Treatment of pediatric acute lymphoblastic leukemia
A technology for acute lymphocytes and leukemia, applied in chemical instruments and methods, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, immunoglobulin, etc., can solve the problem of overall curative effect decline, tolerance reduction, Increased morbidity and other problems, to achieve the effect of eliminating the risk of recurrence and effective anti-leukemia effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
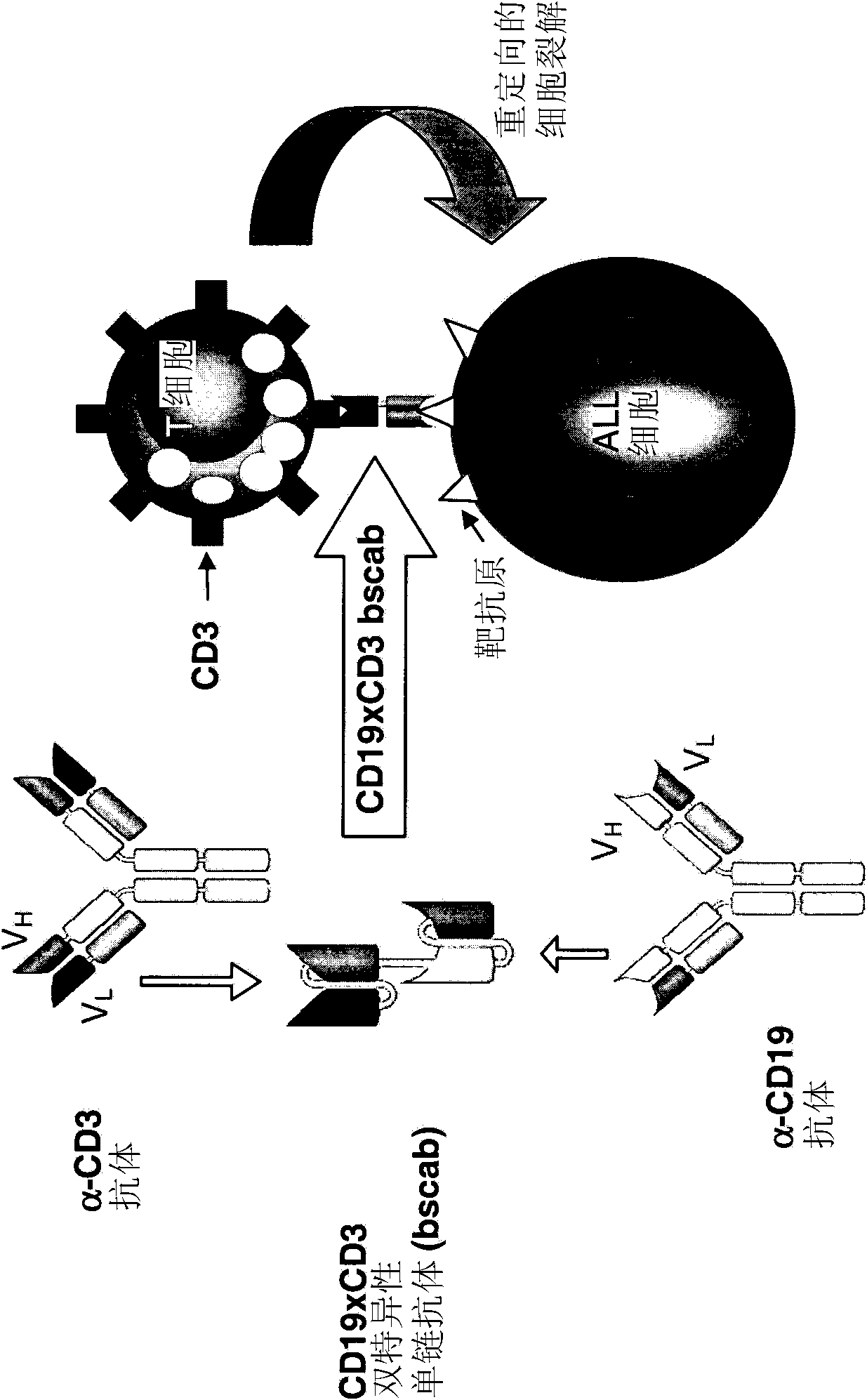
[0162] 1. CD19xCD3 bispecific single chain antibody
[0163] WO 99 / 54440 describes the production, expression and cytotoxic activity of a CD19xCD3 bispecific single chain antibody. The corresponding amino acid and nucleic acid sequences of the CD19xCD3 bispecific single chain antibody are shown in SED ID NO.1 and 2, respectively. The VH and VL regions of the CD3-binding domain of the CD19xCD3 bispecific single-chain antibody are shown in SED ID NO.7-10, respectively, while the VH and VL regions of the CD19-binding domain of the CD19xCD3 bispecific Shown in SED ID NO.3-6.
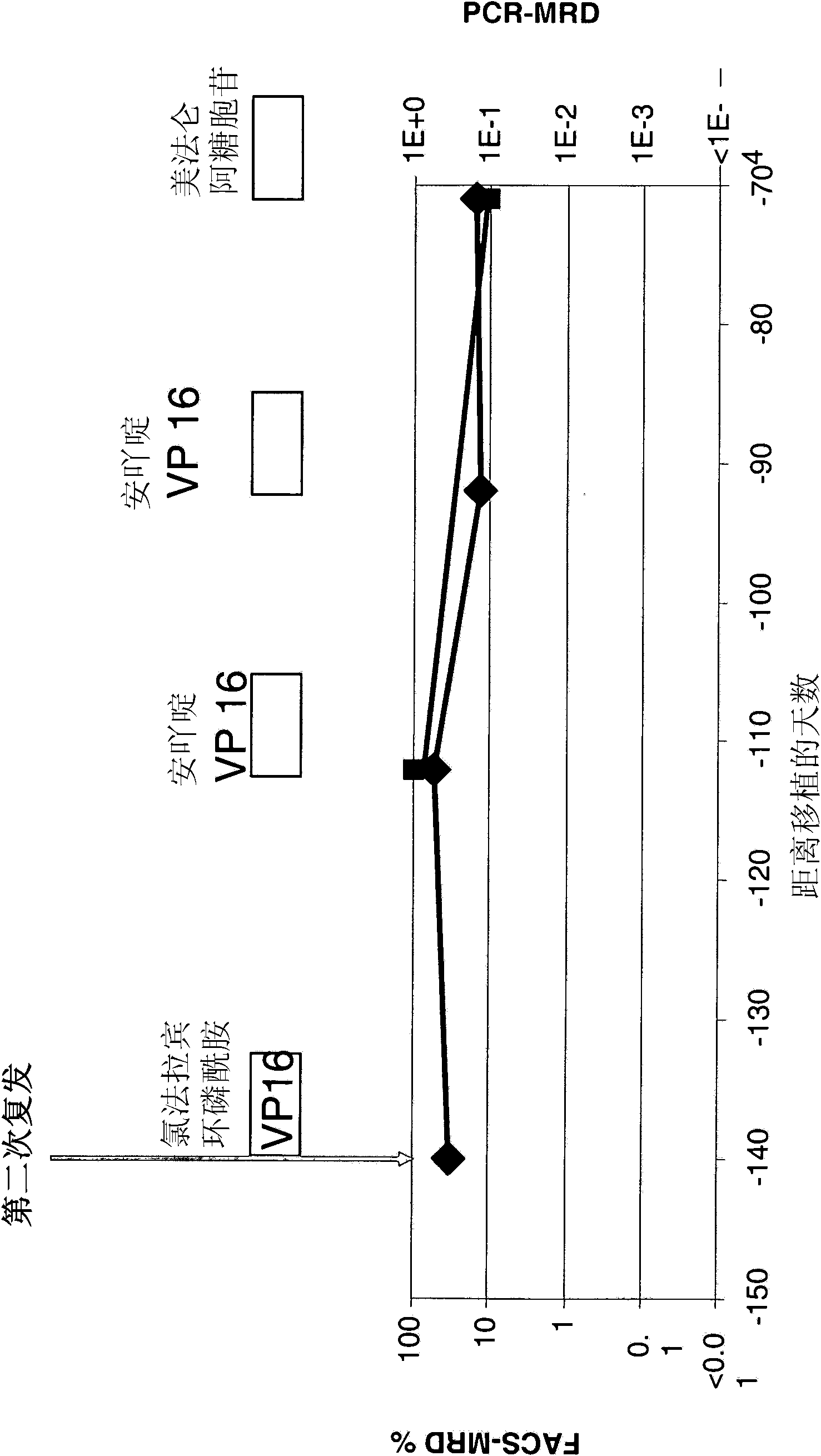
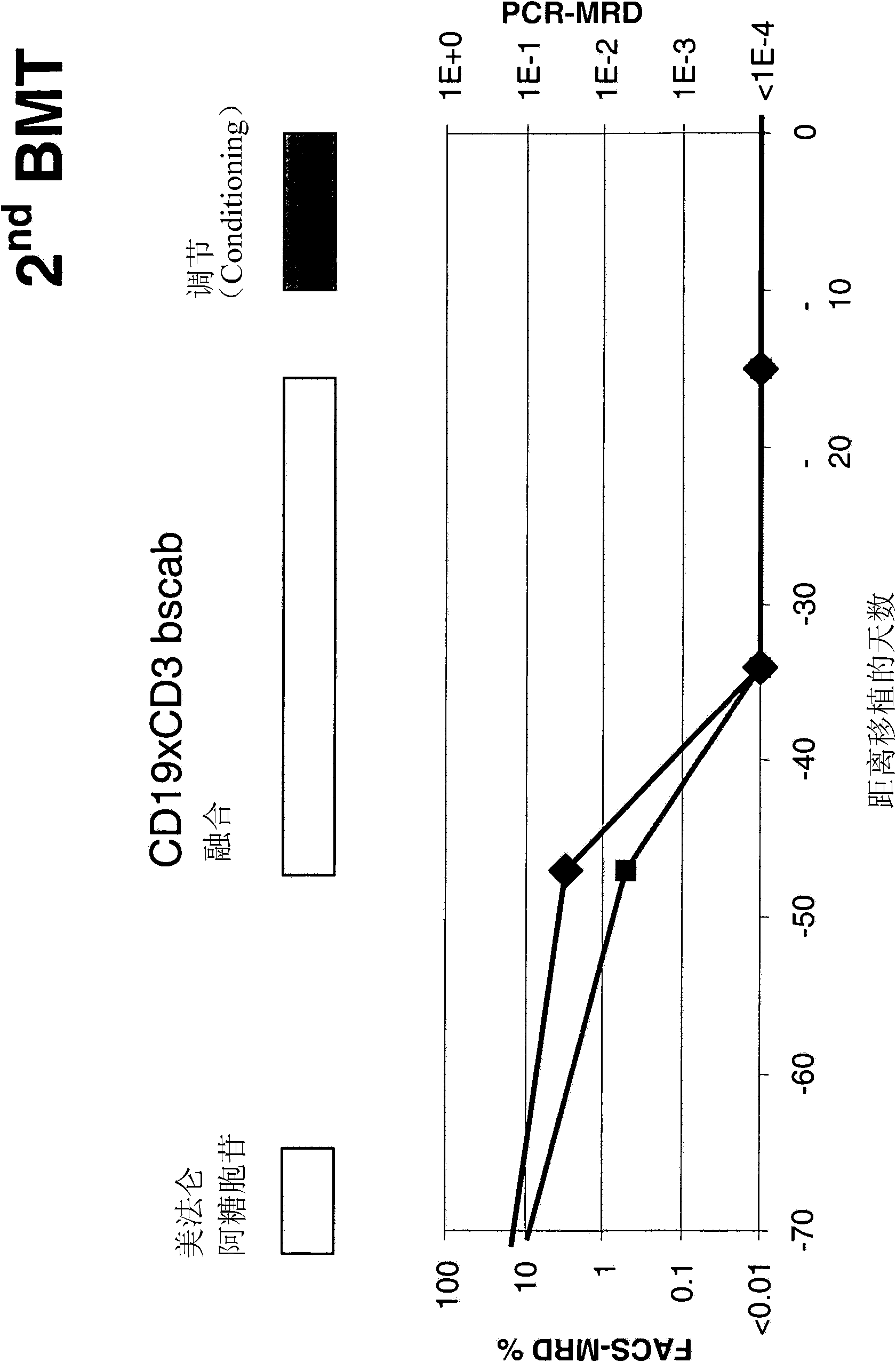
[0164] 2. Lymphocyte phenotype analysis and chimerism analysis
[0165] Before, during, and after treatment with the CD19xCD3 bispecific single-chain antibody, blood was collected from patients using collection tubes containing EDTA for lymphocyte phenotype analysis and chimerism analysis. The absolute number of lymphocytes in the blood sample was determined by performing differential blood analysis (diff...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com