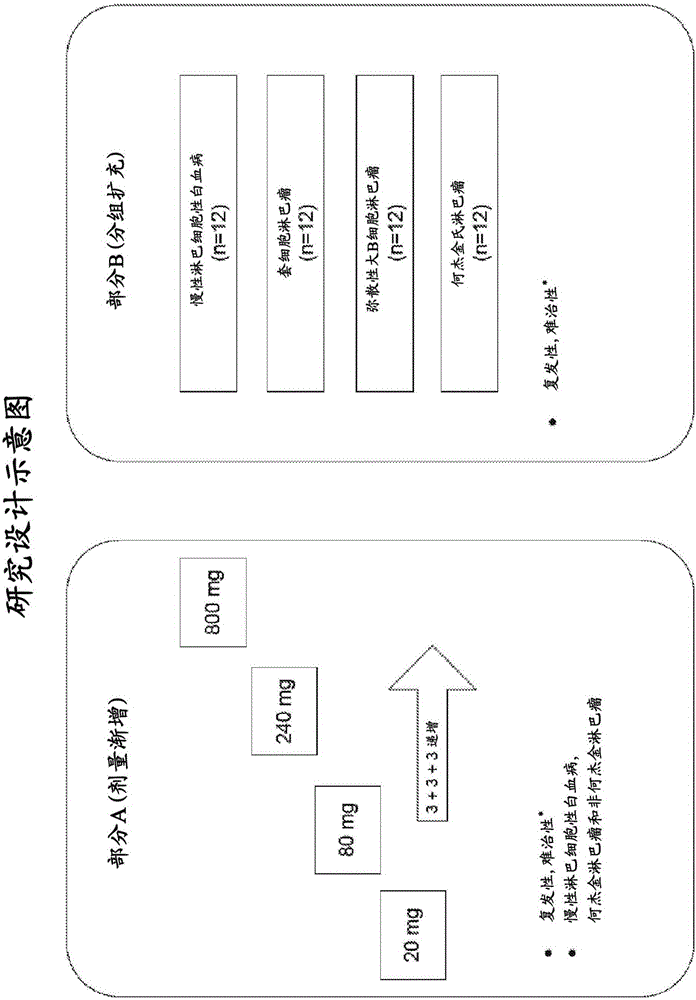
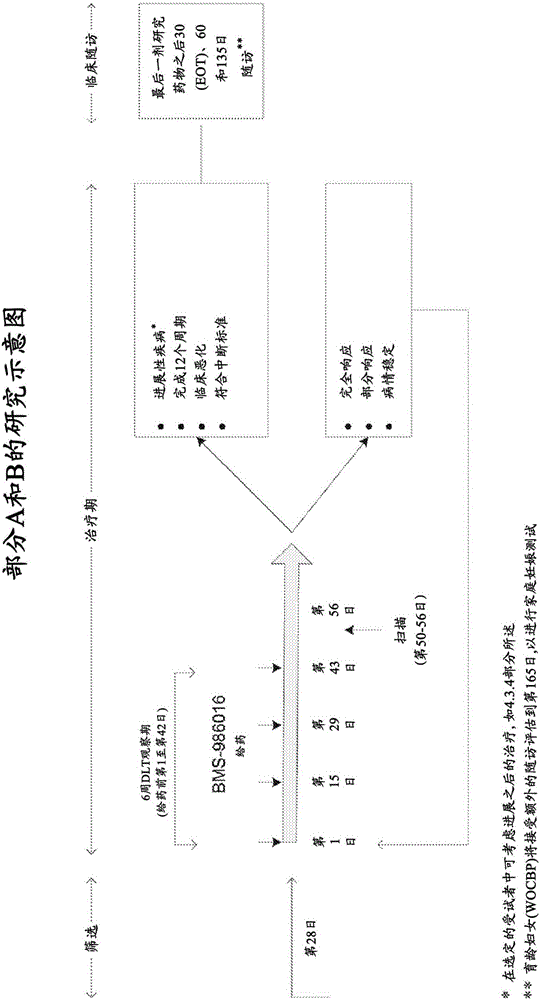
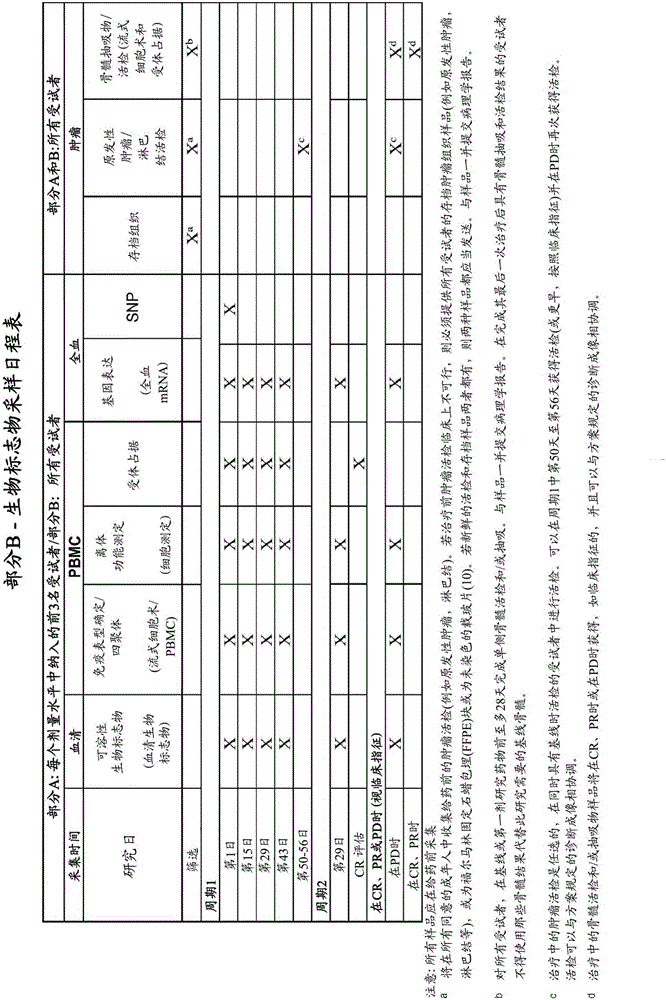
Anti-lag-3 antibodies to treat hematological malignancies
A LAG-3, malignant tumor technology, applied in the direction of antibodies, anti-tumor drugs, antibody medical components, etc., can solve the problems of not binding to class II MHC and unknown functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Example 1: Preclinical Pharmacology of Anti-LAG-3 Antibody (BMS-986016)
[0113] Anti-LAG-3 antibody, BMS-986016
[0114] BMS-986016 is a fully human antibody specific for human LAG-3, isolated from immunized transgenic mice expressing human immunoglobulin genes. It is expressed as an IgG4 isotype antibody and contains a stabilizing hinge mutation (S228P) to attenuate Fc receptor binding to reduce or exclude the possibility of antibody- or complement-mediated killing of target cells. The heavy chain and light chain amino acid sequences of BMS-986016 are set forth in SEQ ID NO: 1 and 2, respectively.
[0115] The ability of BMS-986016 to bind recombinant human LAG-3 antigen was determined using Biacore and enzyme-linked immunosorbent assay (ELISA). Binding to human and primate LAG-3+ transfectants and to activated human or primate T cells was measured using flow cytometry and Scatchard analysis. BMS-986016 with high affinity (K D = 0.12-0.5 nM) binds to human LAG-3...
Embodiment 2
[0116] Example 2: Toxicity of anti-LAG-3 antibody (BMS-986016)
[0117] The following preclinical toxicology studies were performed:
[0118] A. A GLP-compliant 4-week intravenous combination toxicity study of BMS-986016 and nivolumab in macaques with a 6-week recovery period
[0119] The relevant results of single agent treatment with BMS-986016 are as follows:
[0120] 1. Single agent BMS-986016 administered up to 100 mg / kg / week produced no adverse changes.
[0121] 2. The NOAEL of single agent BMS-986016 is considered to be 100 mg / kg / week (mean AUC[0-168h]=474,000 μg·h / mL). The dose administered (100 mg / kg BMS-986016) was > 10 times the maximum dose suggested by the current study.
[0122] B. GLP-compliant tissue cross-reactivity study of BMS-986016 in human and selected macaque tissues
[0123] The relevant results for cross-reactivity are as follows:
[0124] 1. Positive staining with BMS-986016-FITC was observed in the plasma membrane or plasma membrane granules of ...
Embodiment 3
[0131] Example 3: Preclinical Metabolism and Pharmacokinetics of Anti-LAG-3 Antibody (BMS-986016)
[0132] Metabolism studies of BMS-986016 in animals have not been performed in accordance with regulatory guidelines for biotechnology-derived pharmaceuticals (ICH Harmonized Tripartite Guideline, S6(R1) Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals. International Conference on Harmonization, 2011). Monoclonal antibodies (mAbs) are expected to be degraded into small peptides and amino acids in vivo via biochemical pathways independent of cytochrome P450 enzymes.
[0133] BMS-986016 demonstrated favorable pharmacokinetic (PK) properties in rhesus monkeys. From both single-dose and repeat-dose IV PK studies, BMS-986016 decays in a bi-exponential manner and exposure is approximately dose-proportional. Systemic clearance (CLTp) varied from 0.12 to 0.22 mL / h / kg, and terminal half-life (T-HALF) varied from 133 to 414 hours. The volume of distribution (Vss) at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com