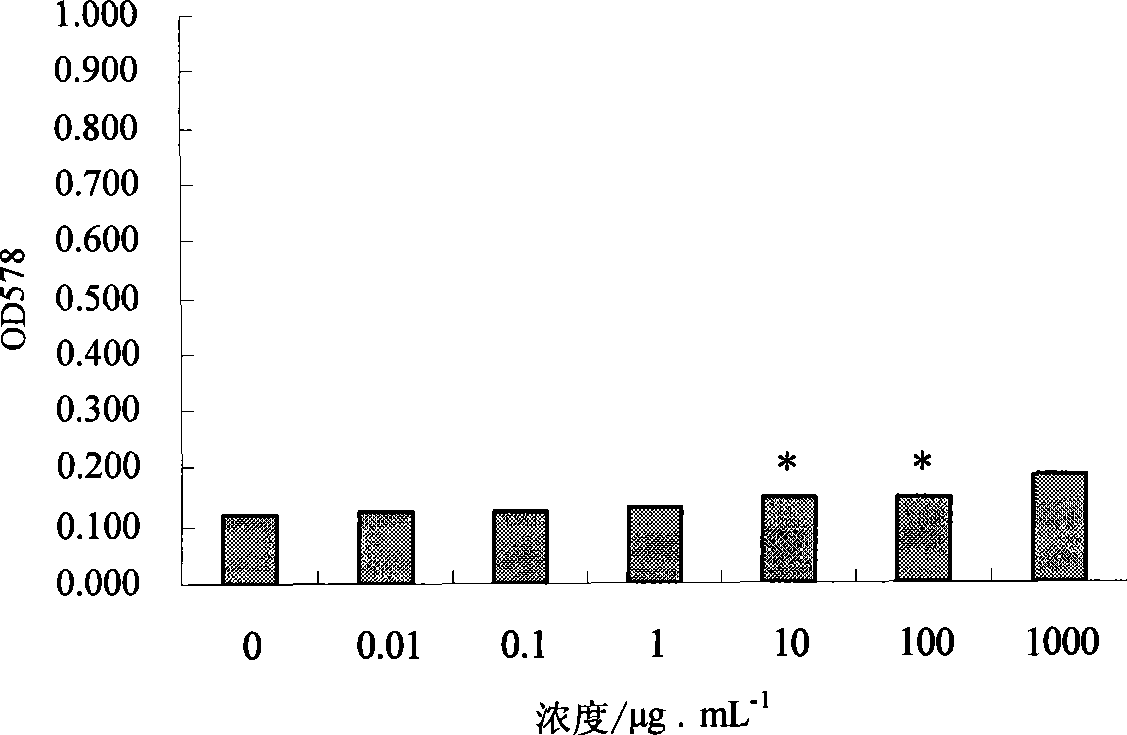
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
186 results about "Lymphocytic cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Biomarkers for Diagnosis and Treatment of Chronic Lymphocytic Leukemia
InactiveUS20110190157A1Improve clinical outcomesMicrobiological testing/measurementLibrary screeningMolecular classificationHer Disease
A molecular classification procedure based on activity levels of modules in protein networks, wherein the proteins are biomarkers for chronic lymphocytic leukemia (CLL), and method for use of the subnetworks to distinguish between patients at low or high risk of progression of their disease.
Owner:RGT UNIV OF CALIFORNIA
Composite Profiles of Cell Antigens and Target Signal Transduction Proteins for Analysis and Clinical Management of Hematologic Cancers
InactiveUS20100261204A1Increased riskDetermining prognosisDisease diagnosisBlood/immune system cellsCellular antigensTarget signal
Owner:BECKMAN COULTER INC
Antigen-Specific T Cell Receptors and T Cell Epitopes
ActiveUS20130273647A1Reliable detectionMeasureVirusesPeptide/protein ingredientsMHC restrictionCellular receptor
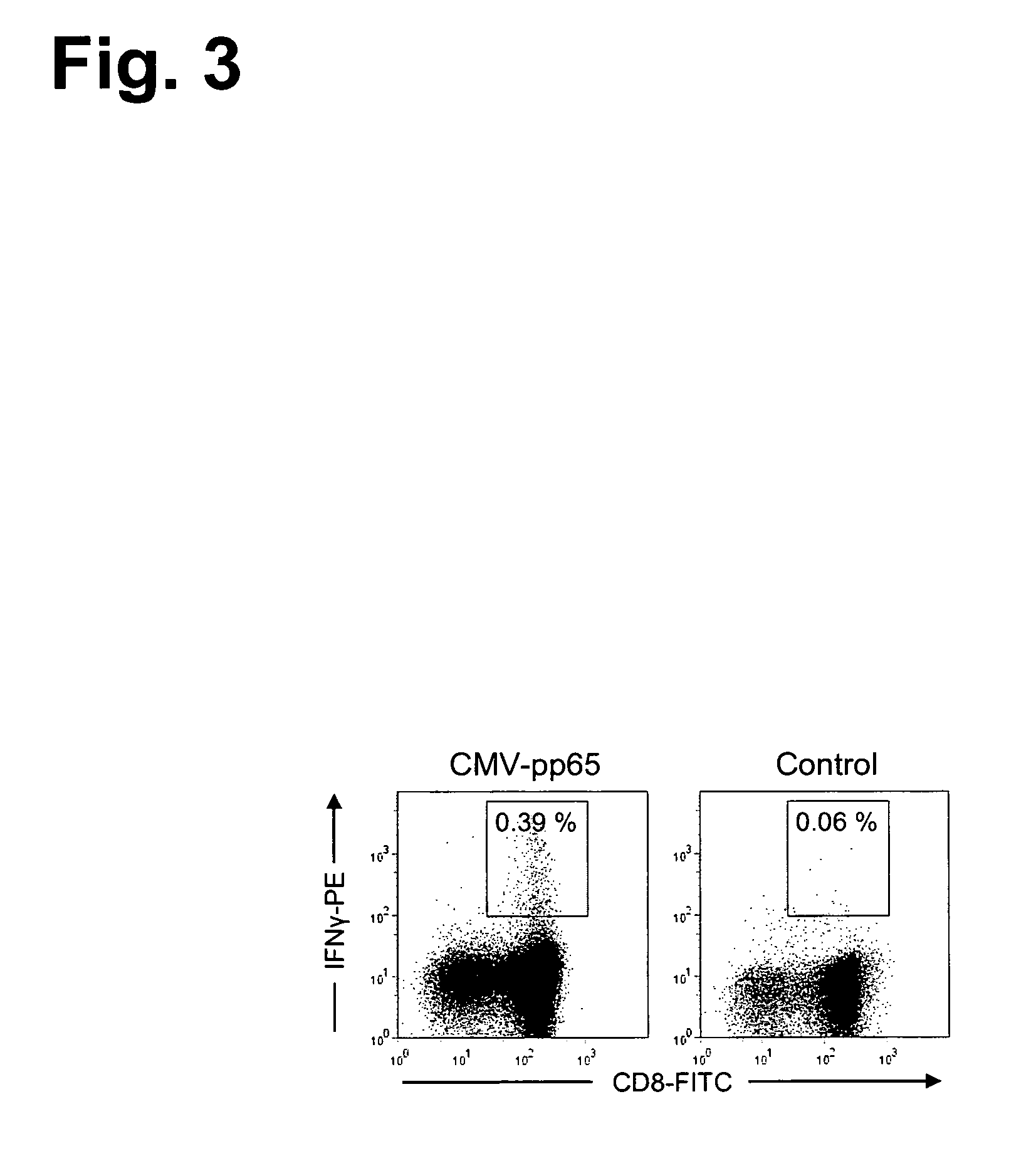
The present invention relates to efficient methods for providing antigen-specific lymphoid cells. These lymphoid cells may be used to provide antigen specific T cell receptors having a defined MHC restriction and to identify immunologically relevant T cell epitopes. Furthermore, the present invention relates to antigen-specific T cell receptors and T cell epitopes and their use in immunotherapy.
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITATSMEDIZIN DER JOHANNES GUTENBERG UNIVERS +1
DNA methylation biomarkers in lymphoid and hematopoietic malignancies
InactiveUS20090264306A1Guaranteed maximum utilizationImprove the detection rateMicrobiological testing/measurementLibrary screeningDNA methylationLymphocytic cell
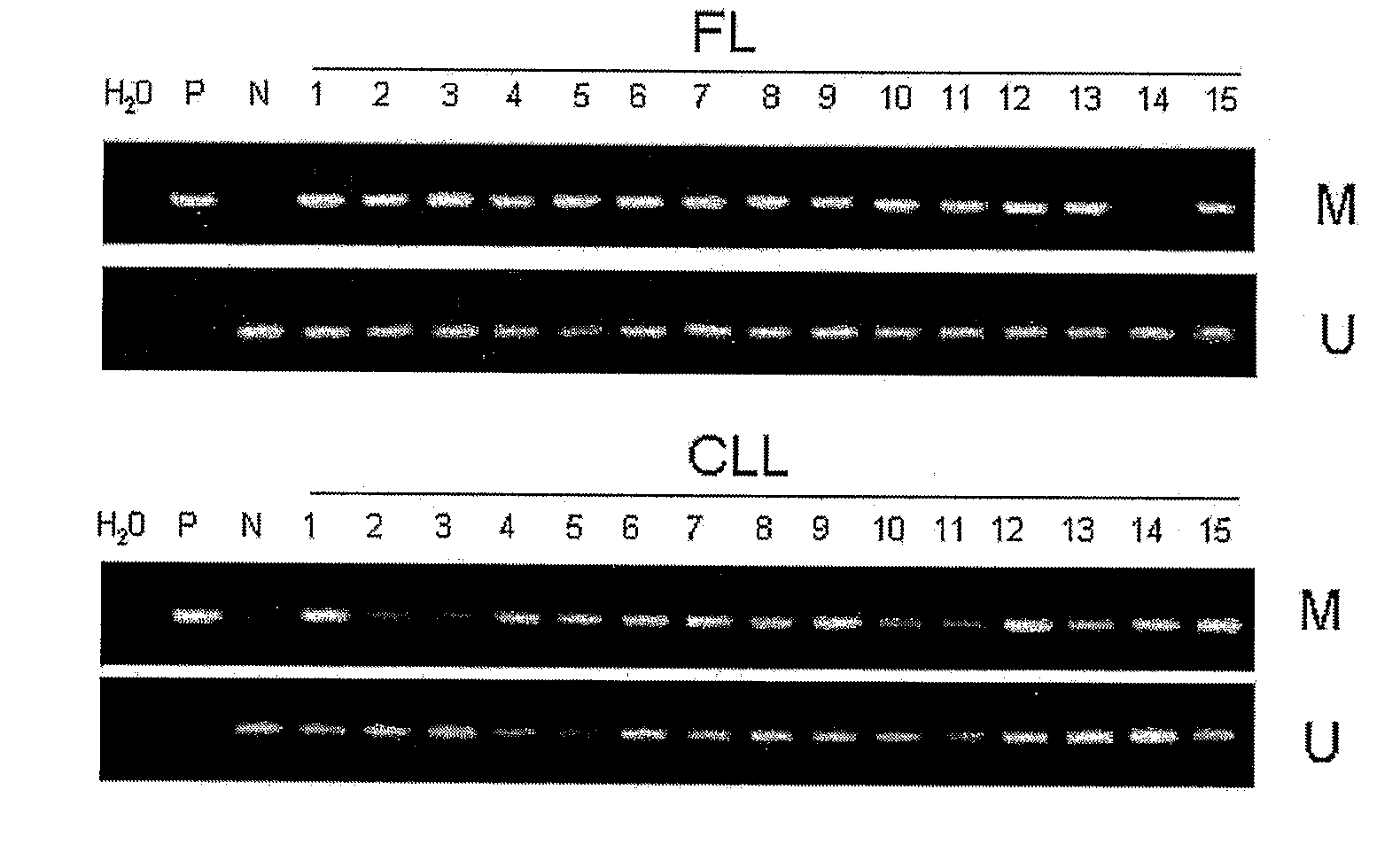
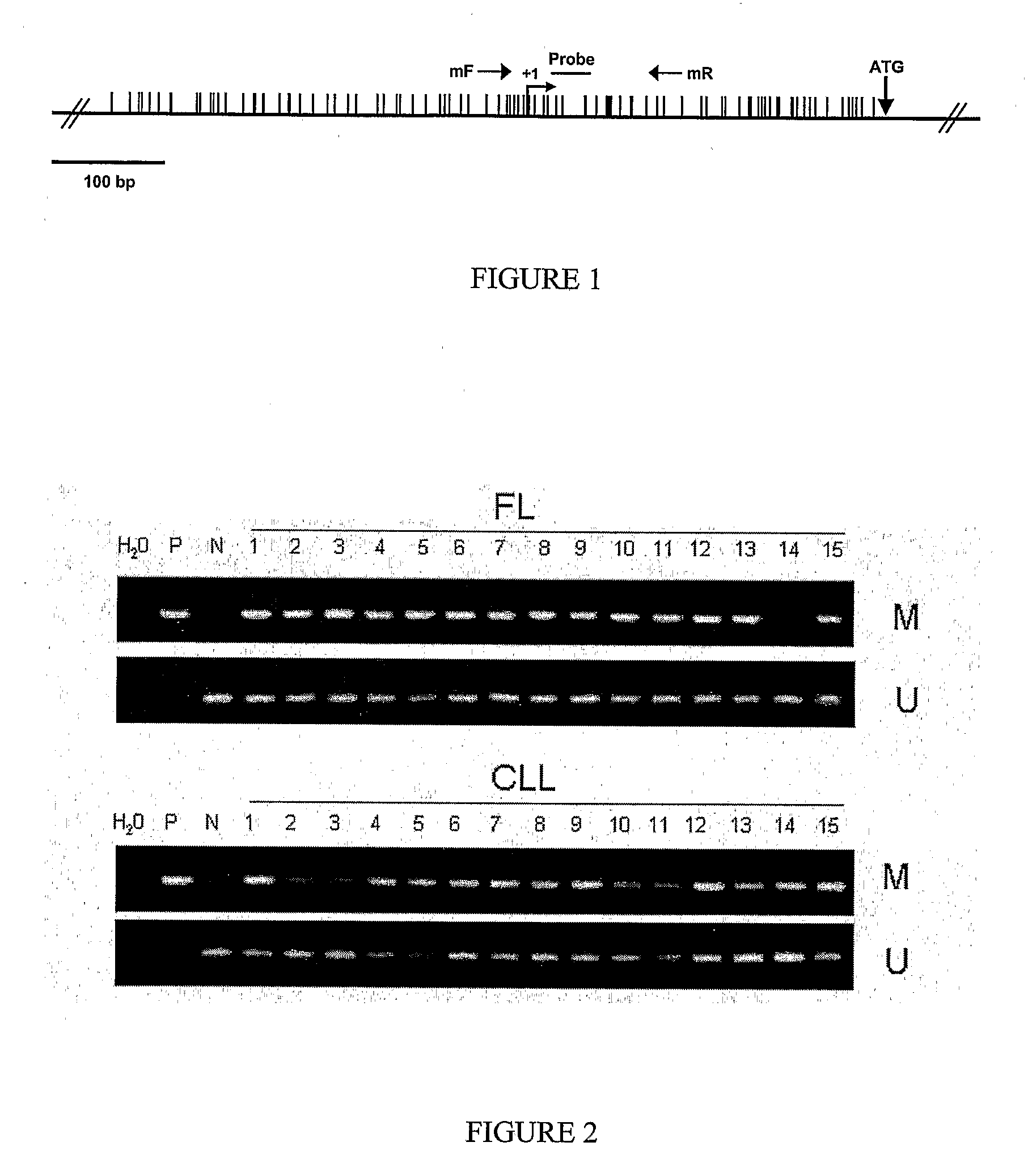
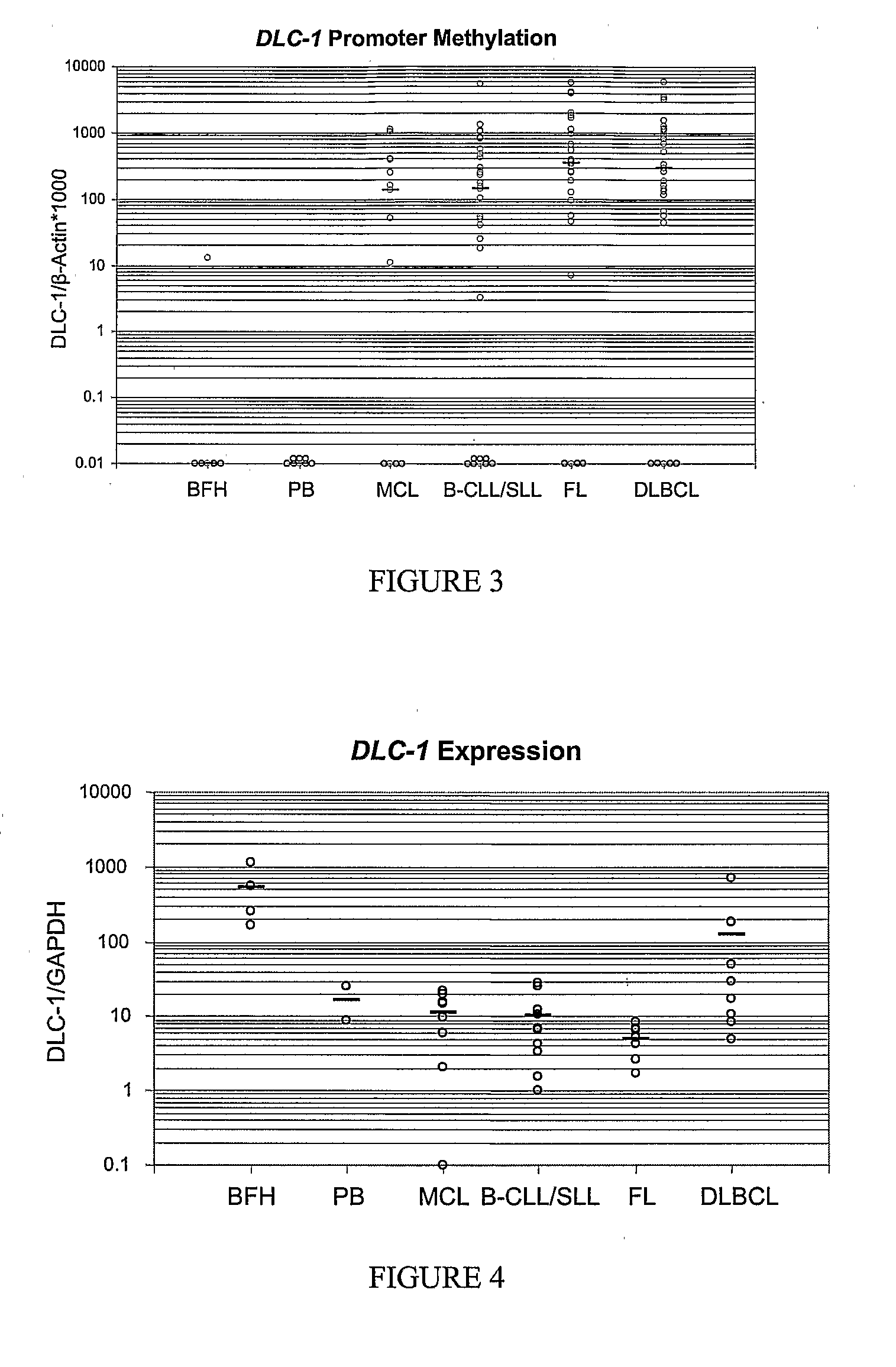
Differential Methylation Hybridization (DMH) was used to identify novel methylation markers and methylation profiles for hematopoieetic malignancies, leukemia, lymphomas, etc. (e.g., non-Hodgkin's lymphomas (NHL), small B-cell lymphomas (SBCL), diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), B-cell chronic lymphocytic leukemia / small lymphocytic lymphoma (B-CLL / SLL), chronic lymphocytic leukemia (CLL), multiple myeloma (MM), acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), etc.). Particular aspects provide novel biomarkers for NHL and subtypes thereof (e.g., MCL, B-CLL / SLL, FL, DLBCL, etc.), AML, ALL and MM, and further provide non-invasive tests (e.g. blood tests) for lymphomas and leukemias. Additional aspects provide markers for diagnosis, prognosis, monitoring responses to therapies, relapse, etc., and further provide targets and methods for therapeutic demethylating treatments. Further aspects provide cancer staging markers, and expression assays and approaches comprising idealized methylation and / or patterns” (IMP and / or IEP) and fusion of gene rankings.
Owner:UNIVERSITY OF MISSOURI
Compositions and Methods for Differential Diagnosis of Chronic Lymphocytic Leukemia
InactiveUS20080280297A1Microbiological testing/measurementImmunoglobulinsReference genesLymphocytic cell
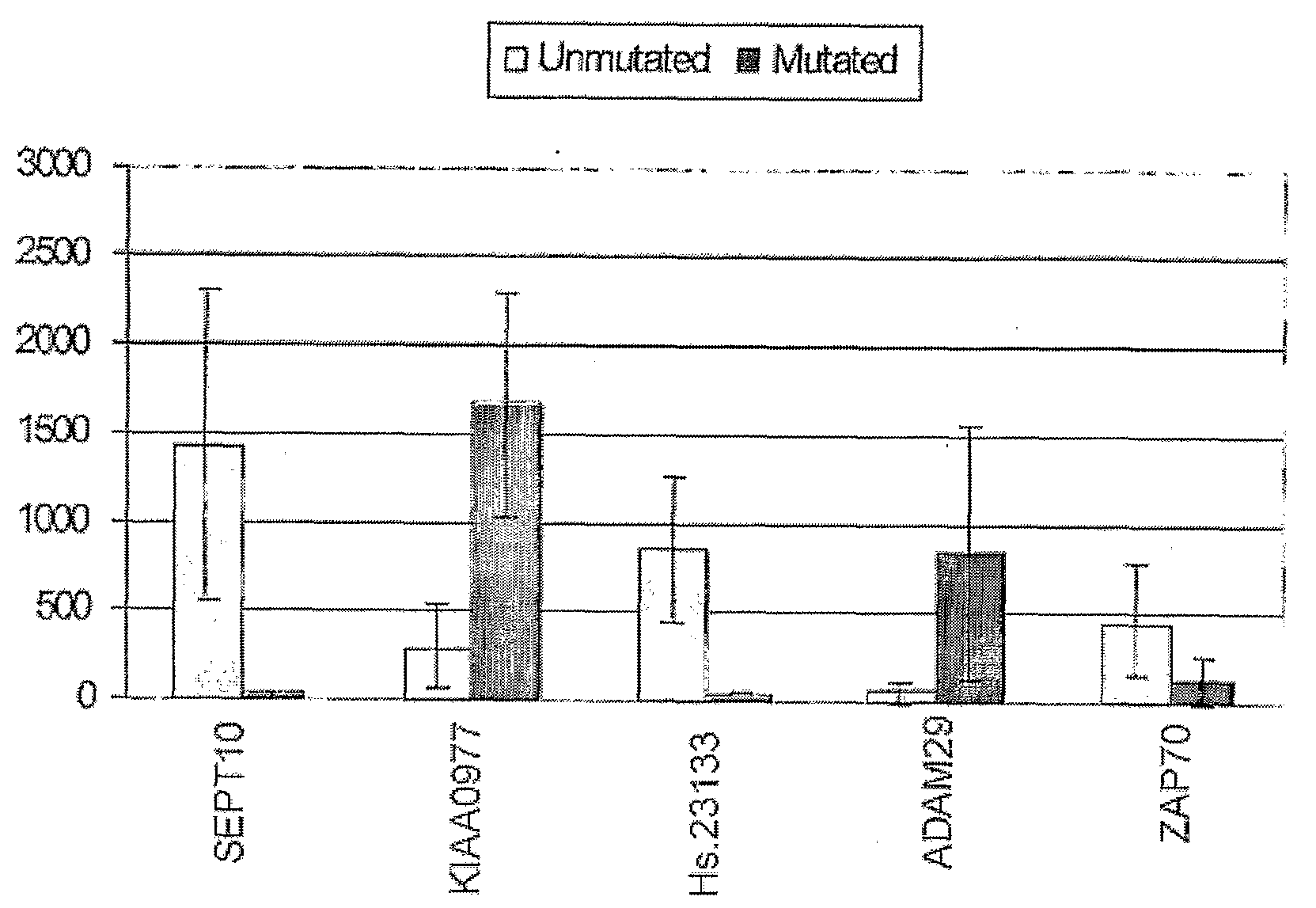
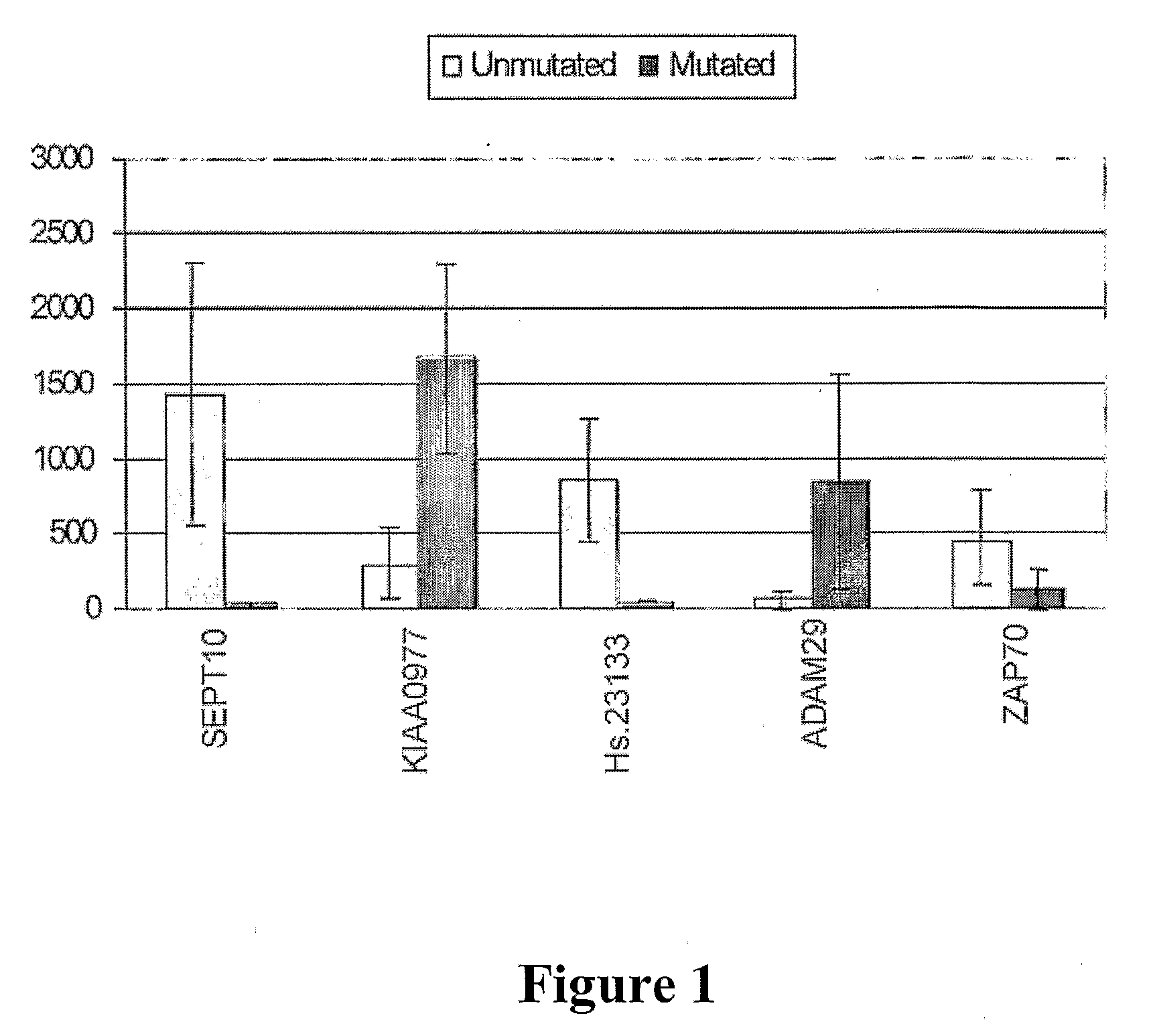
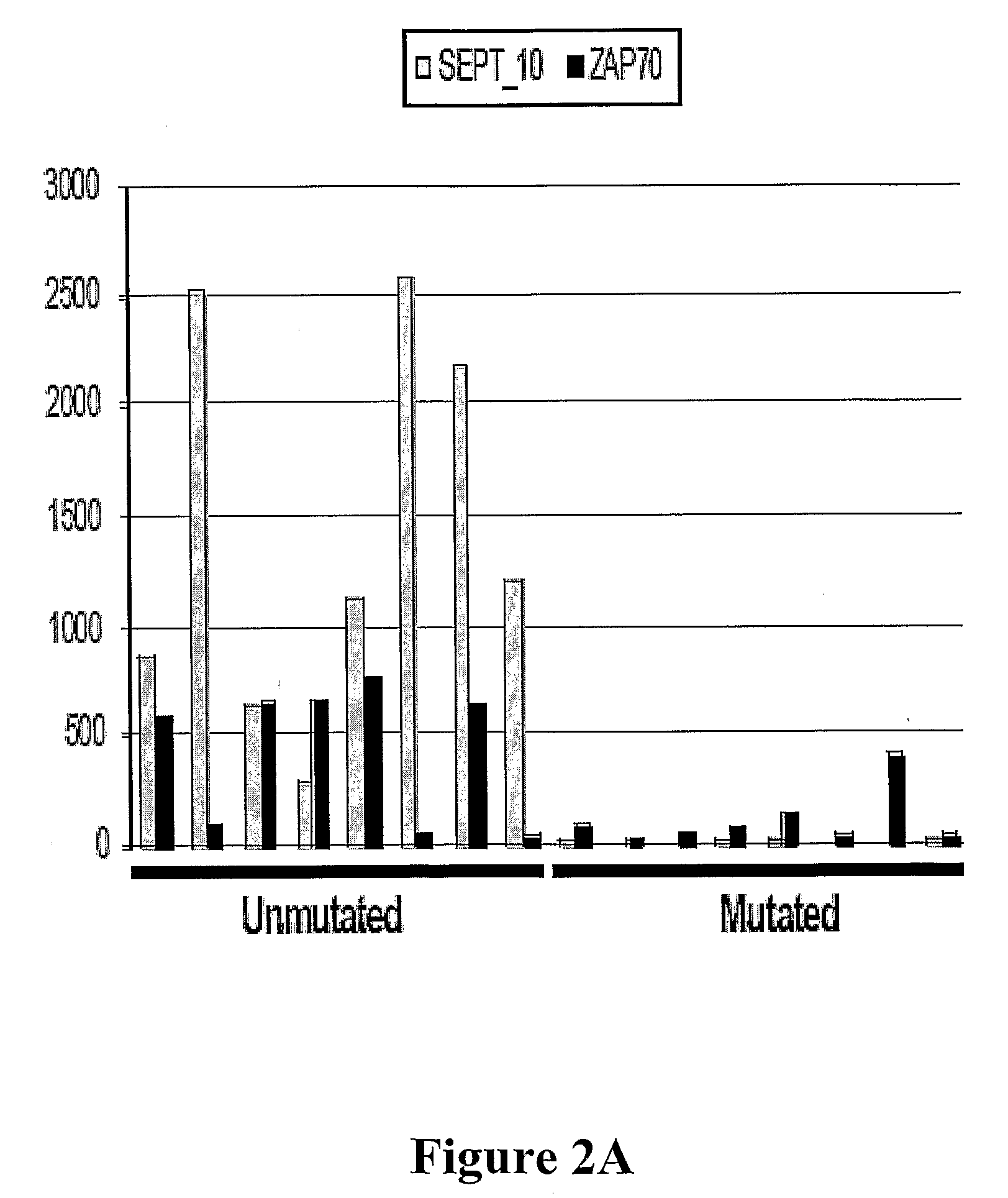
The invention provides compositions and methods for determining a prognosis of a B cell chronic lymphocytic leukemia (CLL) in a subject based on the level of expression of at least one marker gene. Marker genes provided by the invention are SEPTlO, KIAA0799, Hs.23133, and ADAM29. The marker genes can be used to differentially diagnose CLL in a subject based on relative gene expression levels in the subject compared to reference gene expression levels established from a clinically characterized population of patients. The invention also provides diagnostic reagents and compositions and kits based on the marker genes.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Anti-lag-3 antibodies to treat hematological malignancies
Provided are methods for clinical treatment of hematological malignancies, such as relapsed or refractory chronic lymphocytic leukemia or lymphoma using an anti-LAG-3 antibody. Particular malignancies include, e.g., chronic lymphocytic leukemia (CLL), Hodgkin lymphoma (HL), or non-Hodgkin lymphoma (NHL).
Owner:BRISTOL MYERS SQUIBB CO
Hat acetylation promoters and uses of compositions thereof in promoting immunogenicity
InactiveUS20100166781A1Improving immunogenicityPromoting surfaceAntibacterial agentsBiocideMHC class IFactor ii
The invention provides processes and compositions for enhancing the immunogenicity of TAP-1 expression-deficient cells by increasing the presentation of MHC Class I surface molecules for detection by cytotoxic T-lymphocyte cells through increased TAP-1 expression, which comprises administering to the TAP-1 expression-deficient cells a TAP-1 expression increasing amount of a bio-acceptable substance that promotes transcription of TAP-1 gene in the cells to cause enhanced MHC Class I surface expression of the cells. The bio-acceptable substance may be a histone H3 deacetylase inhibitor, such as trichostatin A, a transcriptional co-activator having intrinsic histone acetyl transferase activity or a histone acetyl transferase comprising at least one member of the CBP / p300 protein family. The process and compositions increase the immunogenicity of the target cells to enhance their destruction by cytotoxic lymphocytes.
Owner:JEFFERIES WILFRED
Treatment of cancer
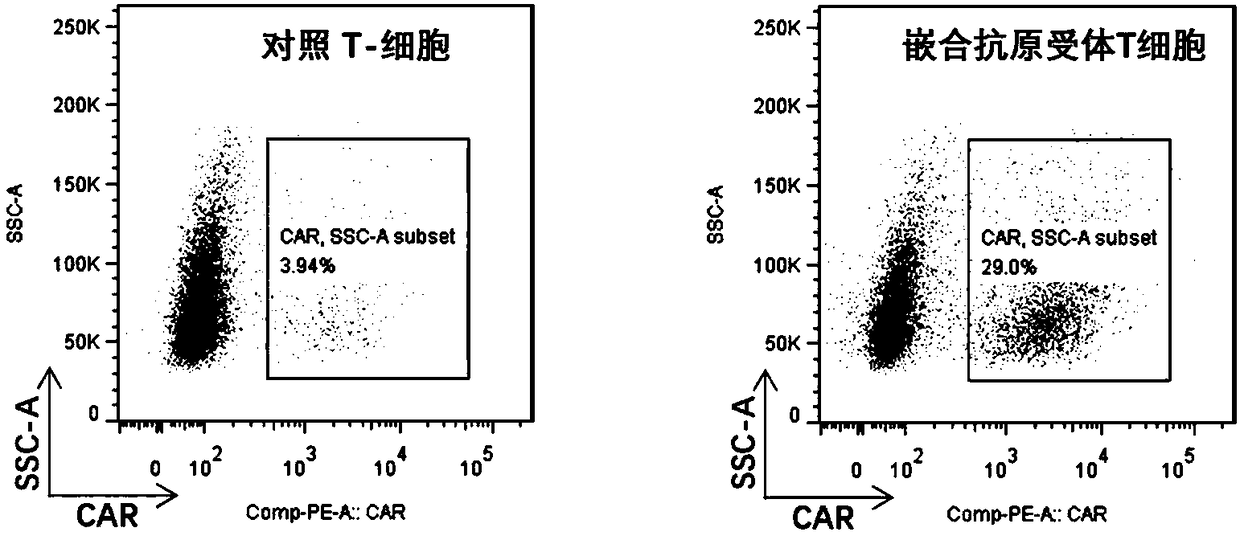
InactiveUS20170319638A1Strong specificityImprove securityViral/bacteriophage medical ingredientsMammal material medical ingredientsLymphocyteChimeric antigen receptor
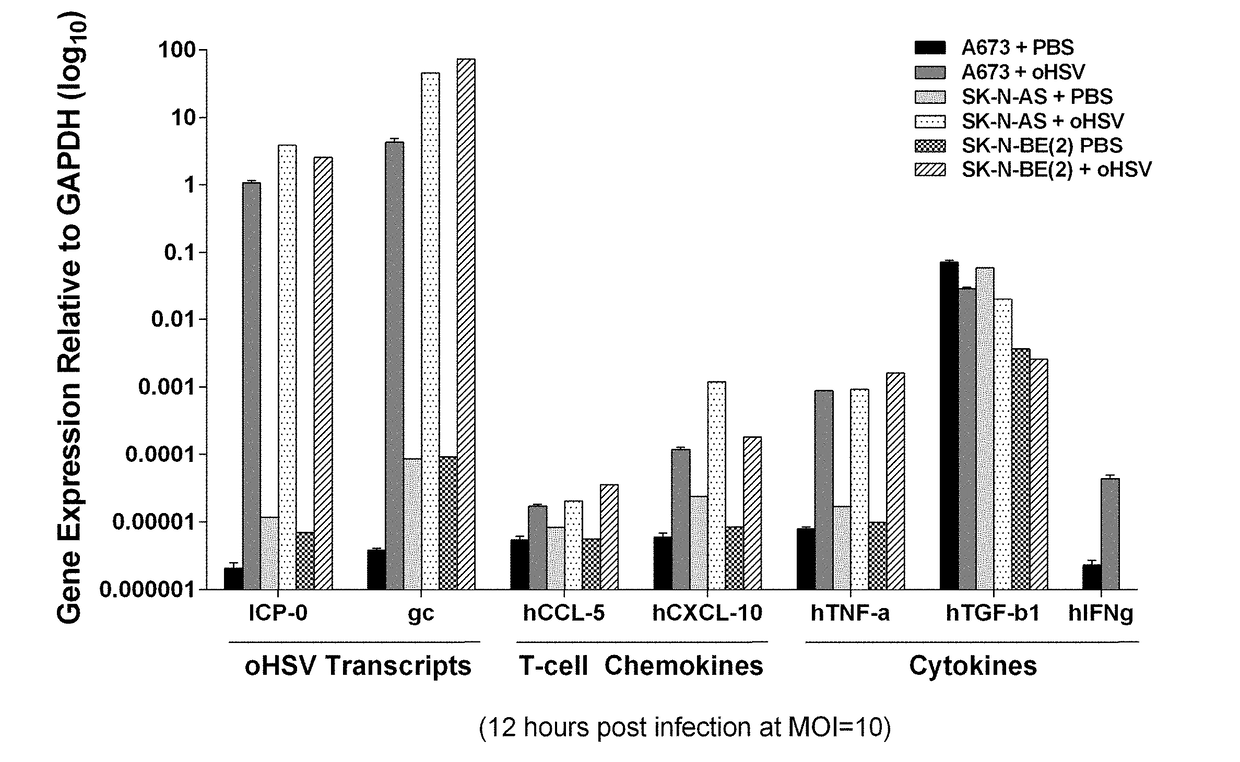
A method of treating cancer in a subject is disclosed, the method comprising administration of an oncolytic herpes simplex virus and administration of lymphocyte cells modified to express a chimeric antigen receptor (CAR) or modified to express a T cell receptor (TCR).
Owner:VIRTTU BIOLOGICS
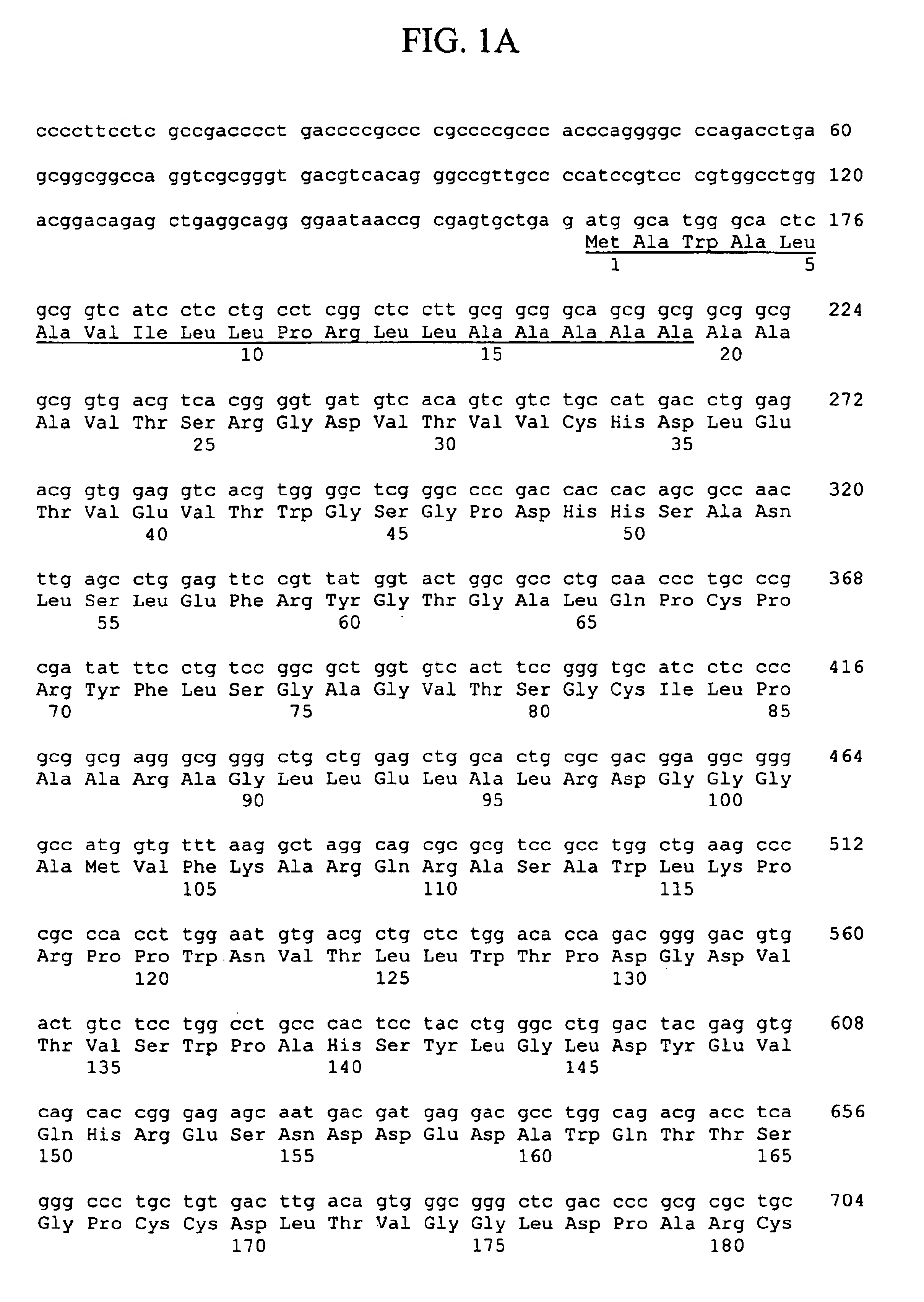
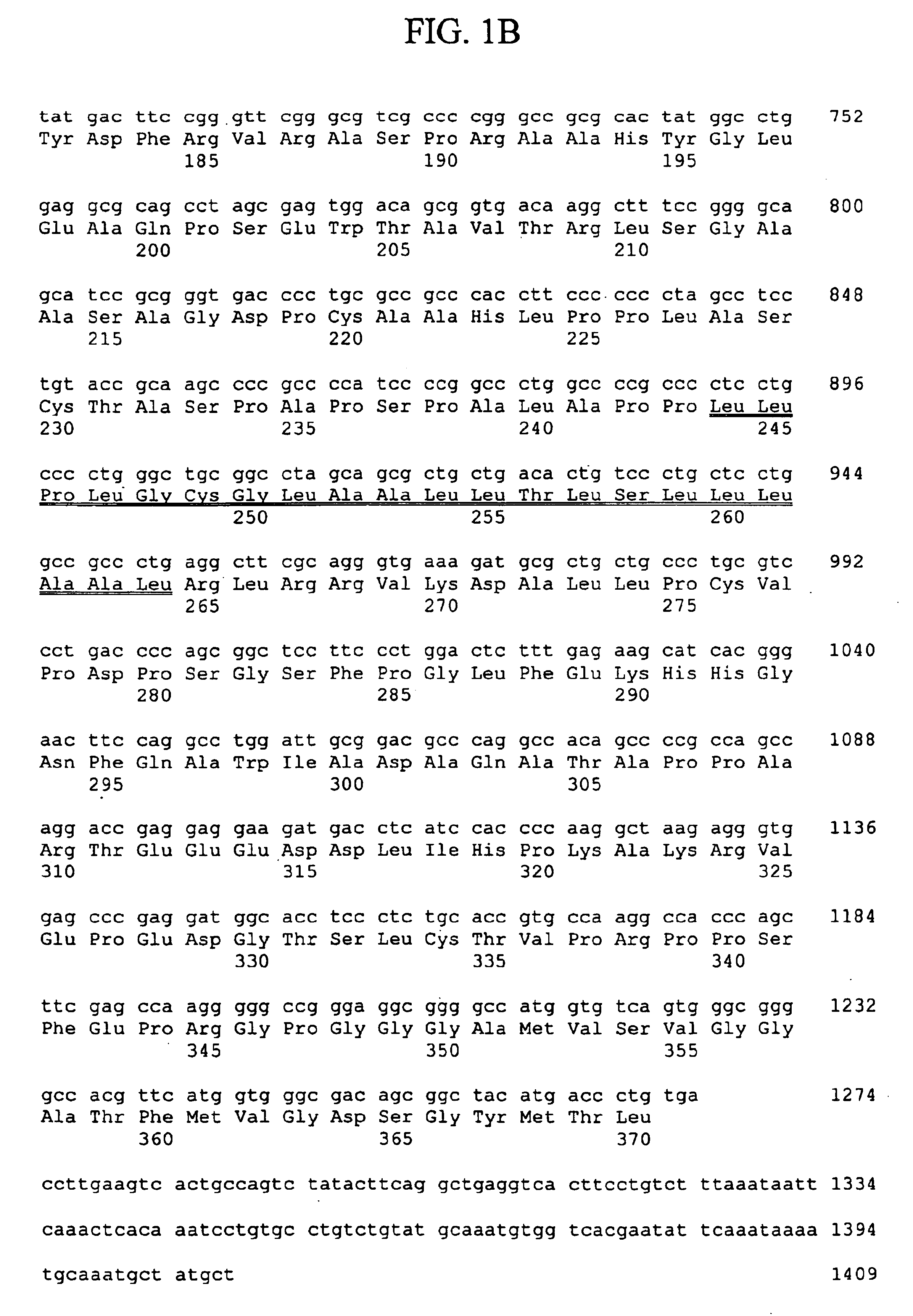
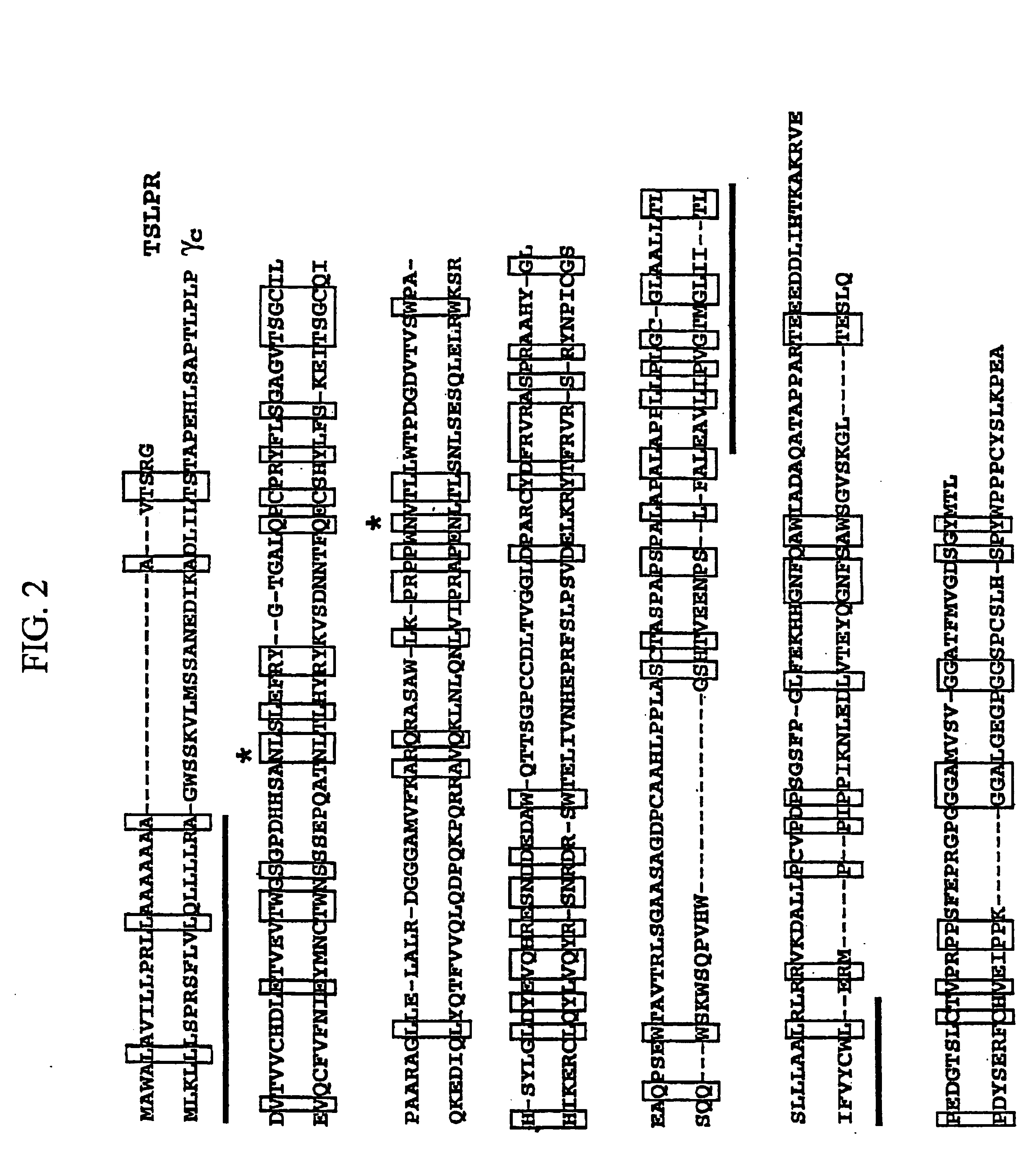
Thymic stromal lymphopoietin receptor molecules and uses thereof
The present invention provides Thymic Stromal Lymphopoietin Receptor (TSLPR) polypeptides and nucleic acid molecules encoding the same. The invention also provides selective binding agents, vectors, host cells, and methods for producing TSLPR polypeptides. The invention further provides pharmaceutical compositions and methods for the diagnosis, treatment, amelioration, and / or prevention of diseases, disorders, and conditions associated with TSLPR polypeptides.
Owner:HEALTH & HUMAN SERVICES THE NAT INST OF HEALTH GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY SEC DEPT OF THE +2
Chimeric antigen receptor T cells targeting CD19, and application of chimeric antigen receptor T cells
InactiveCN108276497AInhibit tumor cell proliferationActivate the killing mechanismMammal material medical ingredientsImmunoglobulinsNH lymphomaInterleukin 2
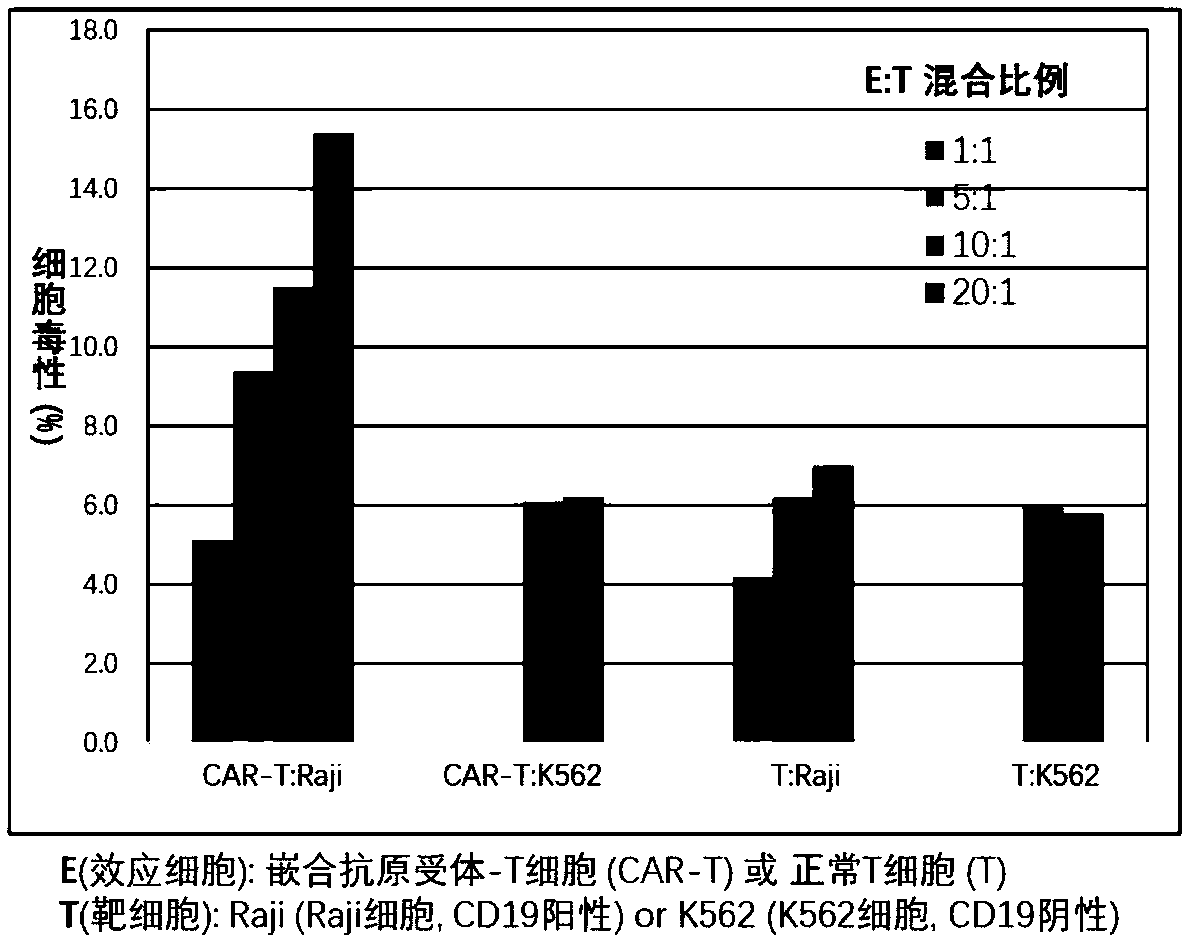
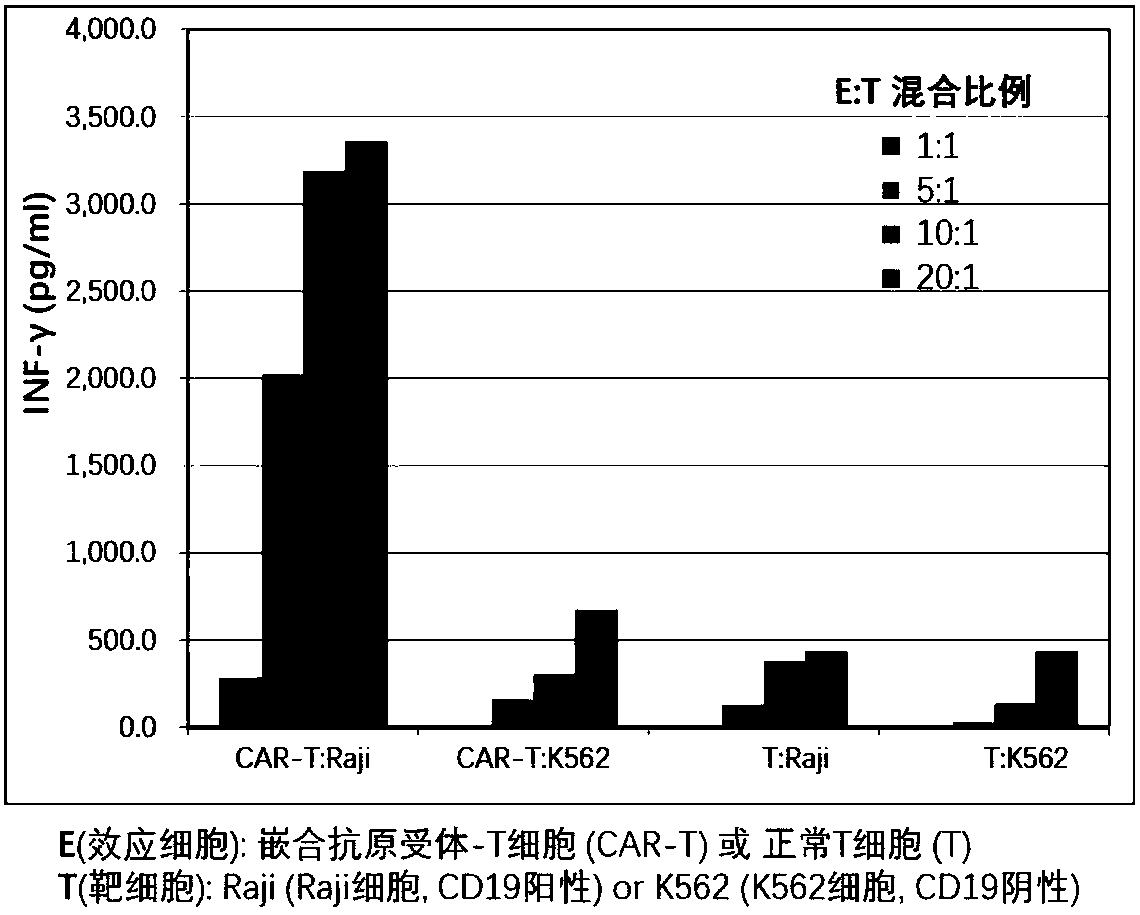
The invention discloses chimeric antigen receptor T cells targeting CD19, and application of the chimeric antigen receptor T cells. A chimeric antigen receptor for preparing the chimeric antigen receptor T cells comprises interleukin 2 signal peptide, an anti-CD19 single chain antibody, a CD8 protein molecular hinge region, a transmembrane region, an intracellular signal structural domain, and anintracellular signal transduction structural domain of CD3 zeta protein molecules which are sequentially connected in series. The chimeric antigen receptor T cells are used for preparing medicines orpreparations for treating hematological malignancies, wherein the hematological malignancies comprise CD19-positive acute B-lymphocytic leukemia, diffuse large B-cell lymphoma and non-Hodgkin lymphoma.
Owner:英普乐孚生物技术(上海)有限公司
Process for producing human-origin immunocompetent cell
It is an object of the present invention to provide an immunodeficient animal capable of generating human-derived lymphoid cells, a human-derived lymphoid cell, and a method for producing a human antigen-specific antibody.The means for solving the aforementioned object is: an immature immunodeficient mammal into which human-derived hematopoietic precursor cells have been transplanted, and which is able to generate said human-derived hematopoietic cells or immunocompetent cells; and a method for producing a human-derived antibody, which is characterized in that it comprises recovering immunocompetent cells from the above-described mammal, culturing the immunocompetent cells, and collecting a human-derived antibody from the obtained culture product.
Owner:KYUSHU UNIV
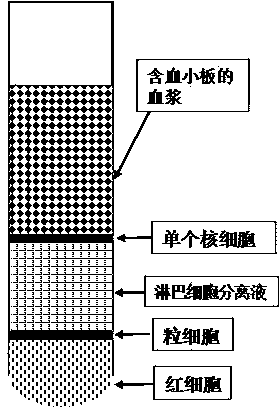
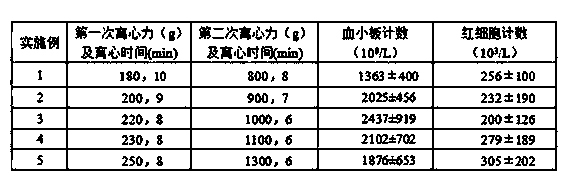
Preparation method for platelet rich plasma (PRP)
ActiveCN104337835AIncrease concentrationReduce in quantityMammal material medical ingredientsBlood collectionLymphocytic cell
The invention belongs to the technical field of blood products and particularly relates to a preparation method for platelet rich plasma (PRP). The method comprises a blood collection step, a lymphocyte separation medium treatment step, a first-time centrifugation step, a second-time centrifugation step and the like. According to the preparation method disclosed by the invention, on the basis of the existing PRP preparation technology, firstly, collected blood is treated by adopting a lymphocyte separation medium, and then, by optimizing a centrifugal force and time of centrifugation, the PRP concentration effect of the prepared PRP is further promoted, and the number of red blood cells is greatly reduced; the preparation method has better practical application value.
Owner:HENAN JINTAI BIOTECH
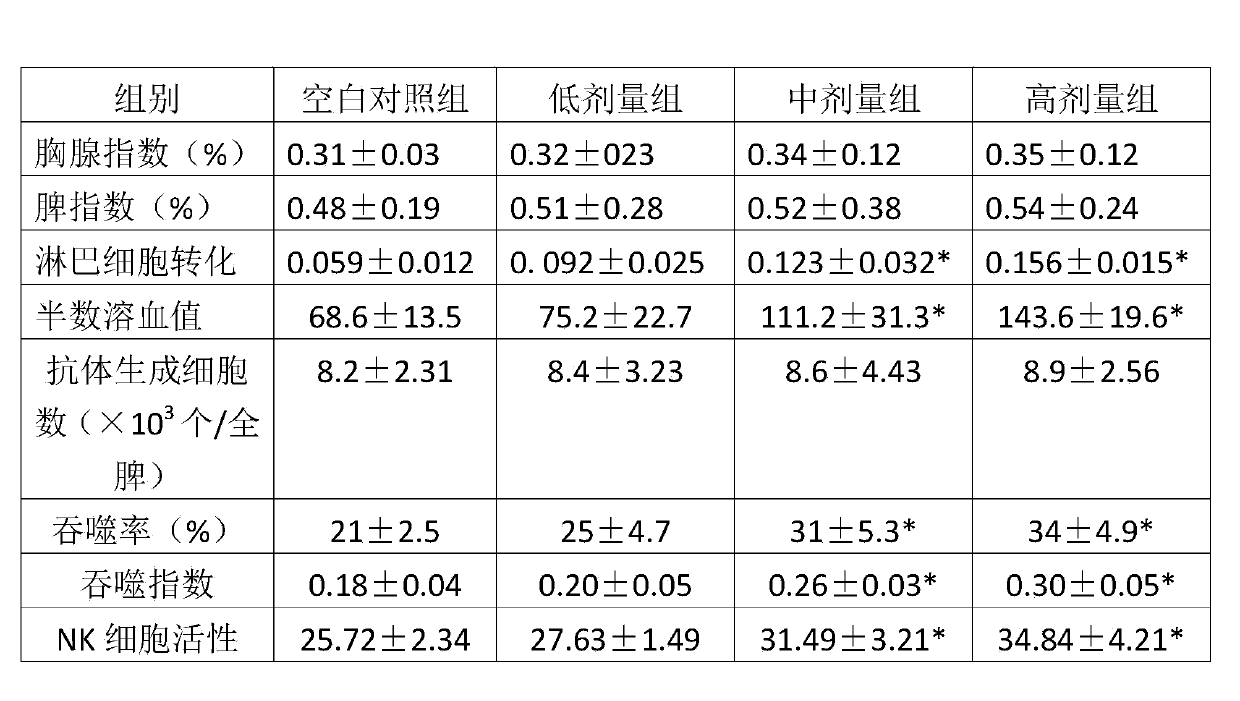
Lactobacillus rhamnosus and application thereof
ActiveCN103421715AGood antibacterial effectPromote conversionBacteriaBacteria material medical ingredientsLymphocyte transformationLactobacillus rhamnosus
The invention aims to provide lactobacillus rhamnosus having bacteriostatic and immunity enhancement function, with the preservation number: CCTCC NO: M 2013355. The lactobacillus rhamnosus provided by the invention can be applied in the biological products which are medicines, food or health products. The lactobacillus rhamnosus has very powerful bacteriostatic ability, can obviously improve the achroacyte conversion and HCso of a mouse, phagocytosis rate and index of macrophage and NK cell activity, can enhance normal mouse immunity, and therefore can be widely applied in the medicines, food or the health products.
Owner:QINGDAO VLAND BIOTECH GRP
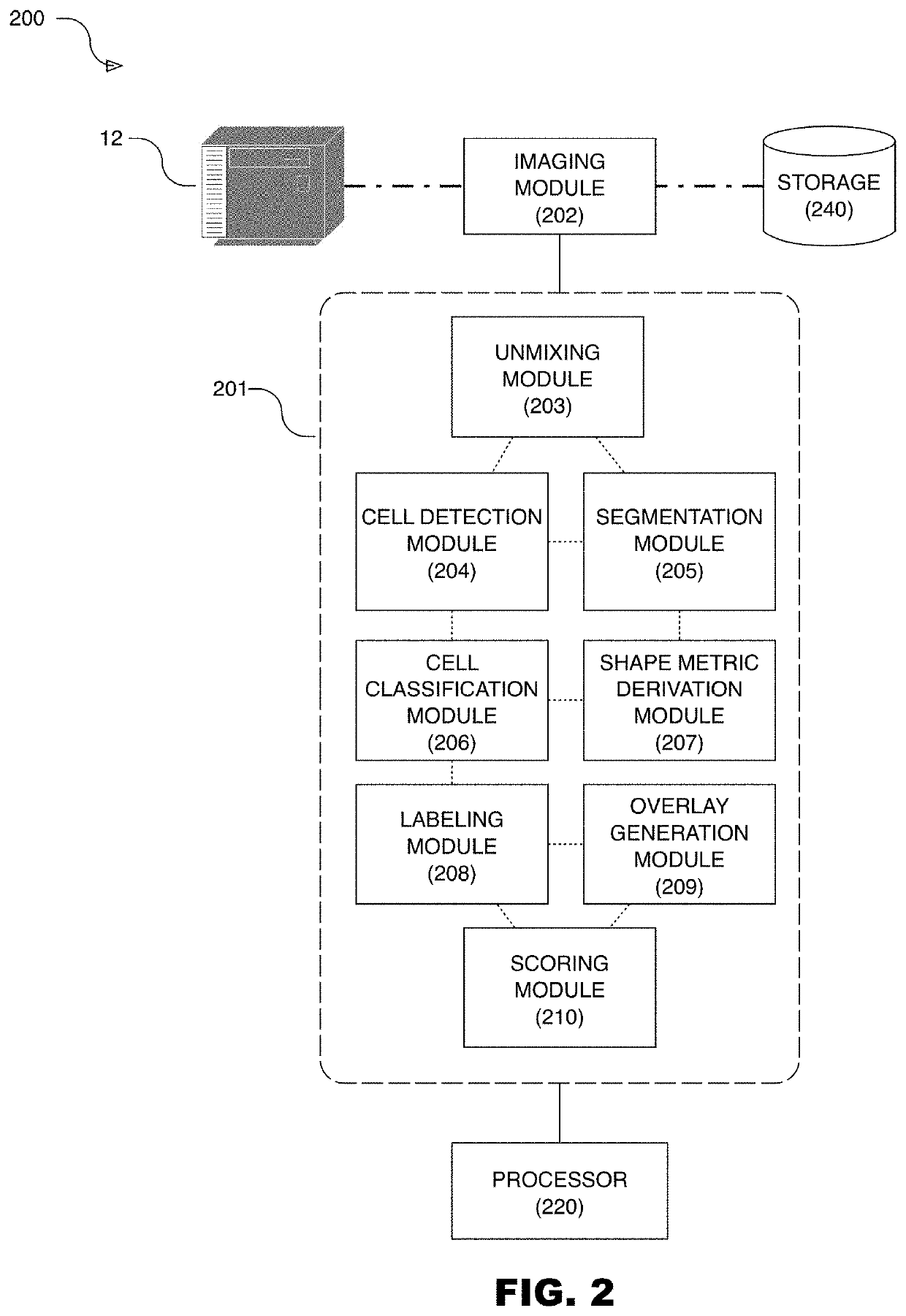
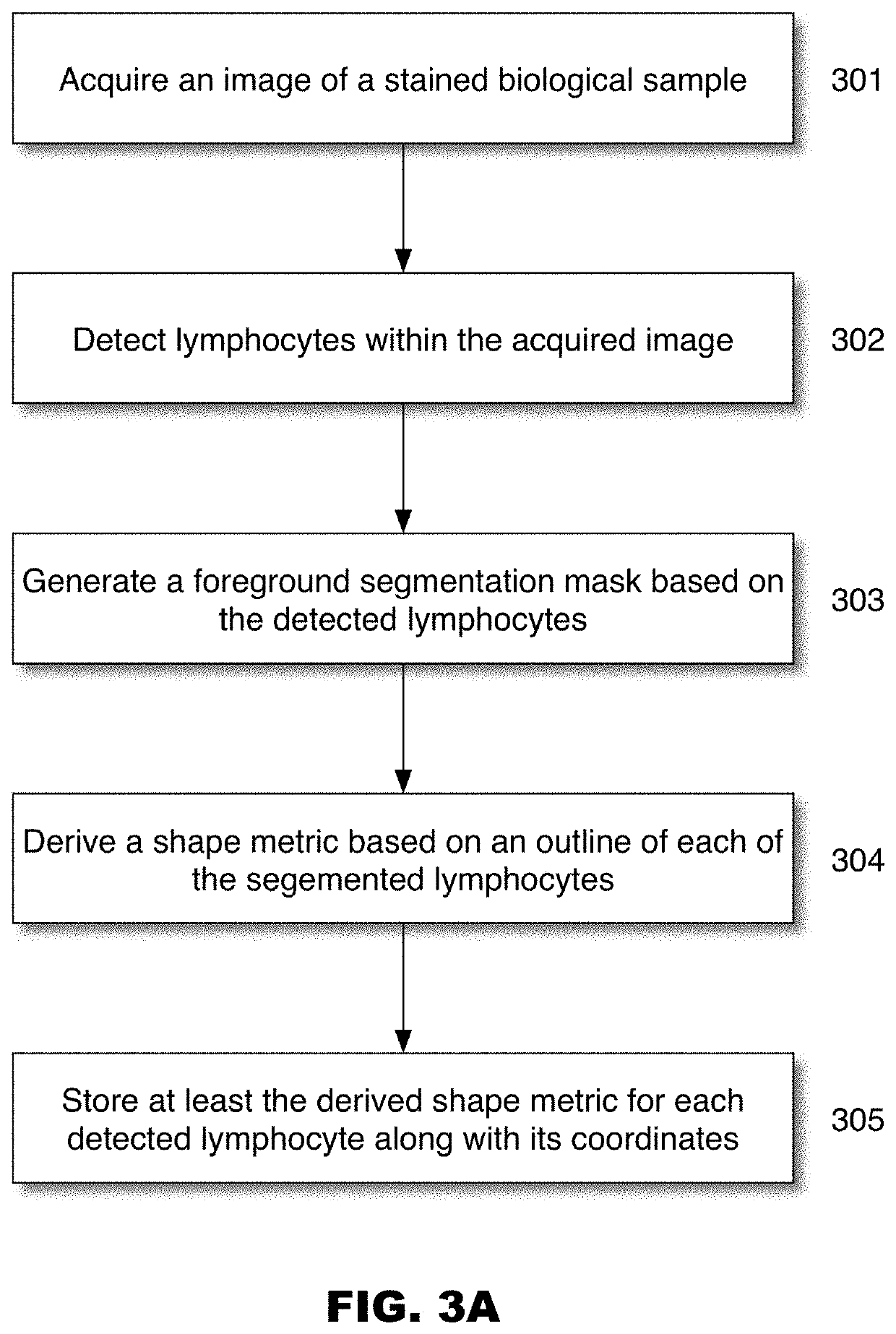
Systems for cell shape estimation
PendingUS20210027462A1Improve efficiencyFacilitate high speed processingImage enhancementImage analysisLymphocytic cellData mining
The present disclosure is directed, among other things, to automated systems and methods for analyzing, storing, and / or retrieving information associated with biological objects including lymphocytes. In some embodiments, a shape metric is derived for each detected and segmented lymphocyte and the shape metric is stored along with other relevant data.
Owner:ROCHE DIAGNOSTICS GMBH +1
BTK inhibitor and uses thereof
ActiveCN105399756AEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryChronic lymphocytic leukemiaFollicular lymphoma grade II
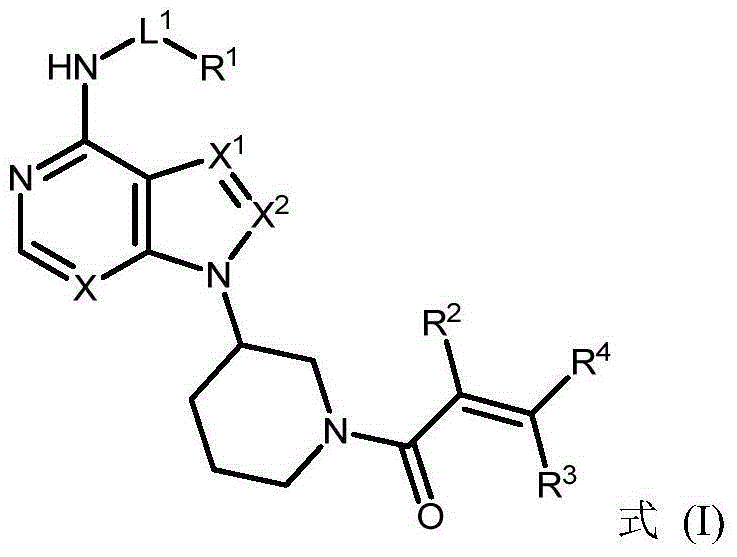
The present invention provides a BTK inhibitor compound (having s structure represented by a formula (I)) and uses of the BTK inhibitor compound in medicines. According to the present invention, the compound and the pharmaceutical composition can be used for treatment of diffuse large B-cell lymphoma, follicular lymphoma or chronic lymphocytic leukemia. The formula (I) is defined in the specification.
Owner:SUNSHINE LAKE PHARM CO LTD
Children acute lymphoblastic leukaemia genotyping diagnosis chip
InactiveCN101525667AReduce complicationsHigh cure rateNucleotide librariesMicrobiological testing/measurementLife qualityA-DNA
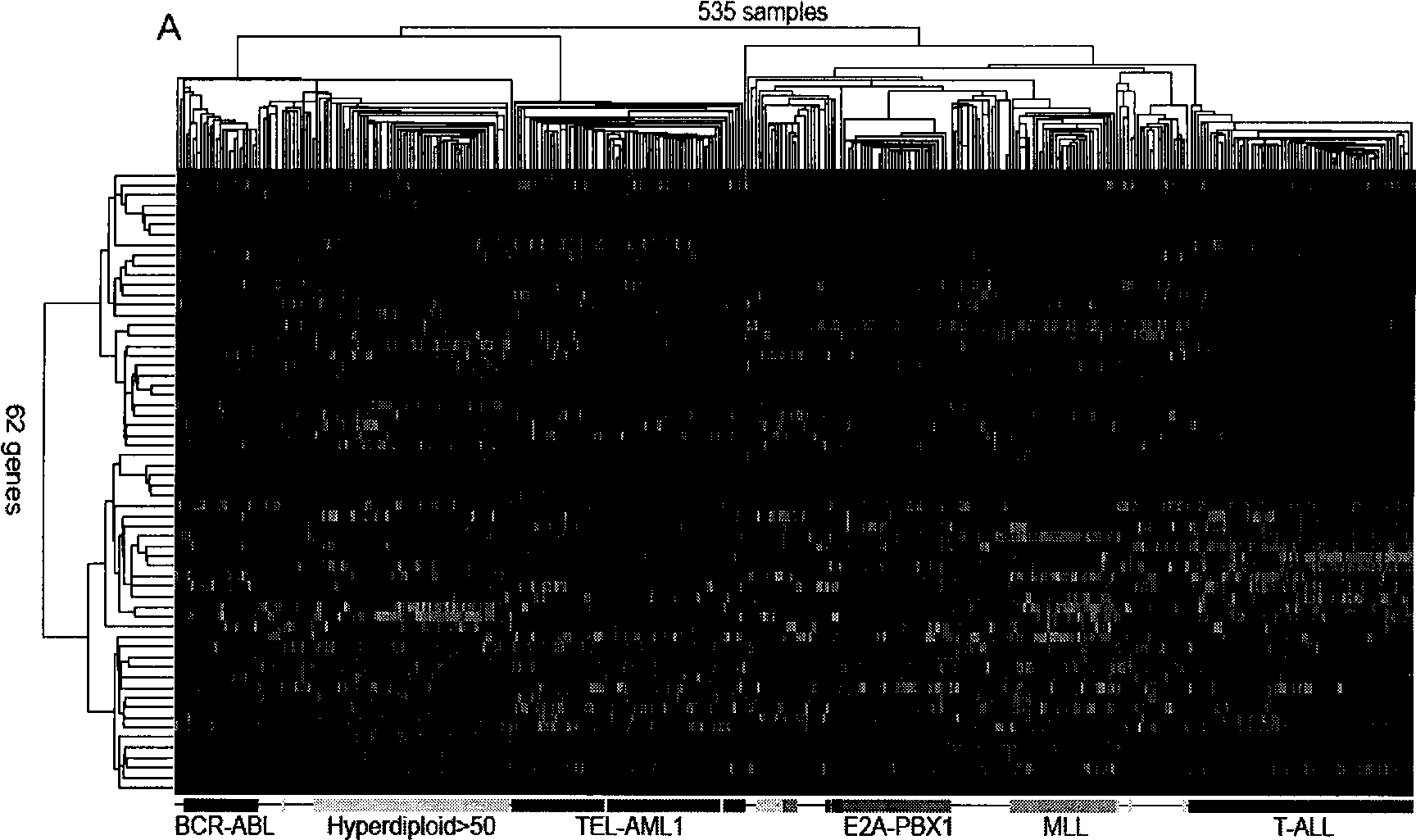
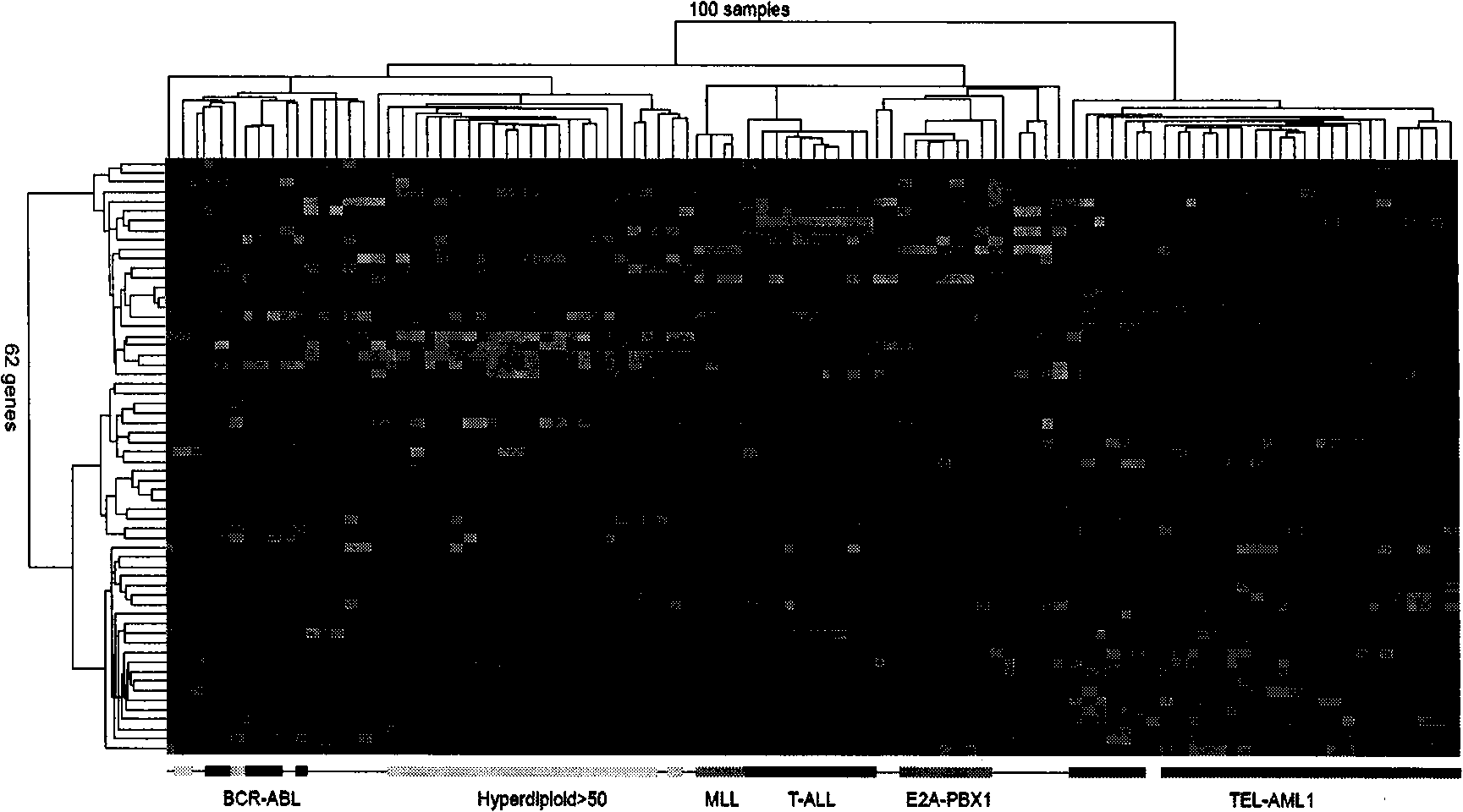
The invention discloses a children acute lymphoblastic leukaemia genotyping diagnosis chip. The children acute lymphoblastic leukaemia genotyping diagnosis chip is a DNA chip which is fixed with 62 DNA fragment arrays on the surface of a carrier. .The nucleotide sequences of the 62 DNA fragment arrays are respectively the sequence 1 to the sequence 62 of the sequence list. The invention further discloses a children acute lymphoblastic leukaemia genotyping method. The genetic chip of the invention can provide precise typing so as to help correctly choose a chemo-treatment plan of appropriate strength, thereby reducing complicating disease and improving curative ratio and life quality. The genetic chip of the invention can be used to carry out genotyping on childhood ALL. Therefore, the genetic chip not only saves diagnosis cost, but also has significance in protecting labour resource and promoting family and society harmony.
Owner:BEIJING CHILDRENS HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV +1
Highly aggressive human acute B lymphocytic leukemia cell strain with add(11)(q23) chromosome abnormality
The invention belongs to the field of microbial animal cell lines, and relates to a new human acute B lymphocytic leukemia cell strain CHH-1. The in vitro isolate of the mononuclear cell of the marrow of a patient with firstly-diagnosed acute B lymphocytic leukemia undergoes cell primary culture to obtain the highly aggressive human acute B lymphocytic leukemia cell strain with add(11)(q23) chromosome abnormality, the preservation number of the cell strain is CGMCC No.11797, and the highly aggressive human acute B lymphocytic leukemia cell strain with add(11)(q23) chromosome abnormality is named as human acute B lymphocytic leukemia cell strain CHH-1, has a same clone source with patient's leukemia cells, can be infinitely and stably passed in vitro, and has the characteristics of clonality add(11)(q23) genetic abnormality, high tumorigenicity and high aggressiveness. The new human acute B lymphocytic leukemia cell strain CHH-1 provides a new good cell model for researches of human acute lymphocytic leukemia with No.11 chromosome long arm structure abnormality and development of biomedicines, and has wide prospect and great practical values in revelation of the pathogenesis of the human acute lymphocytic leukemia and development of medicines.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Methods for inducing a sustained immune response against a b-cell idiotype using autologous Anti-idiotypic vaccines
InactiveUS20120114634A1Eliminating and substantially reducing non-HodgkinEliminating and substantially reducingAntibody ingredientsCancer antigen ingredientsChronic lymphocytic leukemiaMantle lymphoma
The present invention relates to methods of inducing and maintaining an immune response against a B-cell idiotype in a subject using an autologous anti-idiotypic vaccine. In one embodiment, the immune response is induced and maintained for treatment of a B-cell derived malignancy selected from among non-Hodgkin's lymphoma. Hodgkin's lymphoma, chronic lymphocytic leukemia, multiple myeloma, and mantle cell lymphoma.
Owner:BIOVEST INT
Hyriopsis cumingii enzymolysis polypeptide, as well as preparation and application thereof
ActiveCN101503727AHigh in proteinEasy to storeHydrolysed protein ingredientsImmunological disordersArginineTert-leucine
The invention provides a Hyriopsis cumingii enzymolysis polypeptide, preparation and application thereof. The enzymolysis method comprises the following steps that: fresh Hyriopsis cumingii meat is crushed and pulped, and added with water of which the weight is 5 to 6 times of that of the fresh Hyriopsis cumingii meat; the mixture is boiled and filtered; the precipitate is dried and degreased to obtain albumen powder of the Hyriopsis cumingii meat; the albumen powder of the Hyriopsis cumingii meat is soaked in water of which the weight is 6 to 8 times of that of the albumen powder of the Hyriopsis cumingii meat for 11 to 12 hours and subjected to superfine grinding and even pulping, and the pH value of the mixed solution is adjusted to between 8.0 and 9.0; based on the albumen content in the albumen powder of the Hyriopsis cumingii meat, 0.3 to 0.6 percent of alkali protease is added in the mixed solution for enzymolysis for 4 to 6 hours at a temperature of between 40 and 60 DEG C; the obtained supernatant is condensed and dried to obtain the Hyriopsis cumingii enzymolysis polypeptide; and by testing, the content of the total albumen of the Hyriopsis cumingii enzymolysis polypeptide is more than or equal to 80 percent, wherein the contents of asparagic acid, glutamic acid, leucine, lysine and arginine are more than or equal to 50 percent, and the molecular weight is mainly concentrated in two zones of 6,100Da and 26,200Da. By a test for culturing mouse lymphocytes in vitro, the Hyriopsis cumingii enzymolysis polypeptide can be used for preparing a medicament for promoting proliferation of the T lymphocute and a health care food for improving the immunity.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
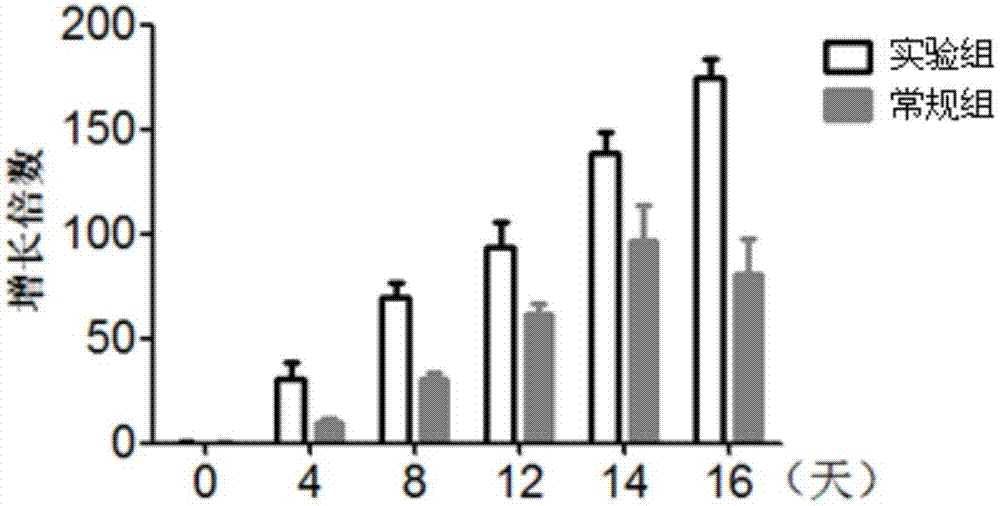
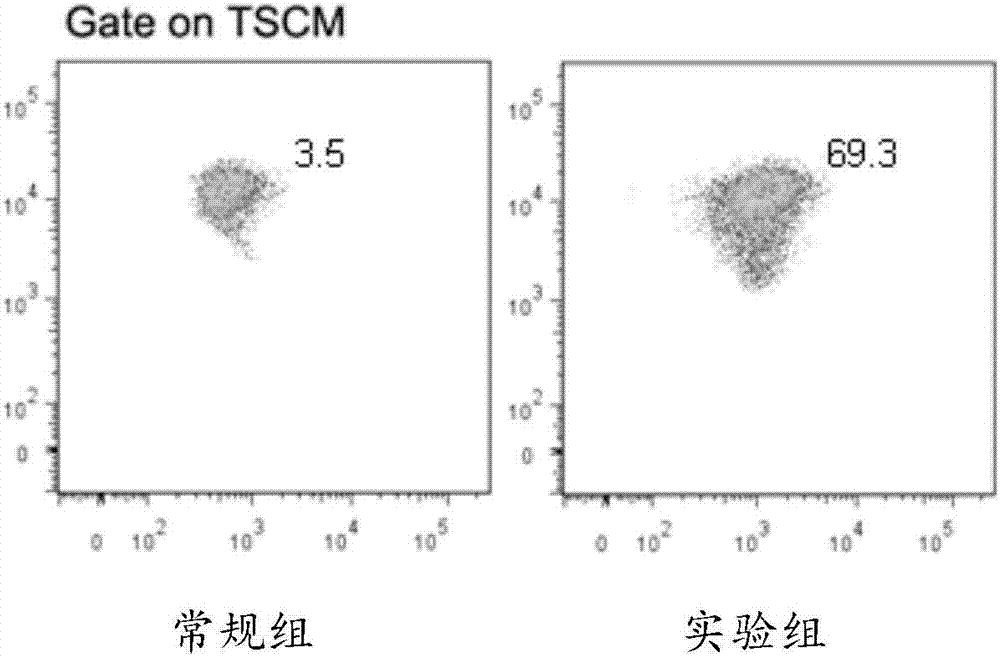
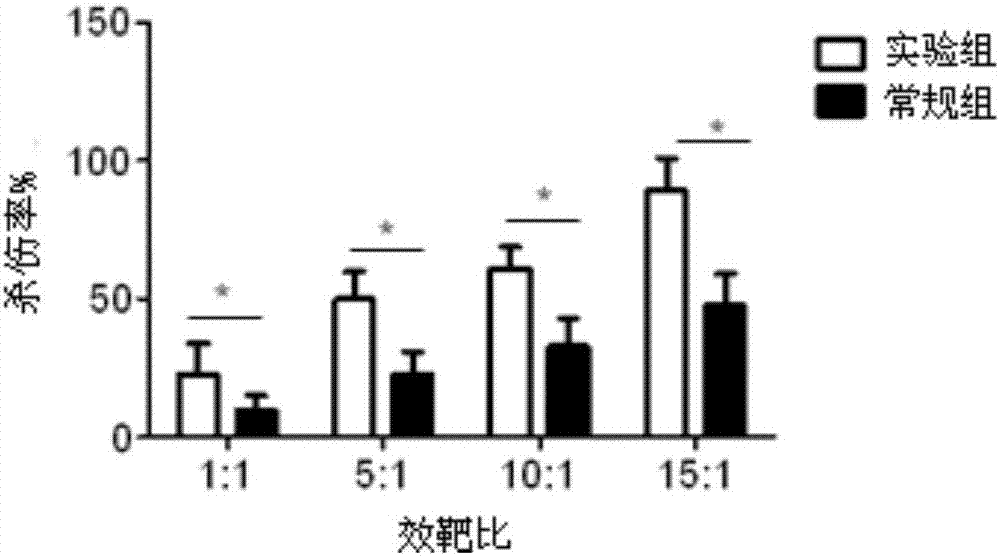
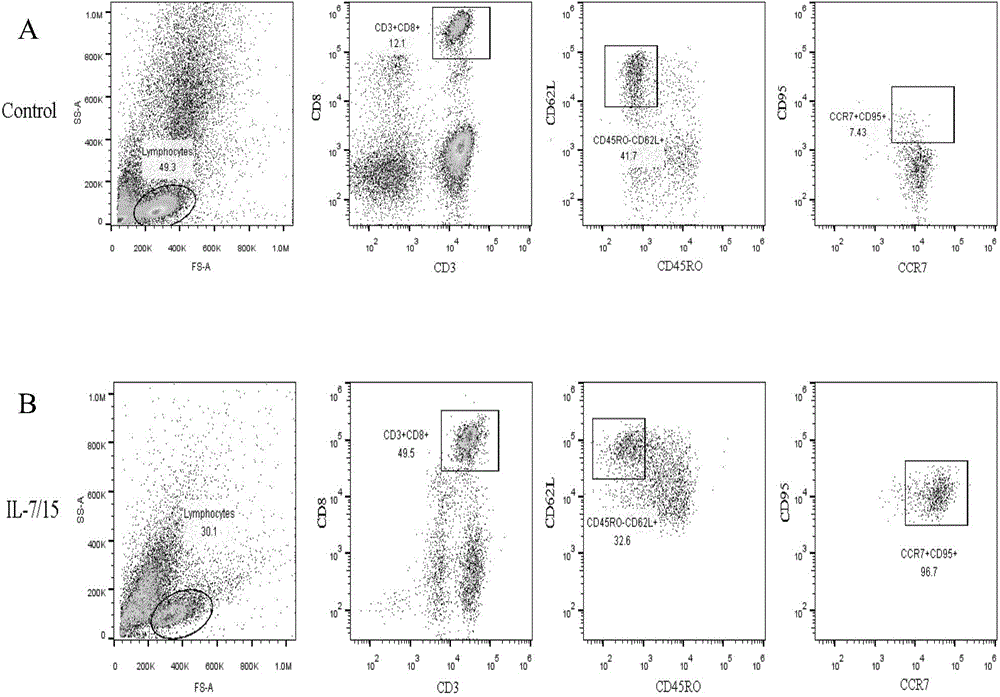
Lymphocyte population with memory stem T cells as main component and in-vitro efficient amplification method of lymphocyte population
ActiveCN107475192AReduce freezingReduce dosageCulture processBlood/immune system cellsSerum free mediaPeripheral blood mononuclear cell
The invention discloses a lymphocyte population with memory stem T cells as a main component and an in-vitro efficient amplification method of the lymphocyte population. The method comprises the steps as follows: peripheral blood mononuclear cells from healthy individuals are transferred into a culture flask precoated with anti-CD3 mAb, anti-CD28McAb and Novonectin, a serum-free medium containing IL-1alpha and IFN-gamma is added, a combination of multiple cytokines including VC, NAC, RAPA, IL-7, IL-15 and IL-21 is added, and then the serum-free medium and the combination of the multiple cytokines are supplemented; and the lymphocyte population with the memory stem T cells as the main component can be obtained after culture for 12 to 16 days. The combined application of the cytokines can have synergistic effect on amplification of lymphocytes, the amplification times are increased, and the in-vitro killing property and survival time are increased obviously; the method has the advantages of high safety, low cost and the like.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT)
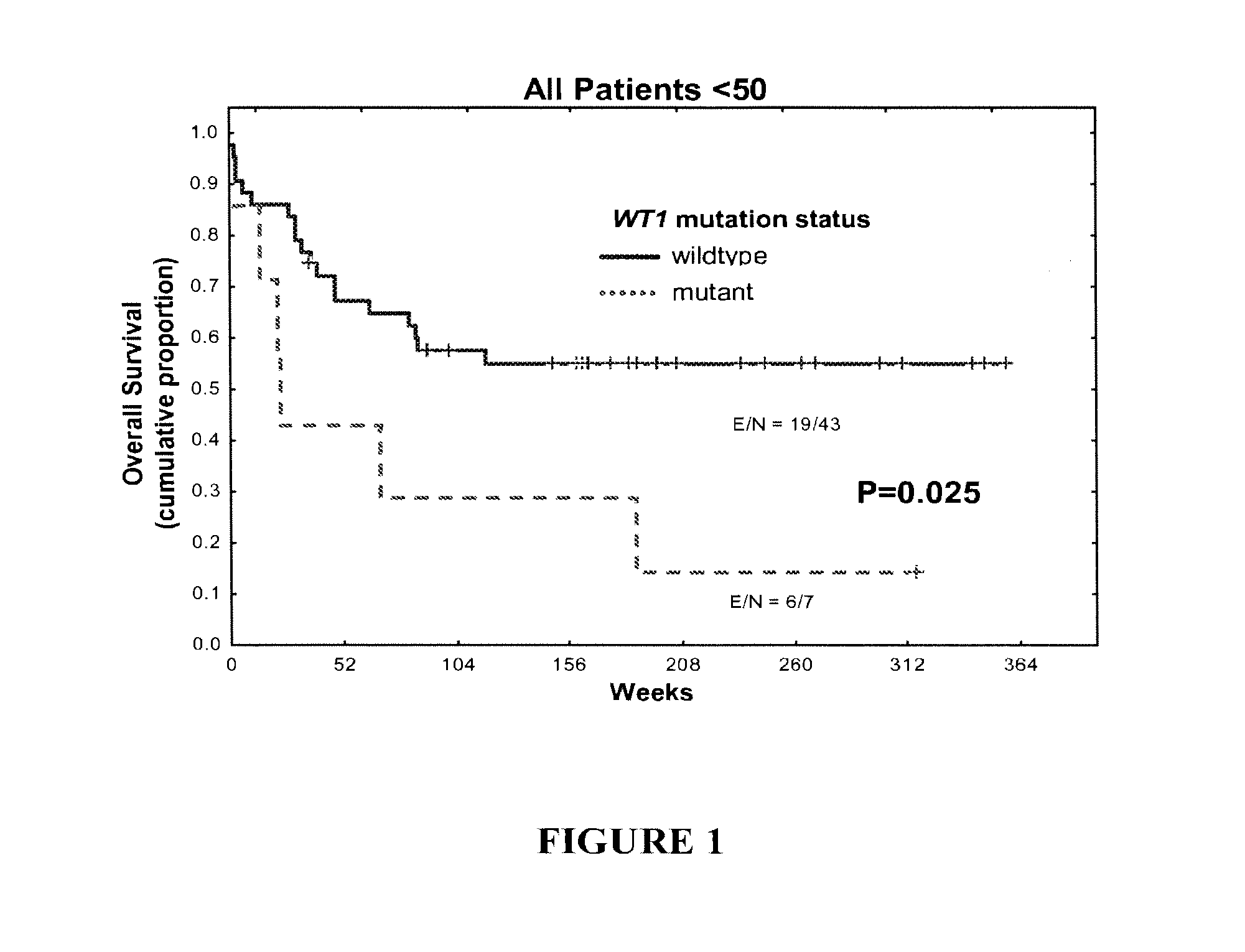
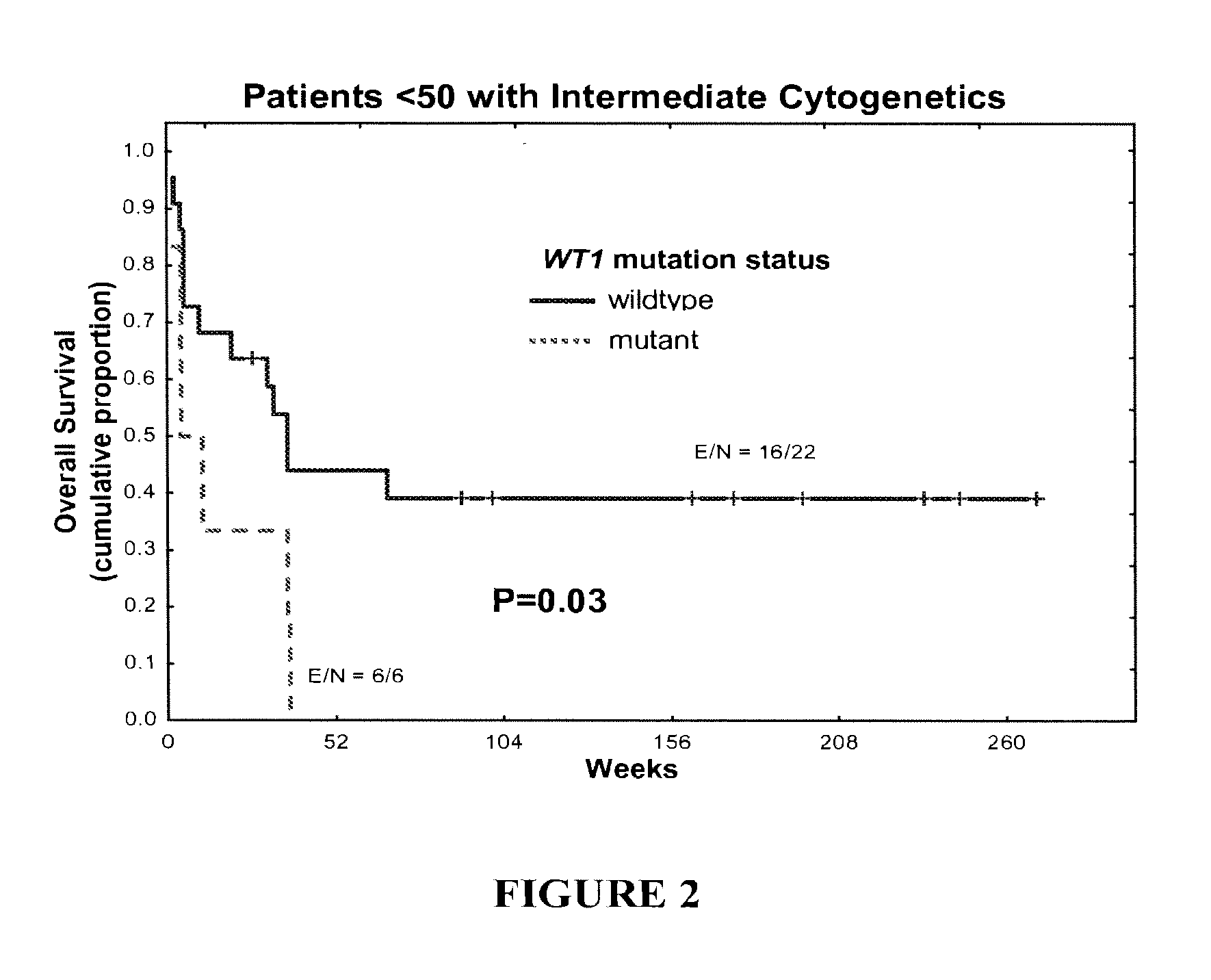
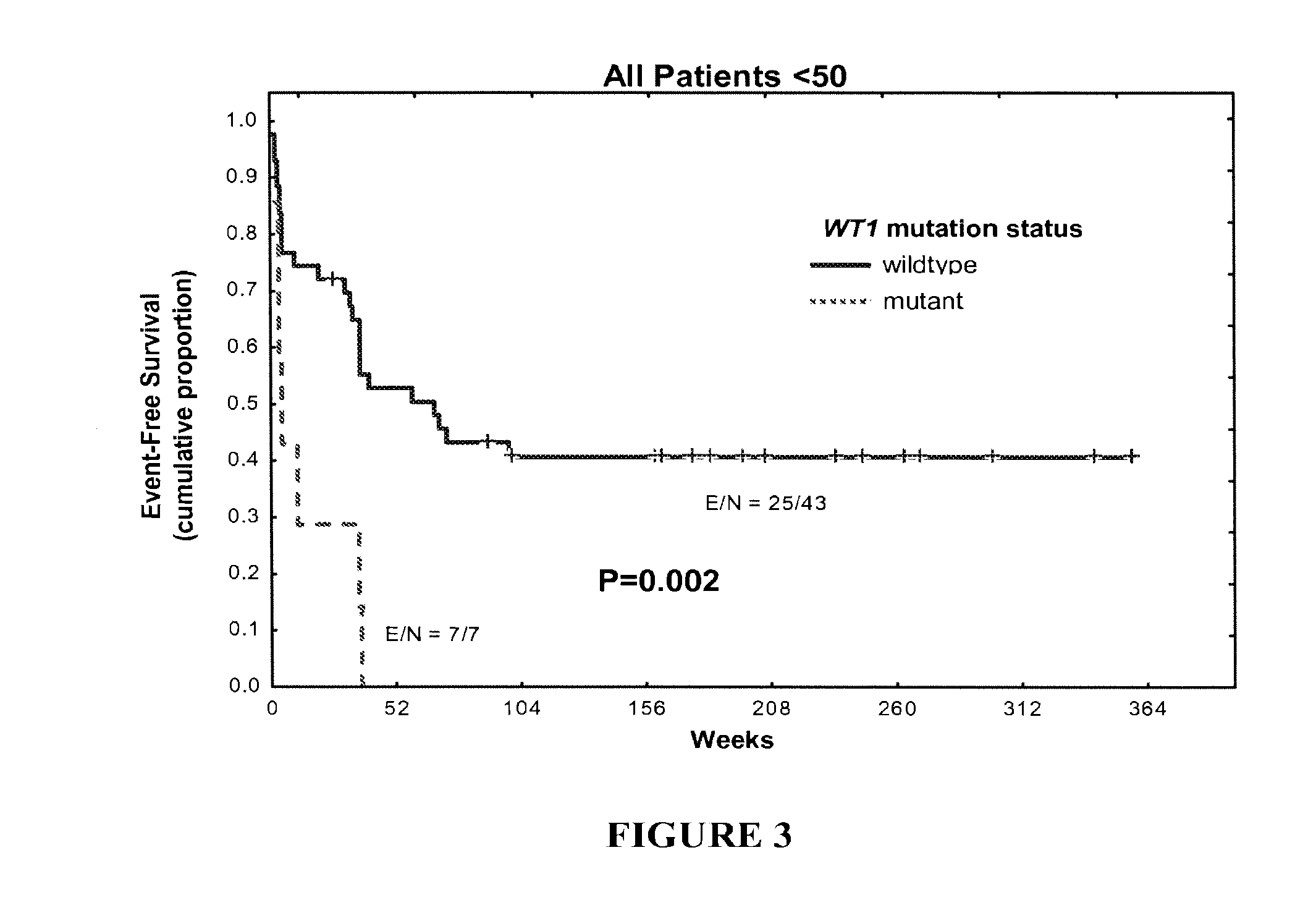
WT1 mutations for prognosis of myeloproliferative disorders
InactiveUS20160201141A1Poor outcomeShorten the construction periodMicrobiological testing/measurementMyeloproliferative DisordersLeukemia
The invention provides methods for determining the prognosis of a patient diagnosed with a leukemia, including B-cell chronic lymphocytic leukemia, by measuring mutations of the WT1 gene in a biological sample. The invention also relates to the diagnosis of leukemia, including B-cell chronic lymphocytic leukemia.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
Adoptive cellular therapy using an agonist of retinoic acid receptor-related orphan receptor gamma & related therapeutic methods
InactiveCN106132422AAntibacterial agentsBacterial antigen ingredientsAdoptive cellular therapyDendritic cell
The invention relates to medical therapy using an agonist of the retinoic acid receptor-related orphan receptor gamma (ROR gamma) and provides adoptive cellular therapies using an agonist of ROR gamma, populations of lymphocyte cells that have been exposed to an agonist of ROR gamma, populations of dendritic cells that have been exposed to an agonist of ROR gamma, pharmaceutical compositions, and methods for enhancing therapeutic effects of lymphocyte cells and / or dendritic cells in a patient by administering an agonist of ROR gamma to a patient.
Owner:LICELLA PTY LTD
B cell surface reactive antibodies
ActiveUS8877199B2Immunoglobulins against cell receptors/antigens/surface-determinantsRadioactive preparation carriersDiseaseB cell
The invention relates to antibodies that are reactive to the cell surface of CD19+ B cells, including B-cell chronic lymphocytic leukemia (B-CLL) cells, and compositions and methods for using such antibodies, including in the diagnosis and treatment of disorders associated with CD19+ B cells, such as B-CLL.
Owner:UNITED STATES OF AMERICA
Application of 5' nucleotide in preparation of medicines and health-care foods for improving weakened immune function
The invention relates to an application of 5' nucleotide in preparation of medicines and health-care foods for improving weakened immune function. The nucleotide is 5' nucleotide mixture which takes ribonucleic acid (RNA) as raw material, produced by enzymic degradation, has the purity of more than 99%, and is prepared by mixing 5' AMP, 5' CMP, 5' GMPNa2 and 5' UMPNa2 according to the mixture ratio of 22.8:26.6:30.2:20.4. The 5'nucleotide can remarkably enhance mouse ConA induced lymphopoiesis capacity, increases the quantity of hemolysis plaque, improves the spleen NK cell percentage and has obvious effect on improving weakened immune function since feed is short of the nucleotide.
Owner:珍奥集团股份有限公司
Lactobacillus rhamnosus with immunomodulatory effect and application of lactobacillus rhamnosus
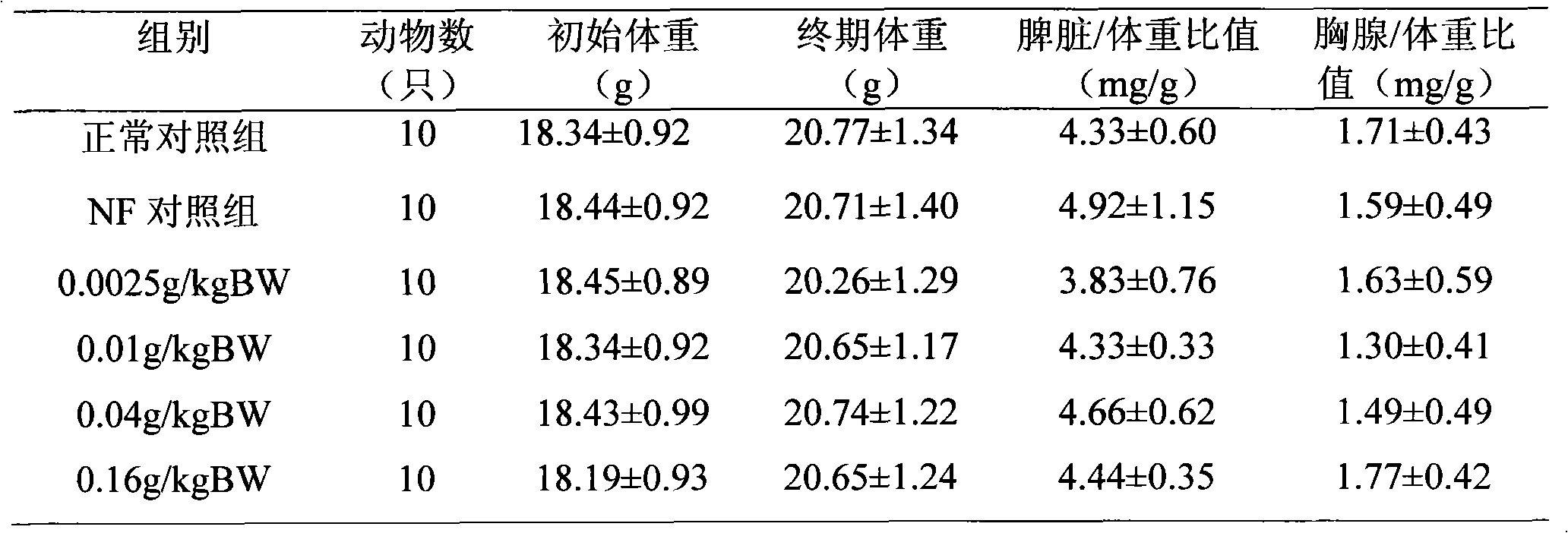
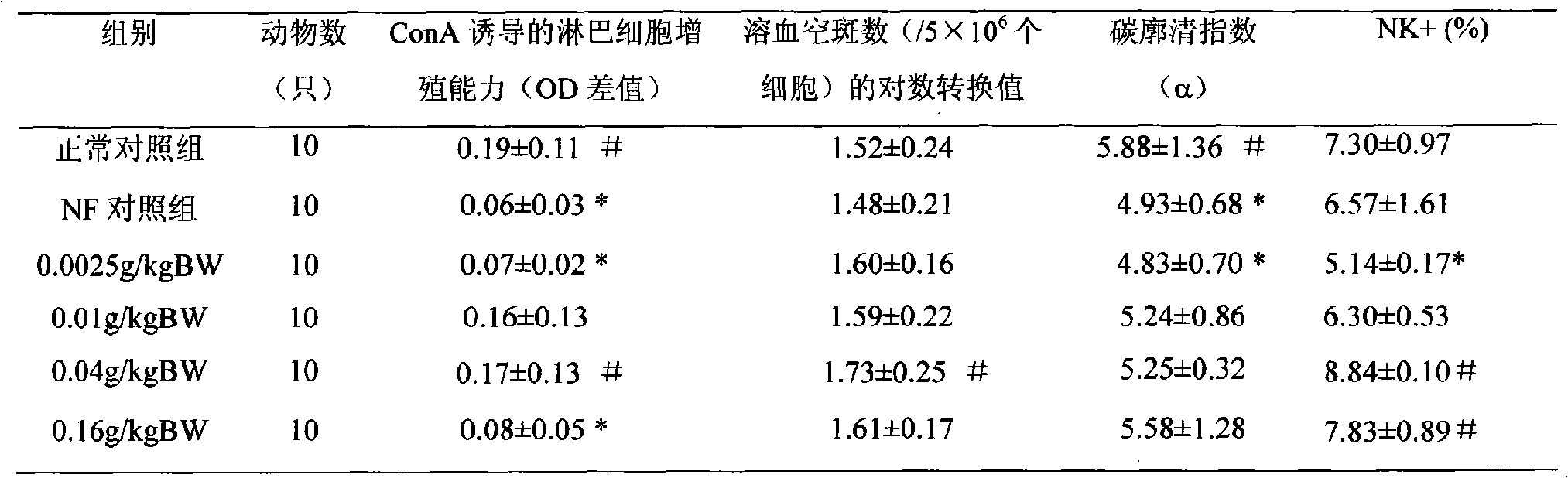
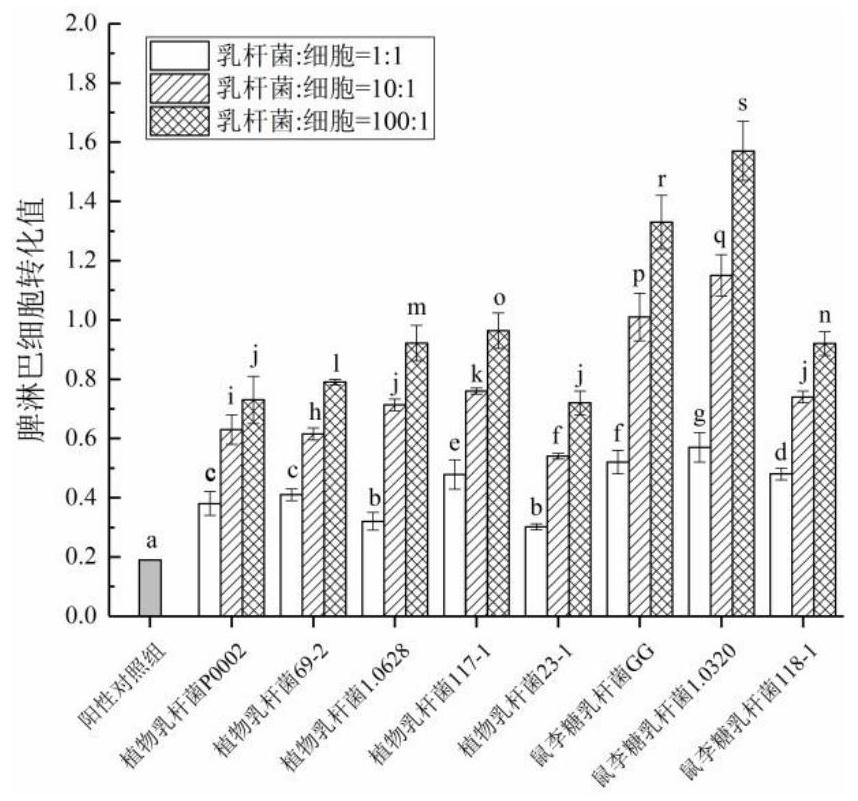
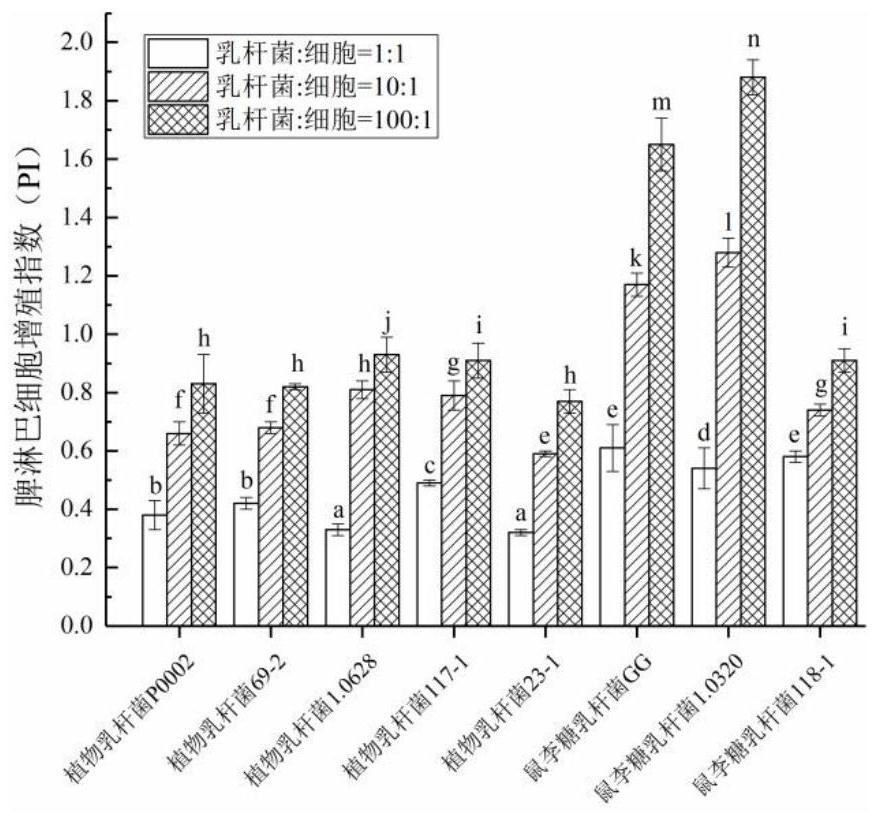
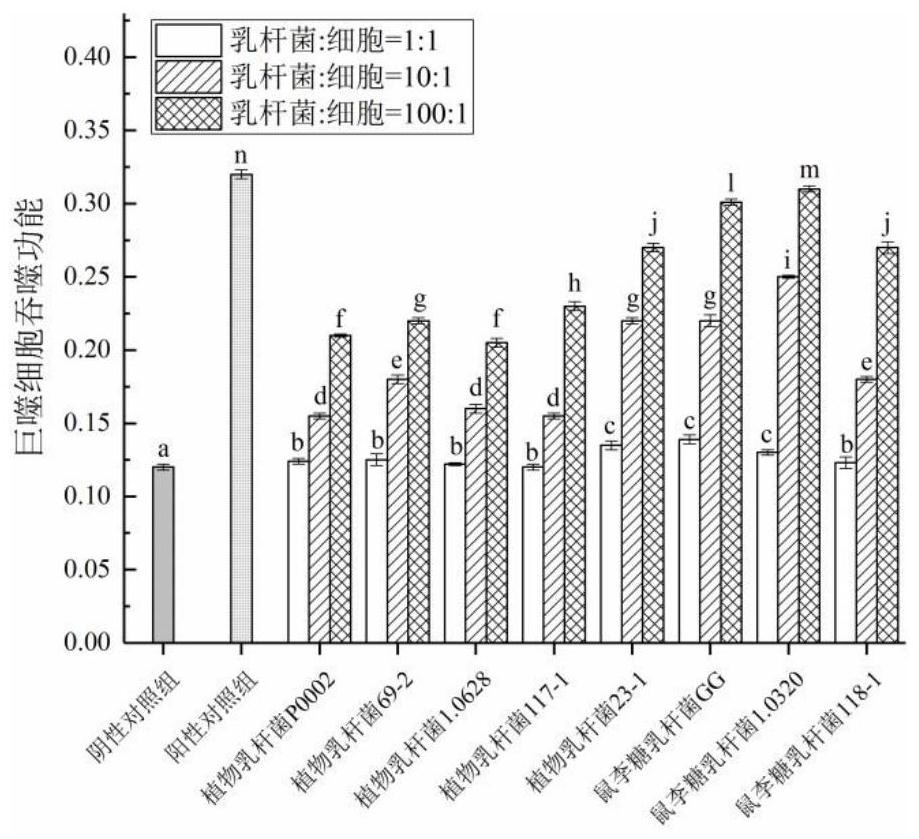
InactiveCN111944721AHigh intestinal wall adhesionEasy to adjustMilk preparationBacteriaBiotechnologyLymphocytic cell
The invention relates to a lactobacillus rhamnosus strain 1.0320 with an immunomodulatory effect and application of the lactobacillus rhamnosus strain 1.0320. The lactobacillus rhamnosus strain 1.0320is applied to the fermentation process of yoghourt or pickles or used for preparing freeze-dried bacterial powder. According to the lactobacillus rhamnosus, the intestinal wall adhesion rate is high(up to 11.76% or above), the lactobacillus rhamnosus resists low pH (pH is lower than 3), and the lactobacillus rhamnosus resists bile salt (the colony count is 10<7> CFU / mL after the lactobacillus rhamnosus is incubated in 0.3% high-concentration bile salt for 4 hours). The lactobacillus rhamnosus 1.0320 (the ratio of lactobacillus to cells is 1: 1) has very strong regulation capability on a splenic lymphocyte transformation value and NO secreted by peritoneal macrophages, and the immunoregulation capability is obviously higher than that of a reference strain lactobacillus rhamnosus GG (p isless than 0.05). The immunoregulation intensity and concentration of the lactobacillus rhamnosus 1.0320 are positively correlated, when the ratio of lactobacillus rhamnosus 1.0320 to cells is 100: 1,the splenic lymphocyte transformation value is 7.5 times that of a positive control group, and the splenic lymphocyte proliferation index reaches 1 or above.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
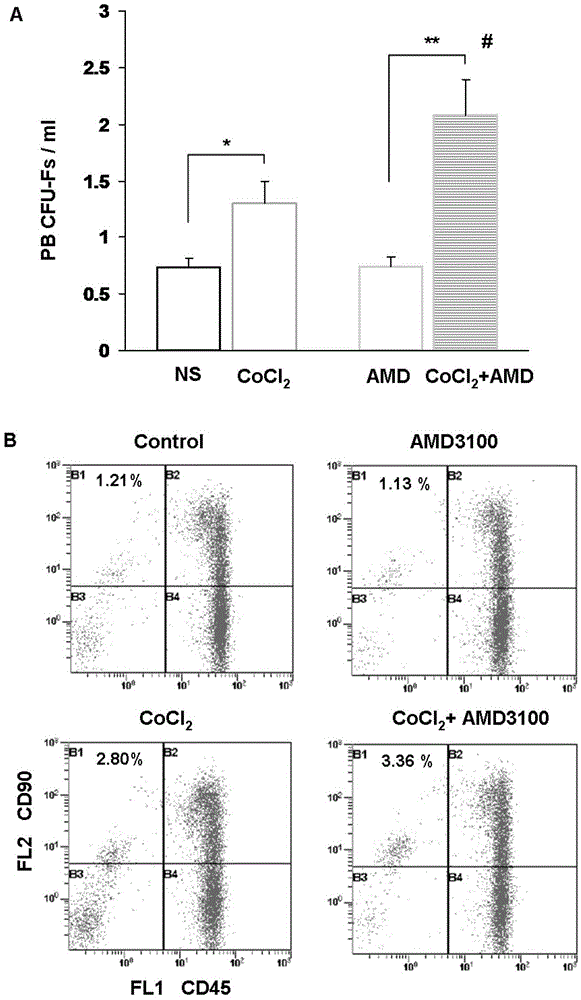
Preparation for mobilizing mesenchymal stem cells and method for separating mesenchymal stem cells
InactiveCN103146646AHigh mobilization efficiencySkeletal/connective tissue cellsLymphocytic cellRegenerative medicine
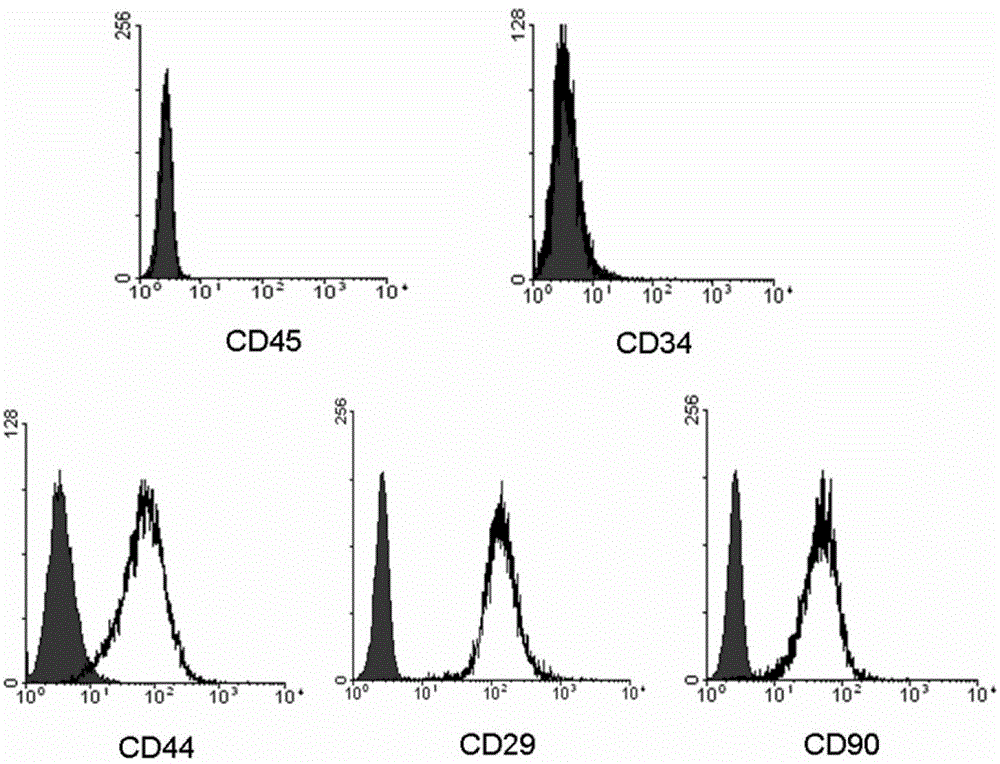
The invention provides a preparation for mobilizing mesenchymal stem cells and a method for separating the mesenchymal stem cells. The preparation for mobilizing the mesenchymal stem cells comprises CoCl2, wherein the CoCl2 is a hypoxia mimetic agent, and the dosage is in a range of 5-20mg / kg. The CoCl2 and AMD3100 are used in a combined manner. The dosage of the AMD3100 is 5mg / kg. According to the method for separating the mesenchymal stem cells by using the preparation, the preparation is used for actuating the mesenchymal stem cells to be mobilized, enter peripheral blood and then be separated. The method comprises the following separation steps of: sampling the periphery blood, separating mononuclear cells by using a lymphocyte separating medium, performing resuspension by using a DMEM (dulbeccos modified eagle medium) containing 20% of fetal bovine serum, inoculating to a culture flask, culturing for 7 days, and changing a culture solution, thus obtaining the mesenchymal stem cells of the peripheral blood on the 10th day. The mesenchymal stem cells of the peripheral blood highly express CD90, CD29 and CD44, do not express CD45 and CD34, and have the capacities of in vitro bone formation, fat formation and cartilage differentiation formation. The preparation is high in MSCs (mesenchymal stem cells) mobilization efficiency, and an effective preparation and an effective method are provided for tissue engineering and regenerative medicine.
Owner:ZHEJIANG UNIV
BTK (bruton tyrosine kinase) inhibitor and purpose of BTK inhibitor
InactiveCN105153154AEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryFollicular lymphomaReceptor tyrosine kinase
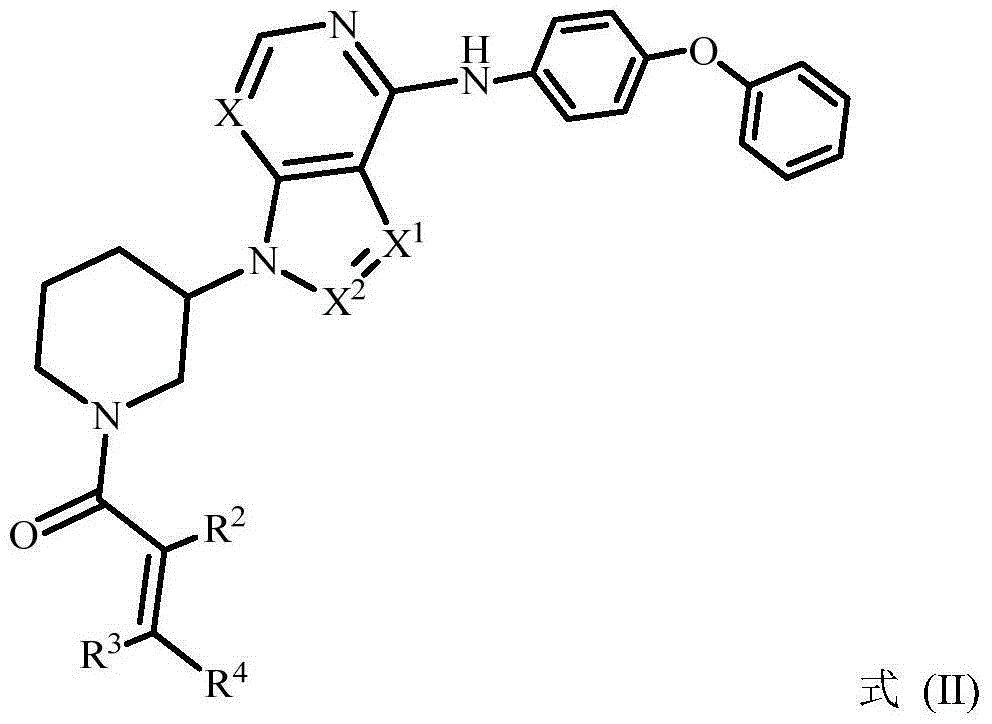
The invention provides a BTK (bruton tyrosine kinase) inhibitor compound (compound shown as a formula (I)) and a purpose of the BTK inhibitor compound in medicine. The compound provided by the invention and a medicine composition of the compound can be used for treating diffuse large B cell lymphoma, follicular lymphoma or chronic lymphocytic leukemia.
Owner:SUNSHINE LAKE PHARM CO LTD
B cell surface reactive antibodies
ActiveUS20120121504A1Improve the level ofRadioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsB cellB-cell chronic lymphocytic leukemia
The invention relates to antibodies that are reactive to the cell surface of CD19+ B cells, including B-cell chronic lymphocytic leukemia (B-CLL) cells, and compositions and methods for using such antibodies, including in the diagnosis and treatment of disorders associated with CD19+ B cells, such as B-CLL.
Owner:UNITED STATES OF AMERICA
Treatment of pediatric acute lymphoblastic leukemia
ActiveCN102209729AEliminate the risk of recurrenceEffective anti-leukemia effectHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsDrugSingle-Chain Antibodies
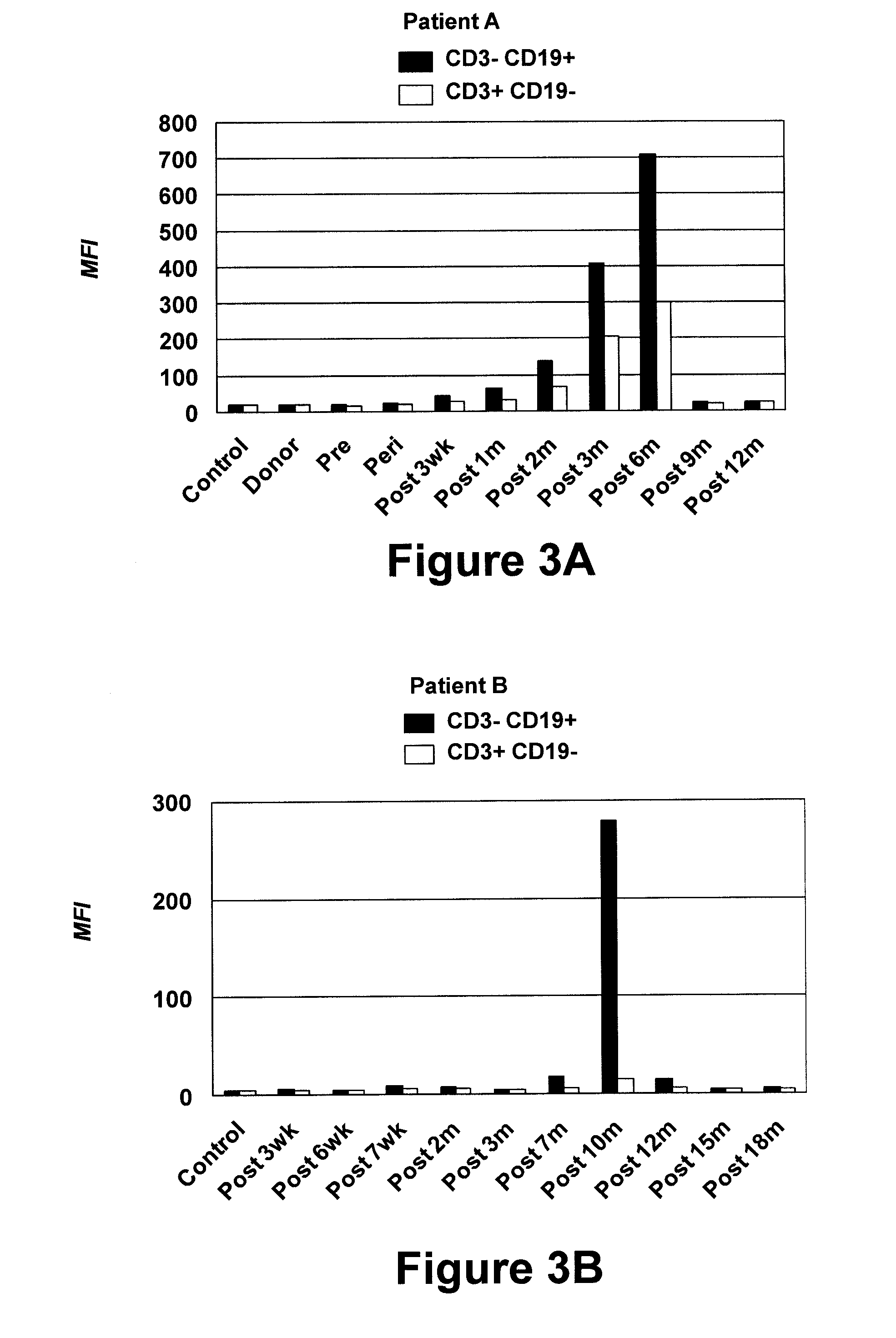
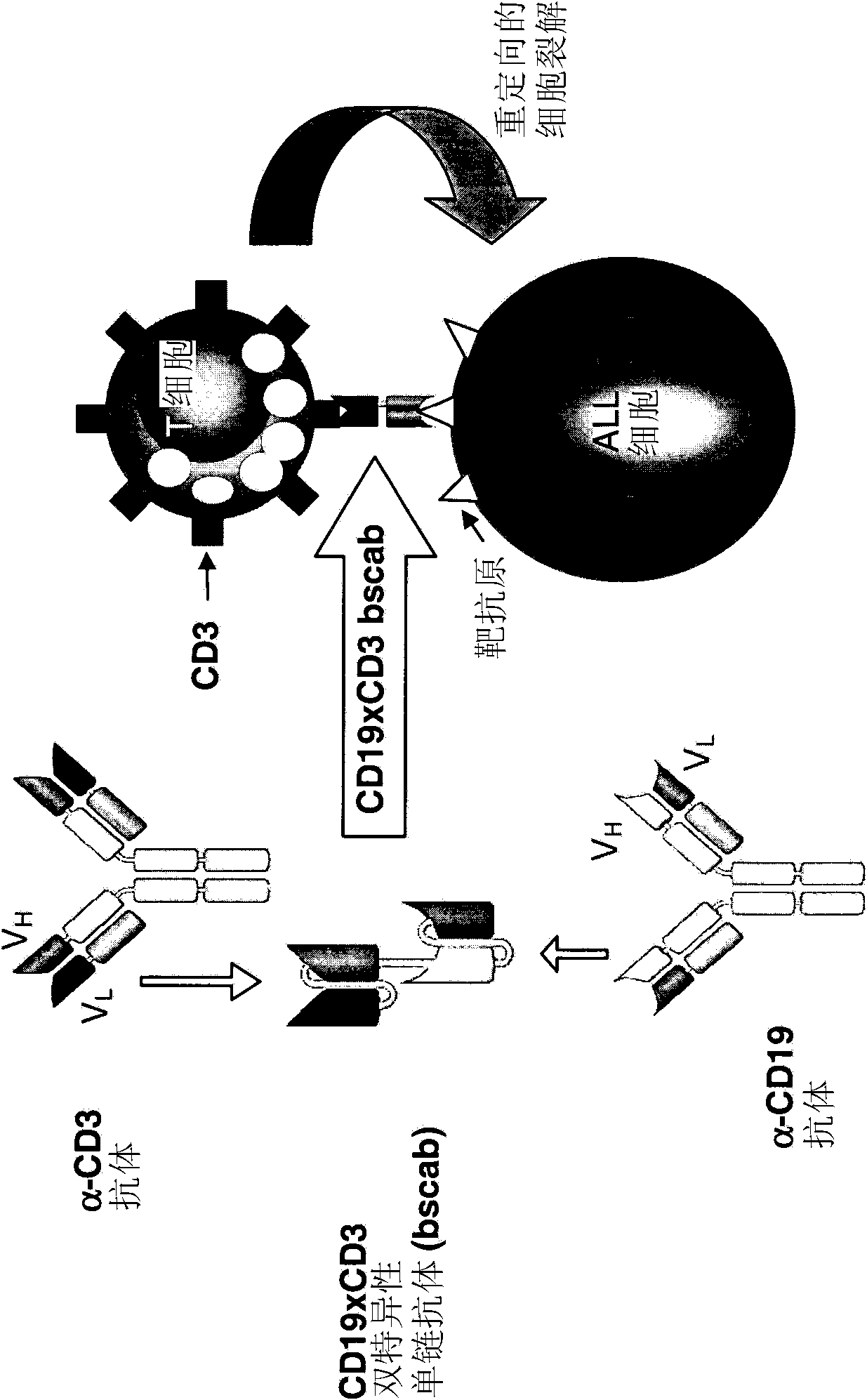
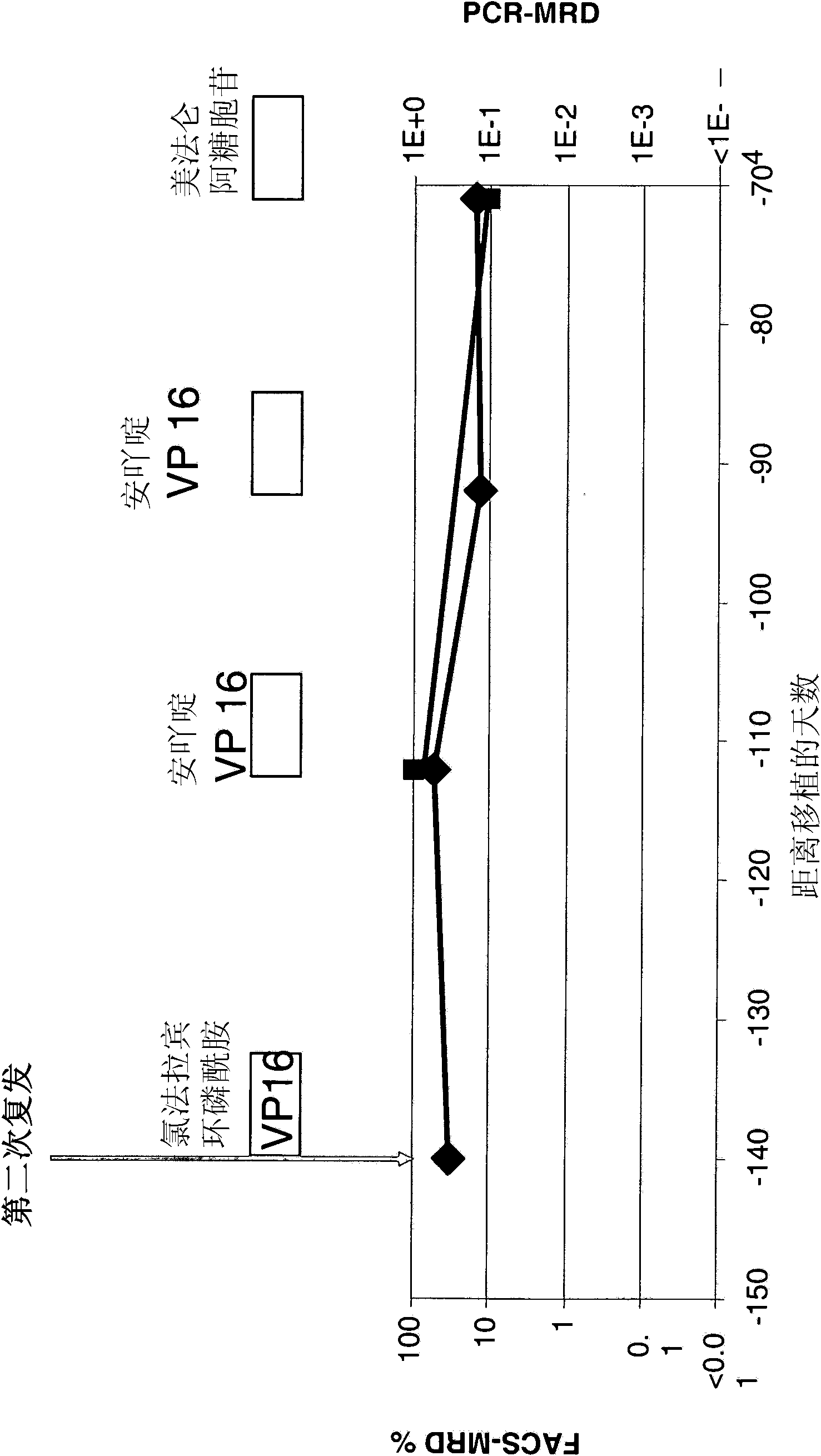
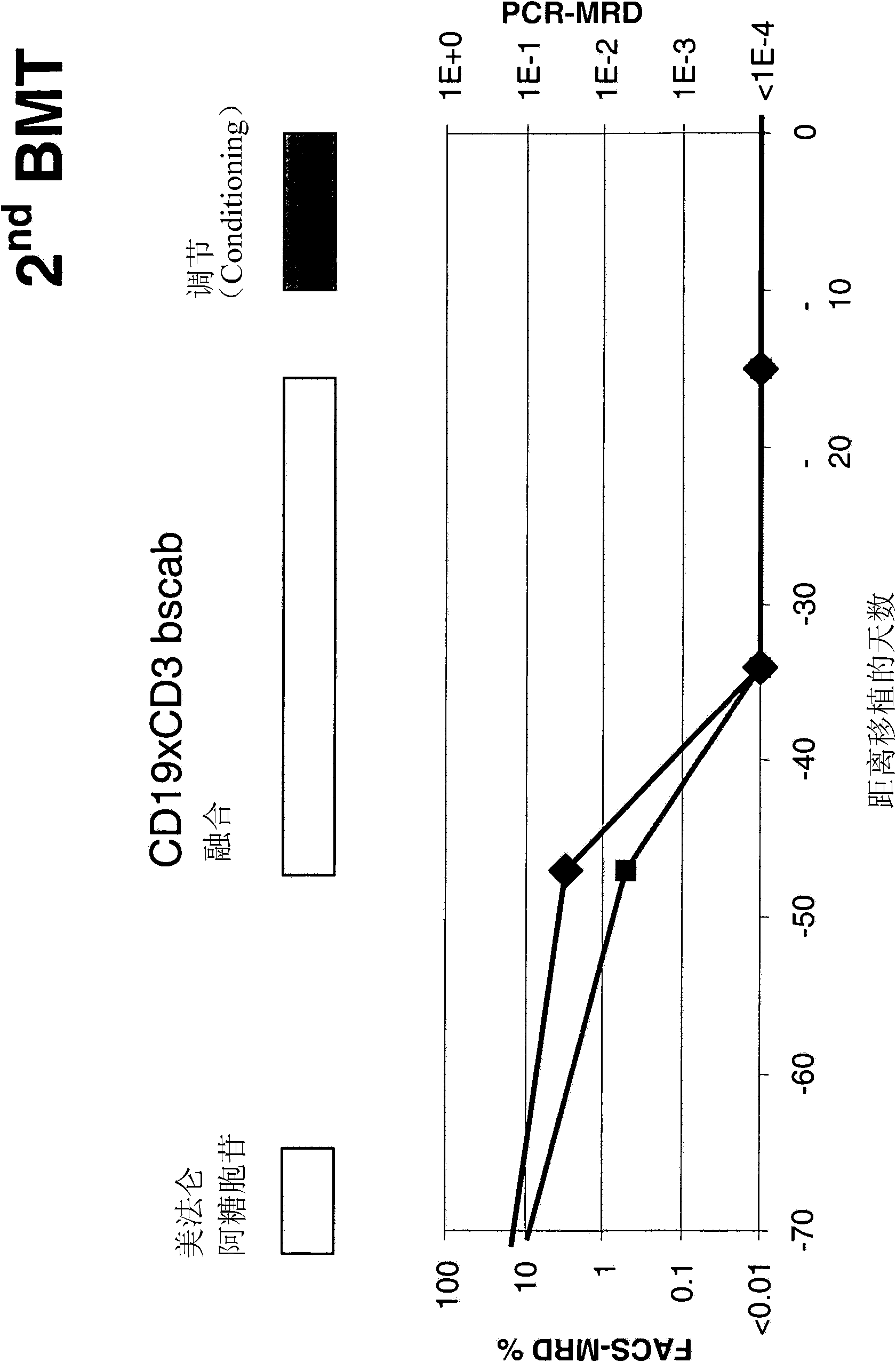
The present invention relates to a method for the treatment, amelioration or elimination of pediatric acute lymphoblastic leukemia (ALL), the method comprising the administration of a pharmaceutical composition comprising a CD19xCD3 bispecific single chain antibody construct to a pediatric ALL patient in the need thereof.
Owner:AMGEN RES (MUNICH) GMBH
Method for in-vitro preparation of tumor antigen-specific CD8+ T memory stem cells
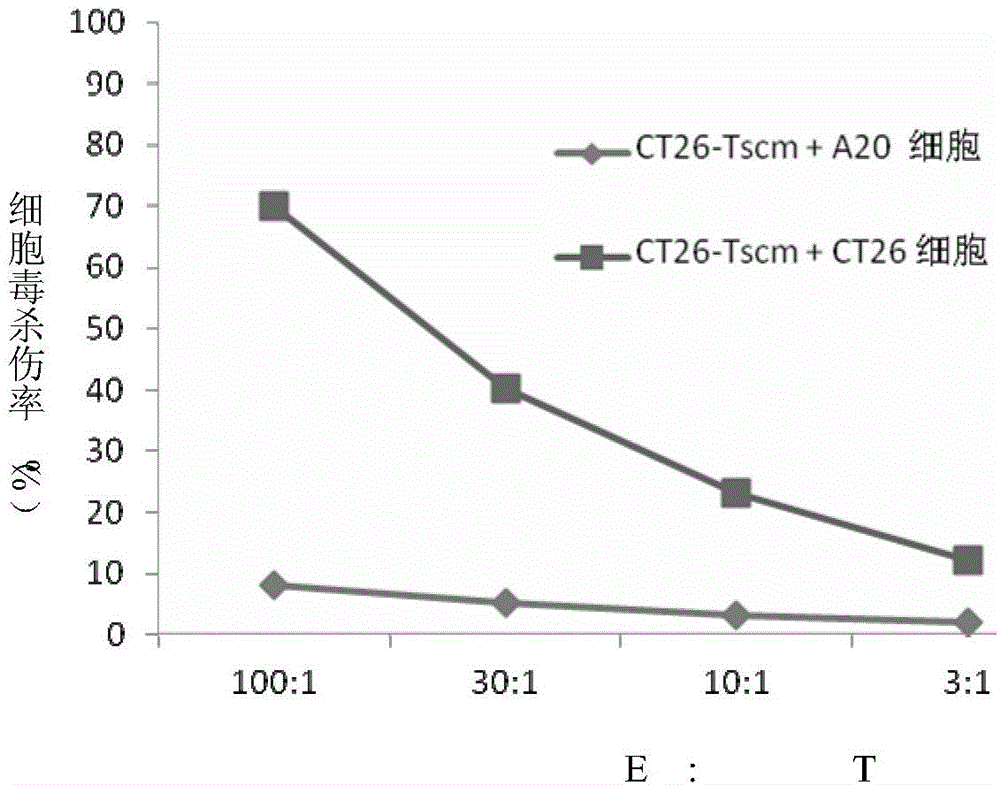
ActiveCN105802908AIncrease intakeSpecific Tscm has good anti-tumor effectBlood/immune system cellsAntibody medical ingredientsDendritic cellLymphocytic cell
The invention relates to a method for in-vitro preparation of tumor antigen-specific CD8+ T memory stem cells and application of the tumor antigen-specific CD8+ T memory stem cells in antitumor immune response. The method comprises the following steps: subjecting tumor antigen and immature dendritic cells to co-incubation so as to obtain mature dendritic cells loaded with specific tumor antigen; subjecting the mature dendritic cells and CD8+ initial T cells to co-culture so as to promote generation of tumor antigen-specific CD8+ T memory stem cells; and separating out the CD8+ T memory stem cells capable of specifically recognizing specific tumor cells. The tumor antigen-specific CD8+ T memory stem cells prepared by using the method has good antineoplastic effect, high specificity and few adverse reaction.
Owner:FUDAN UNIV SHANGHAI CANCER CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com