Preparation for mobilizing mesenchymal stem cells and method for separating mesenchymal stem cells
A technology of mesenchymal stem cells and preparations, applied in the field of preparation and separation of mobilized mesenchymal stem cells, can solve the problems of limited cell sources, cumbersome culture steps, pollution, etc., and achieve the effect of high mobilization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
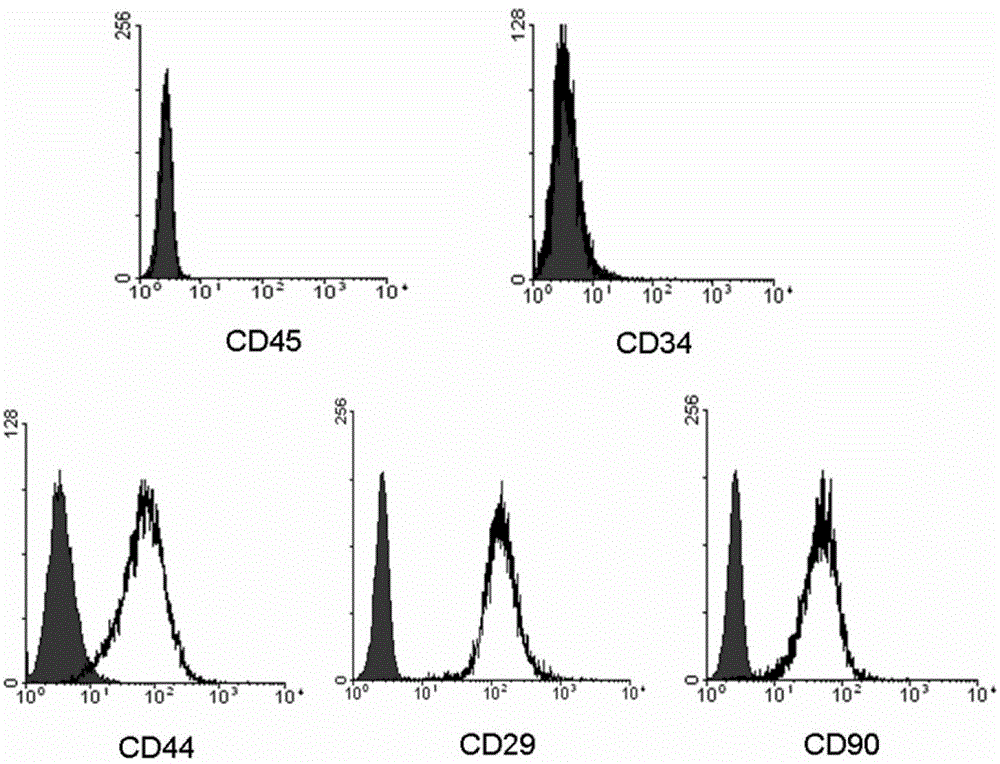
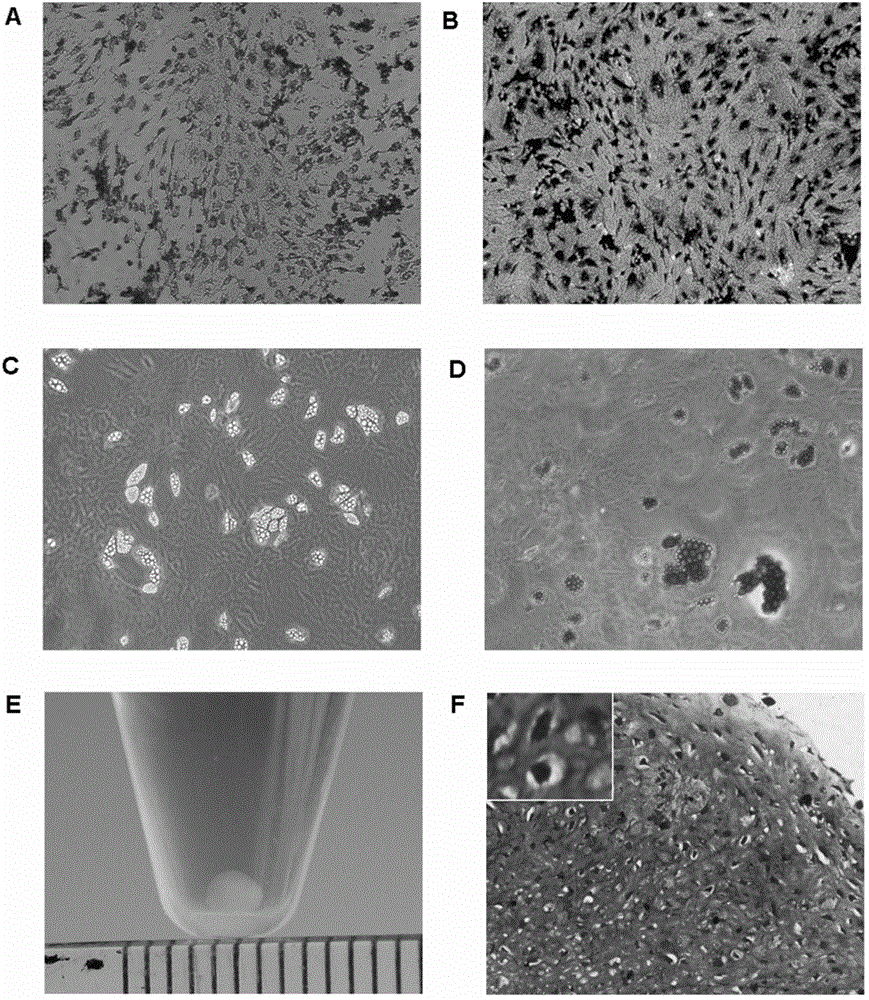
Examples
Embodiment 1
[0037] 1. Reagent preparation
[0038] (1) CoCl 2 : Weigh 400mg CoCl 2 Add 10ml of normal saline to the powder to make a 40mg / ml storage solution, filter it through a 0.22μm filter and store in -20°C. When animals were administered, the stock solution was diluted 10 times with physiological saline to adjust the concentration to 4 mg / ml.
[0039] (2) AMD3100: 5g of powder plus 5ml of normal saline to make a 1mg / ml solution, filtered through a 0.22μm filter, and stored at -20°C. Physiological saline was diluted 4 times to 1.25mg / ml for administration to mice.
[0040] 2. Grouping of animals
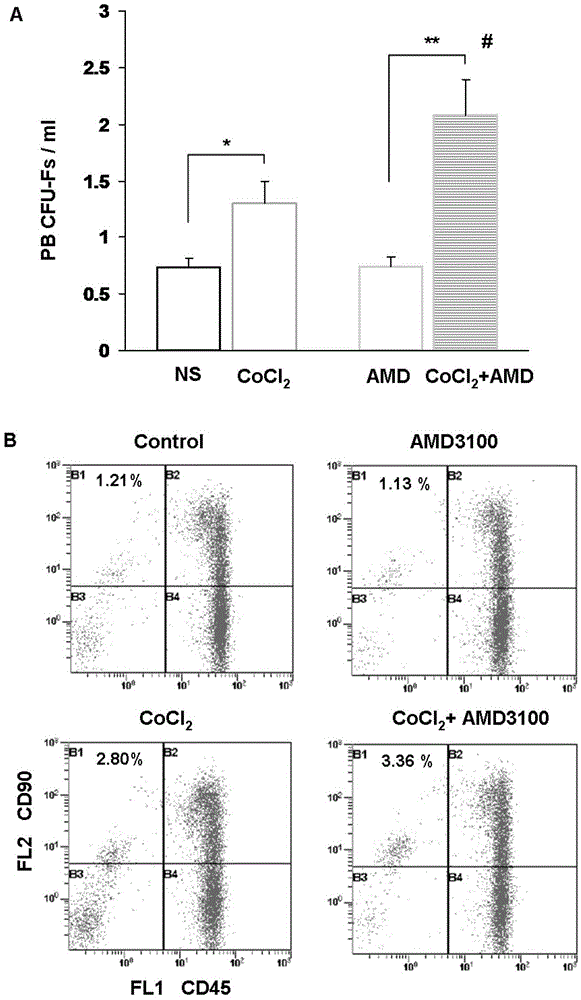
[0041] (1) To detect CoCl 2 and CoCl 2 Combined with the mobilization effect of AMD3100 on MSCs, the rats were divided into 4 groups with 5 rats in each group, which were as follows: ①Normal saline group (NS group): intraperitoneal injection of normal saline (with CoCl 2 same volume), a total of 7 days; ②CoCl 2 Group 7d: intraperitoneal injection of CoCl every day 2 10 mg / kg solut...
Embodiment 2
[0071] To detect CoCl 2 For the effective concentration of MSCs mobilization, we used the CFU-F method to study the daily intraperitoneal injection of CoCl in rats. 2 Solution 5mg / kg, 20mg / kg for 7 days, the number of CFU-Fs in the peripheral blood of rats, the results show that: CoCl 2 5mg / kg×7 days, CoCl 2 20mg / kg×7 days had a mobilization effect on rat MSCs, and the peripheral blood CFU-F increased compared with the normal saline control group, but the mobilization efficiency was lower than that of CoCl 2 10mg / kg×7 days group. The specific result is: CoCl 2 5mg / kg×7 days group vs. normal saline group (2.02±0.12 vs.1.27±0.08 CFU-Fs / ml, P2 20mg / kg×7 days group vs. normal saline group (1.68±0.08 vs.1.27±0.08 CFU-Fs / ml, P<0.05).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com