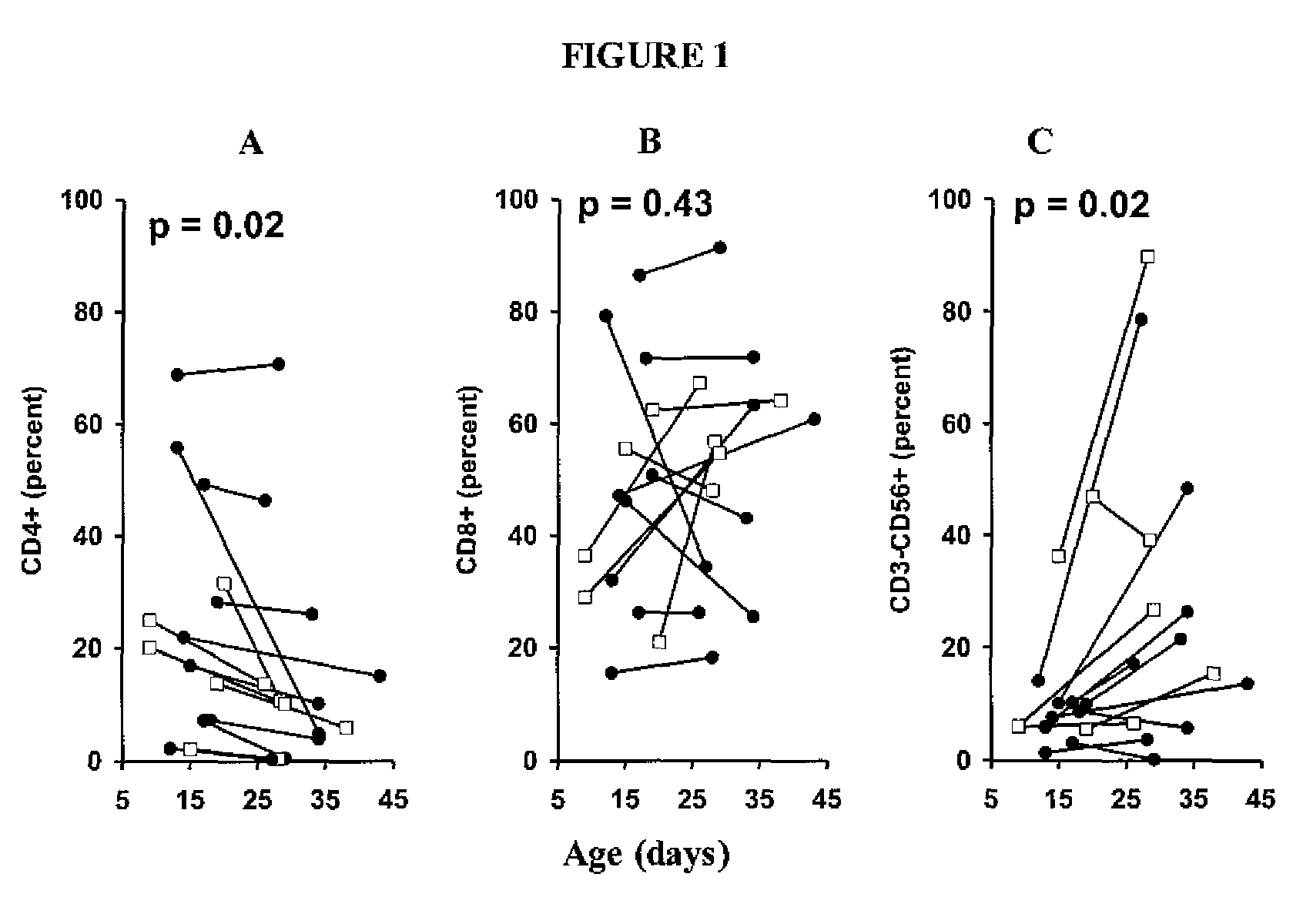
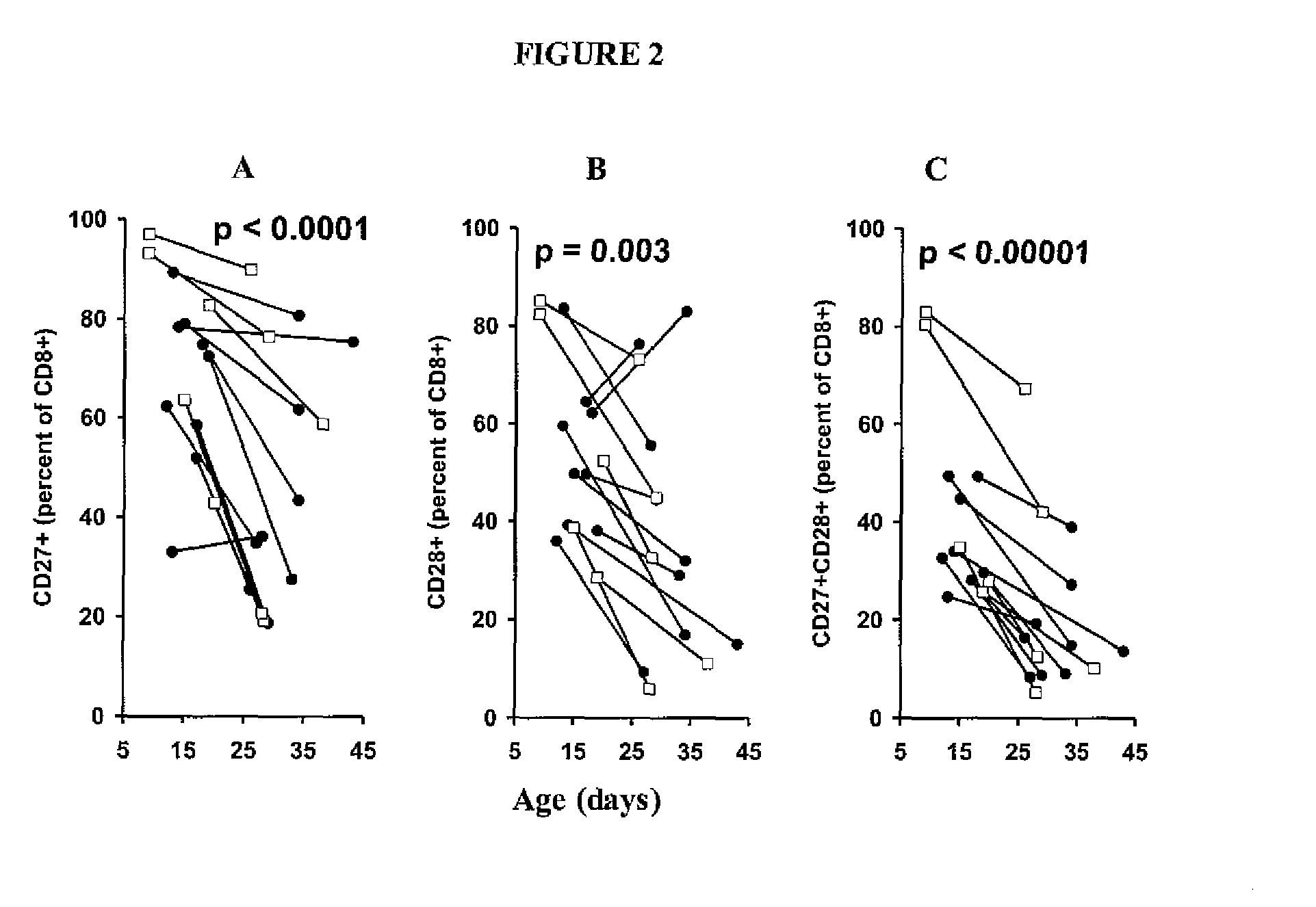
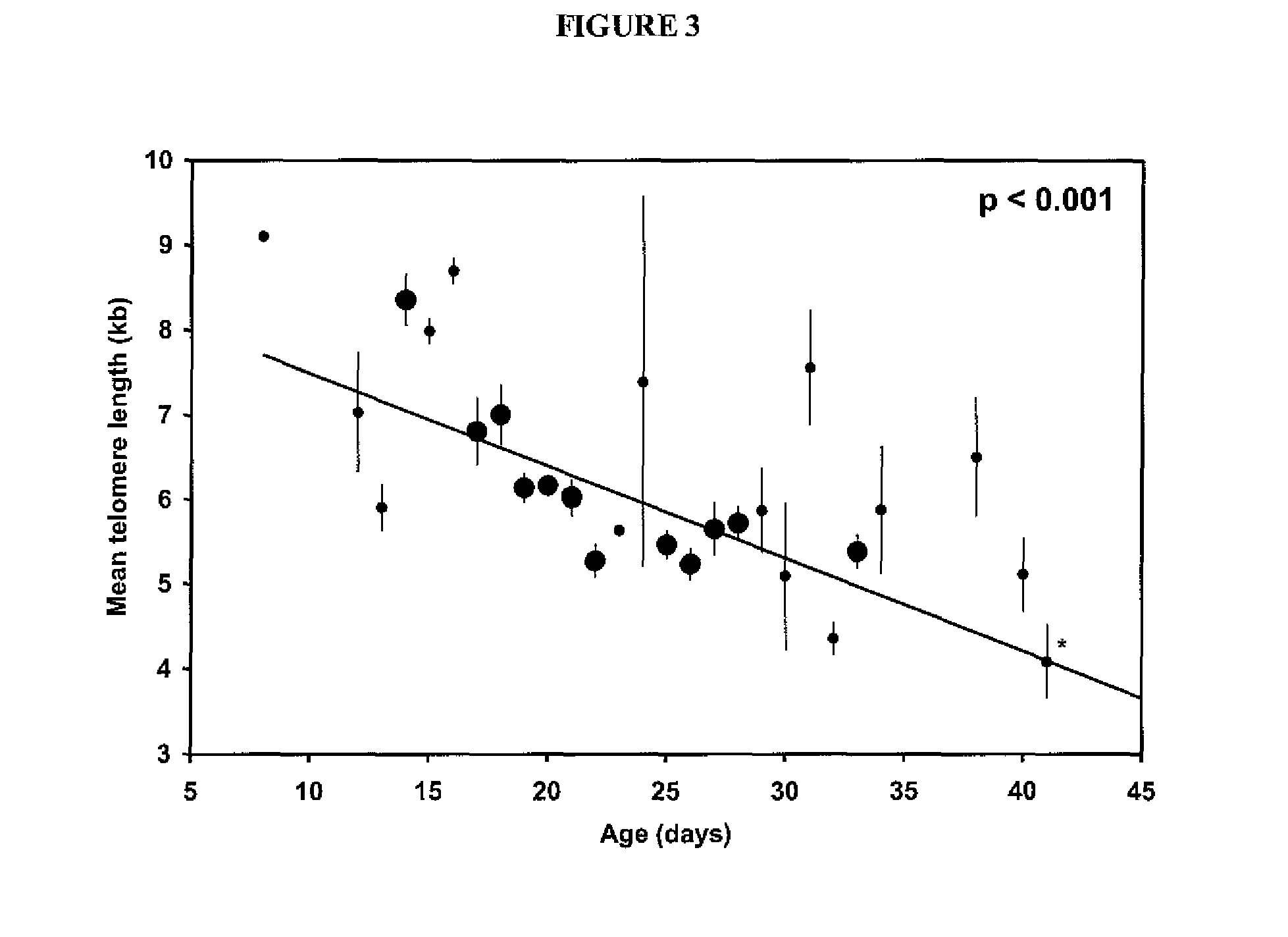
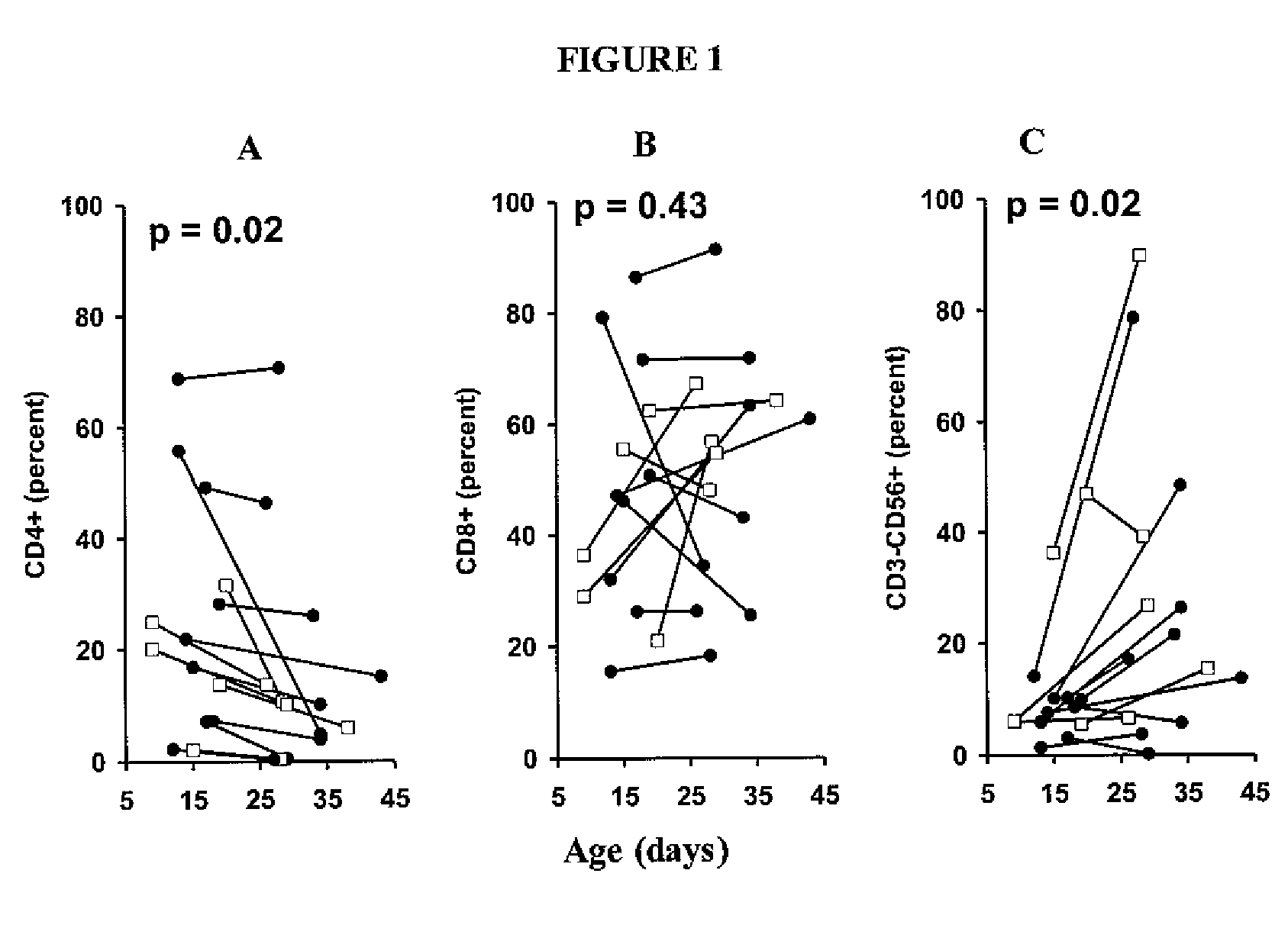
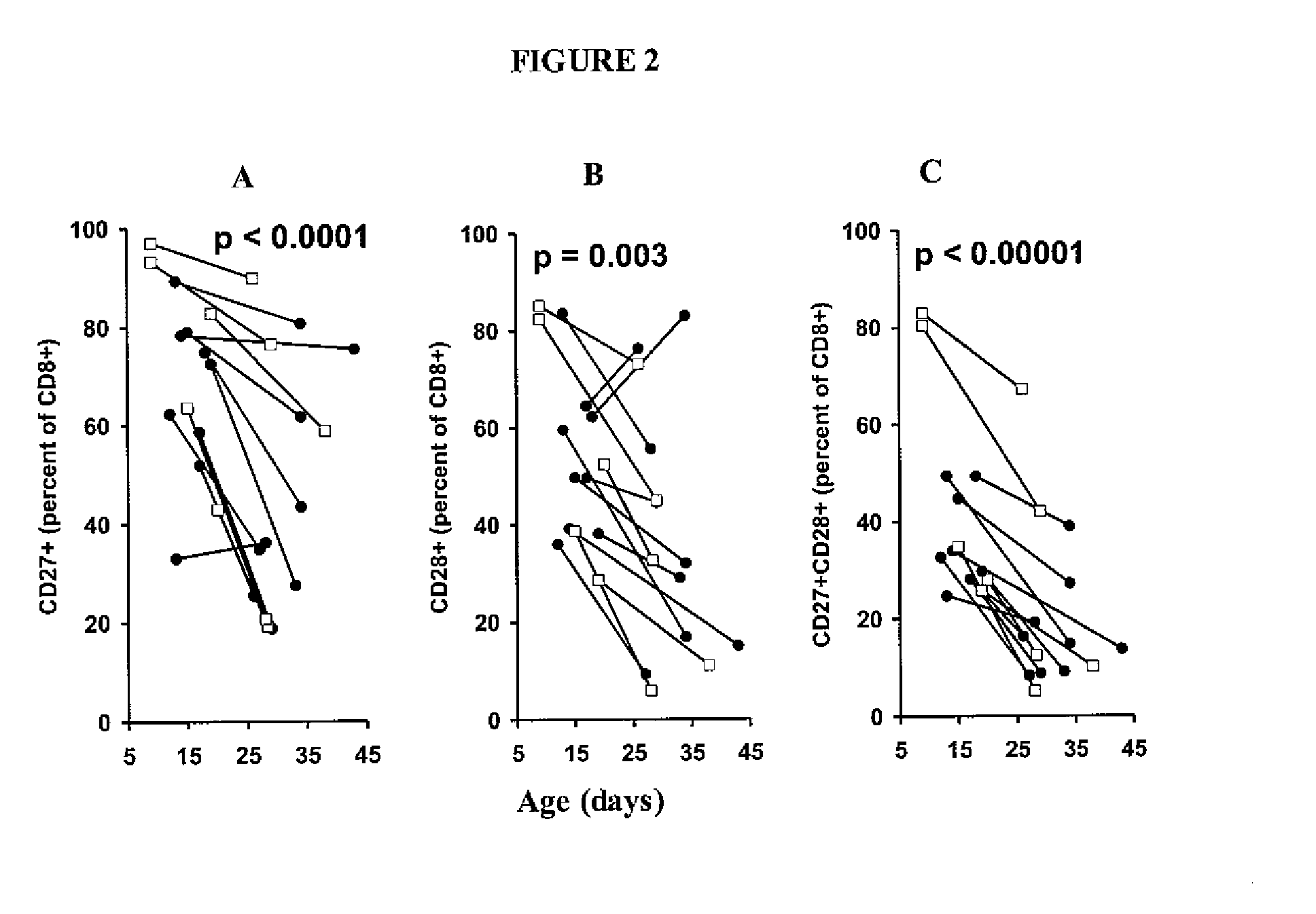
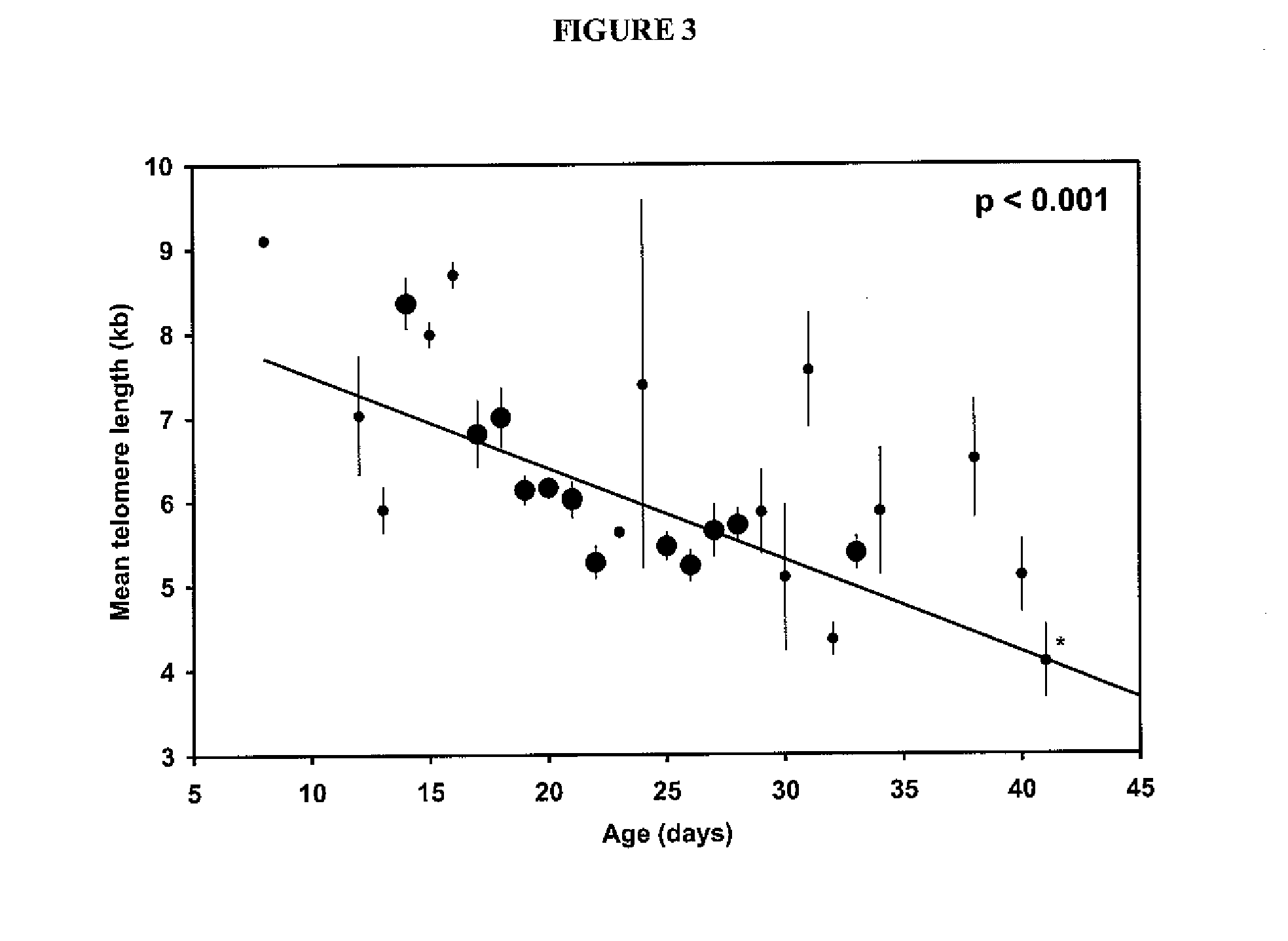
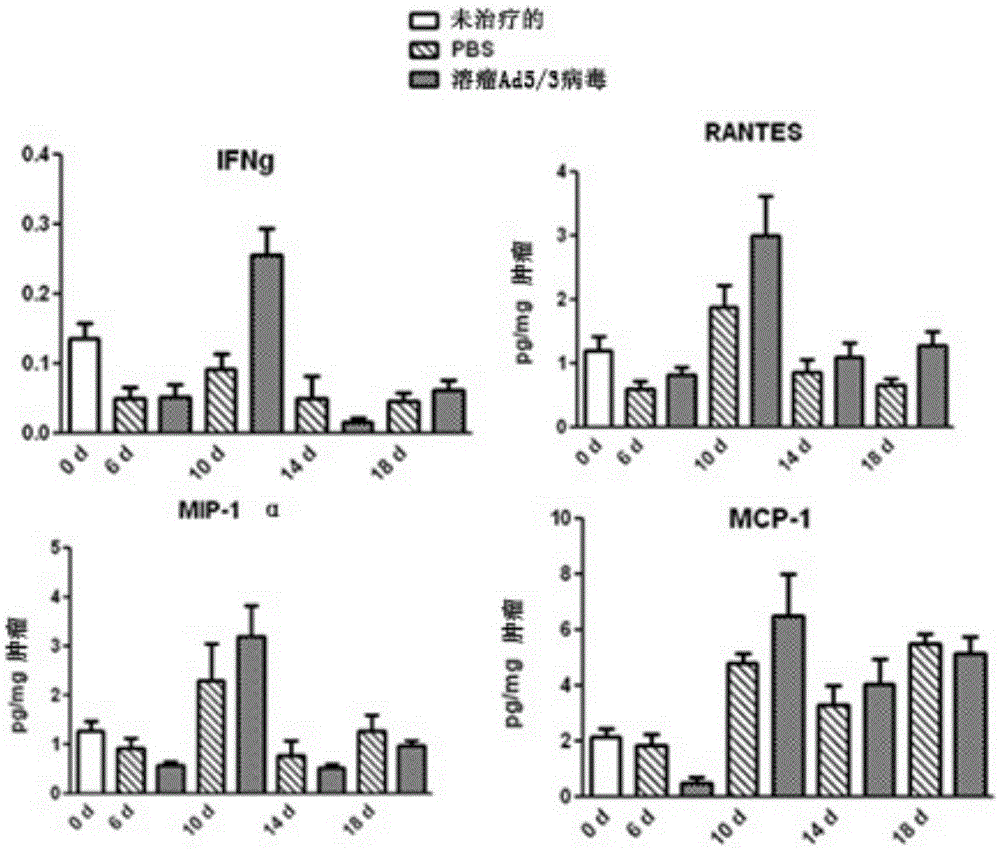
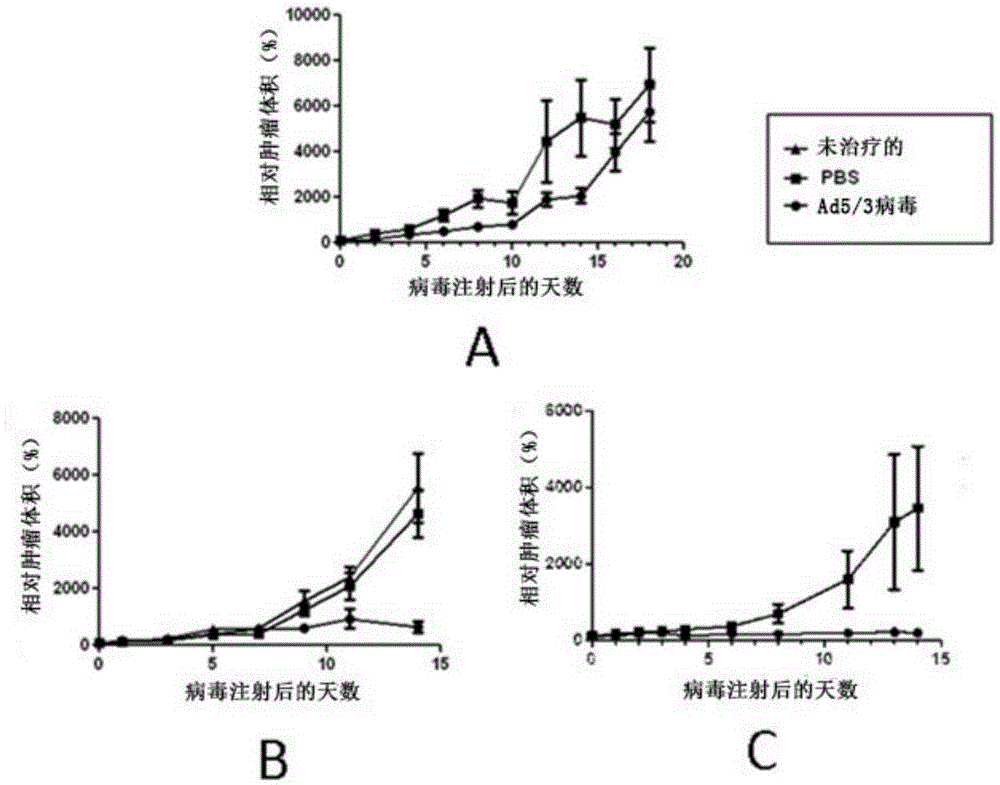
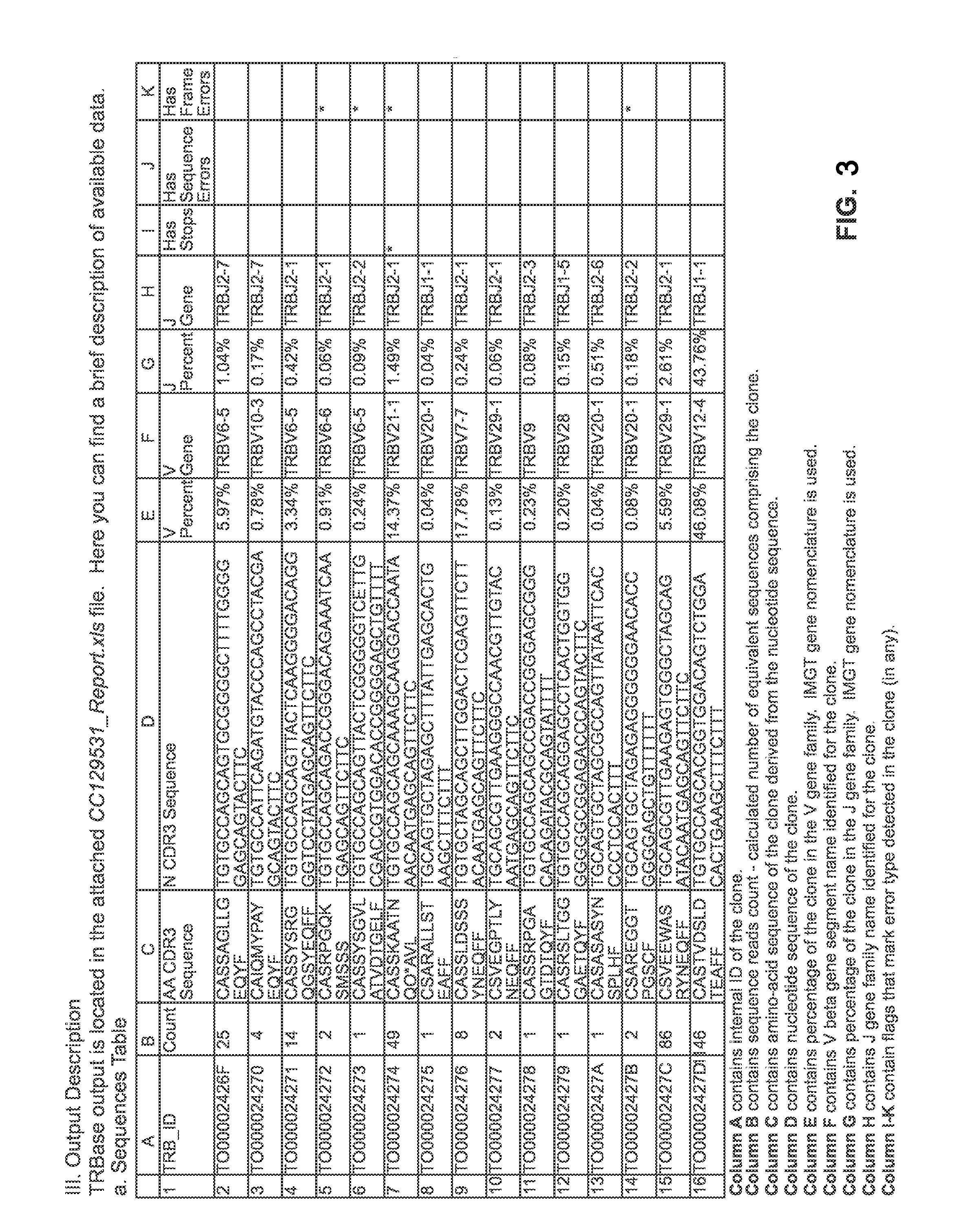
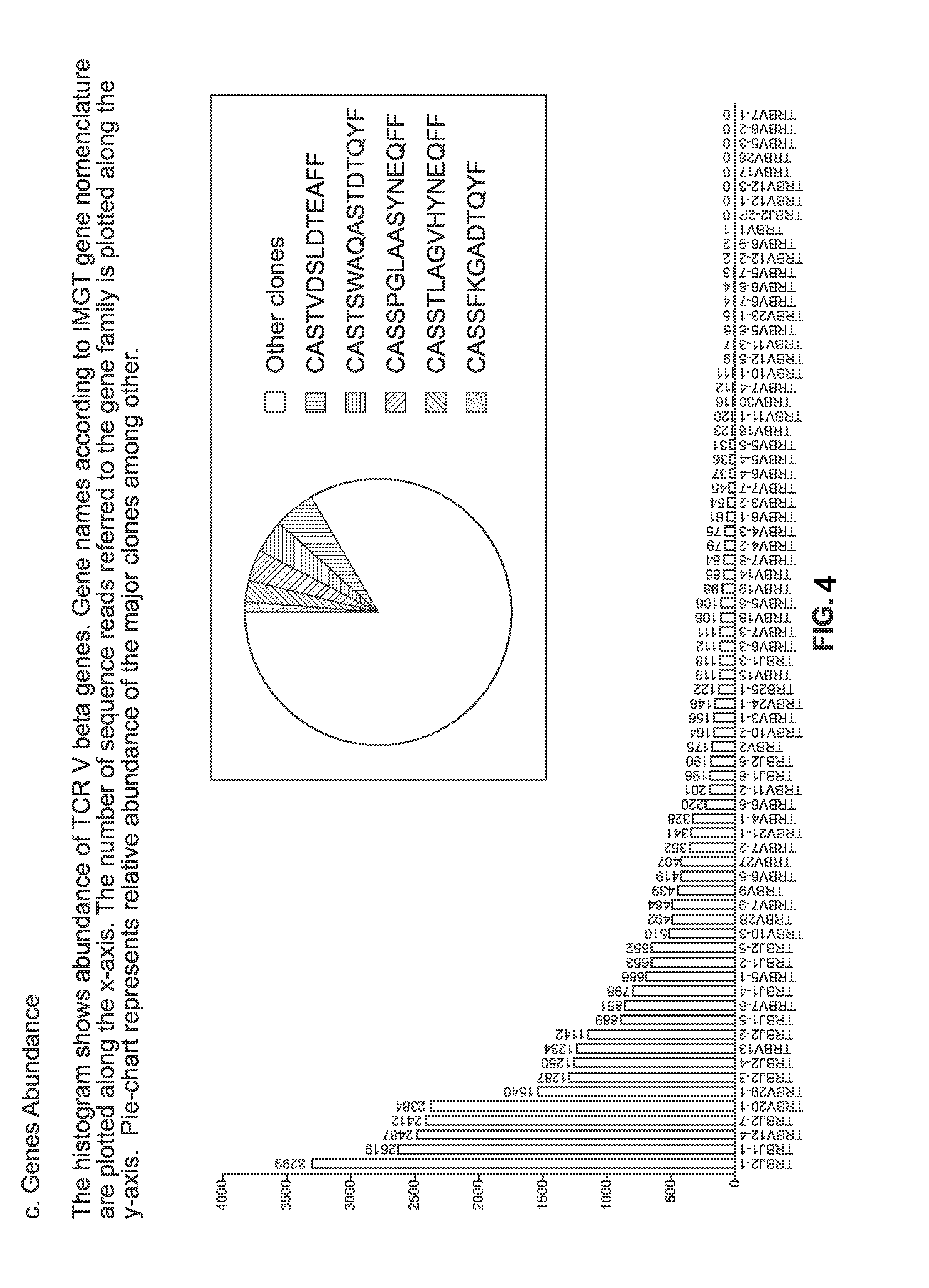
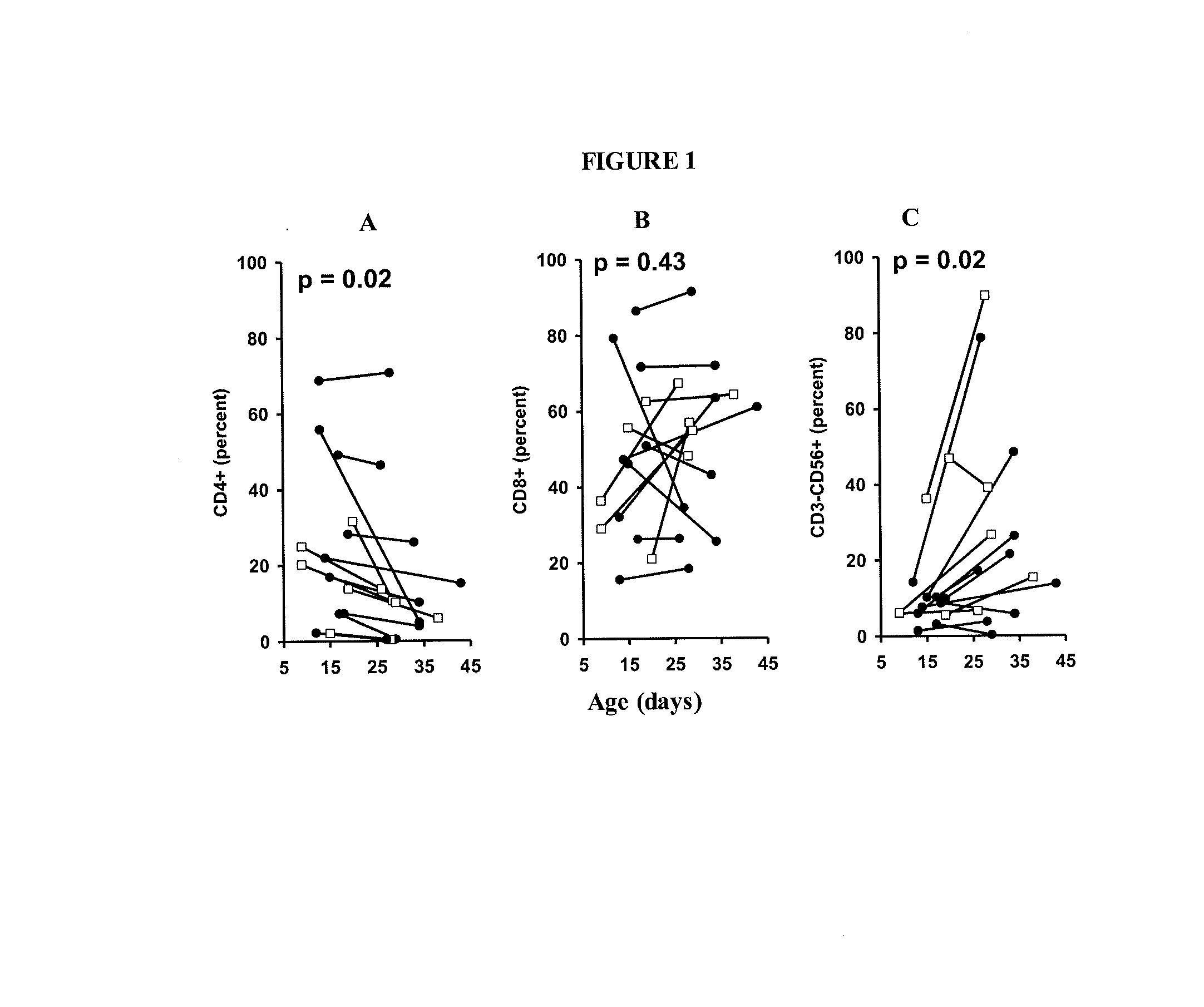
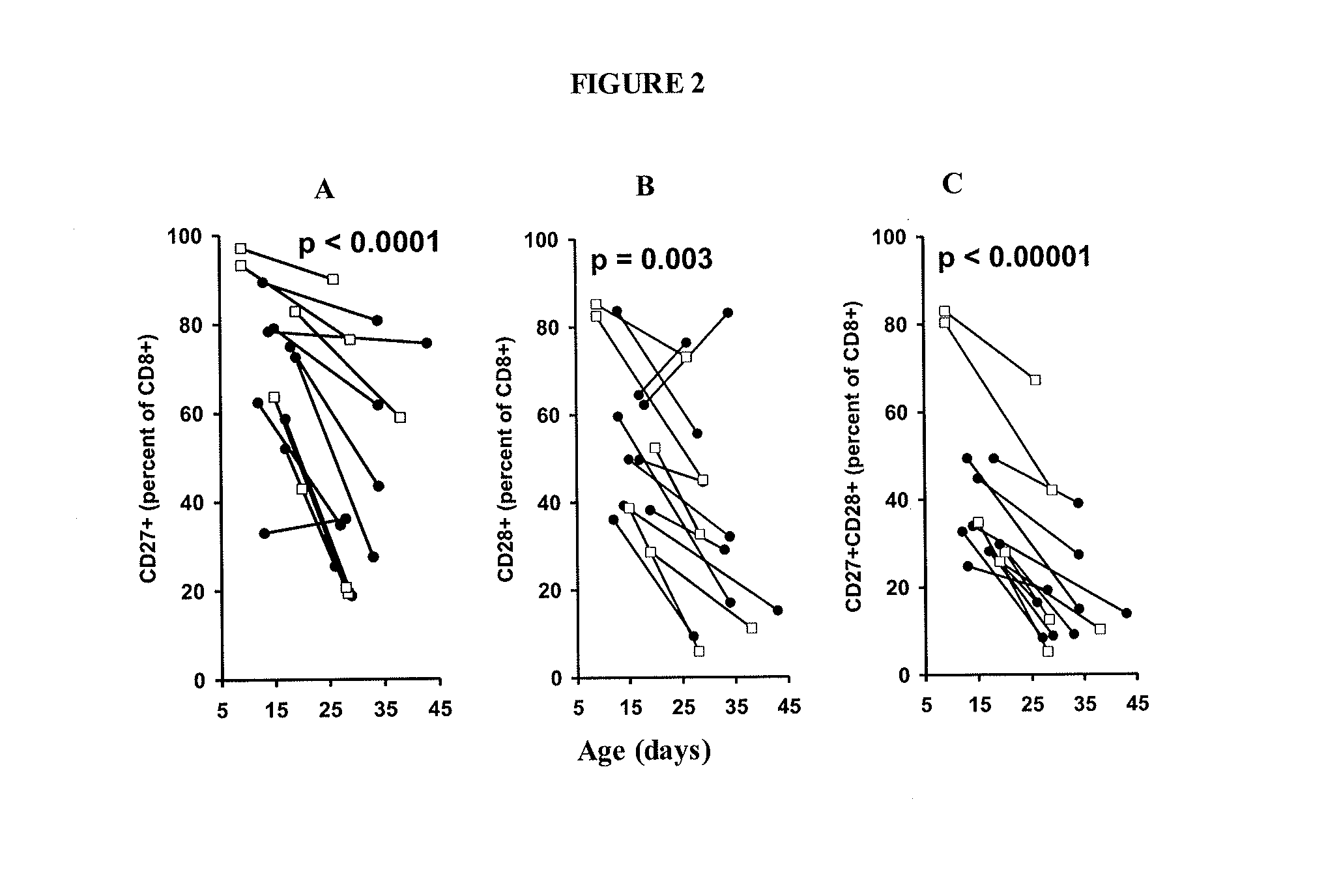
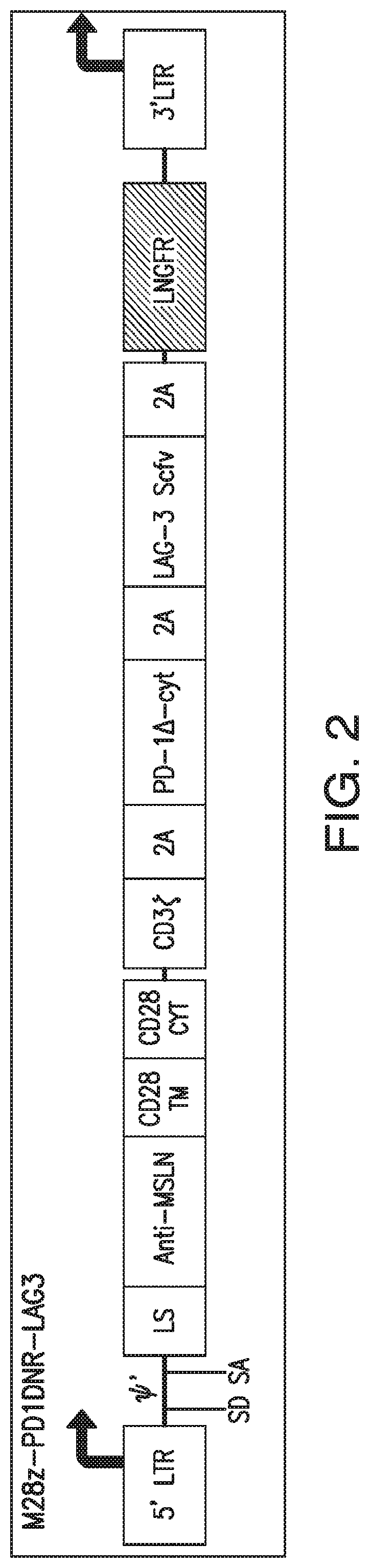
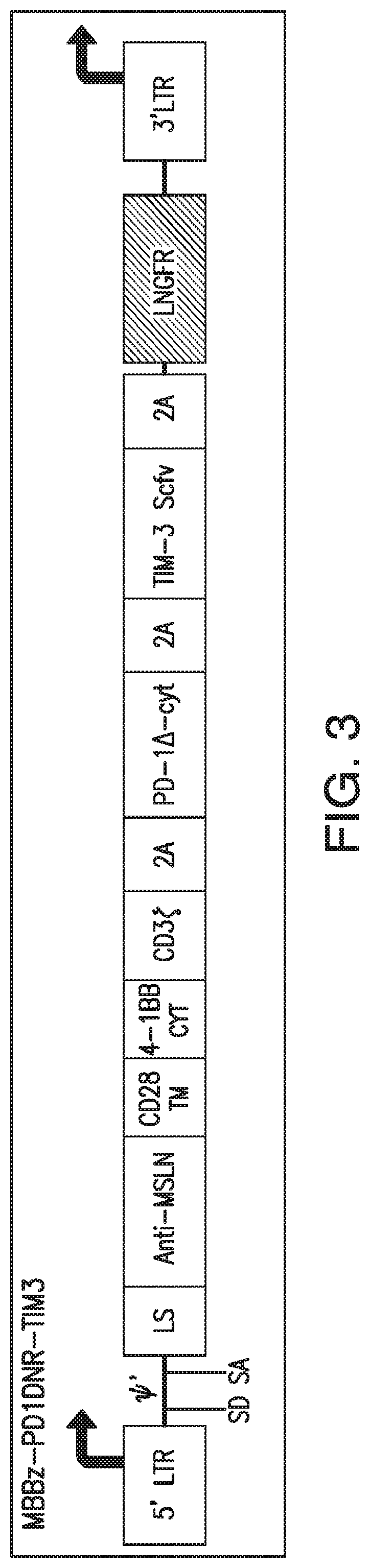
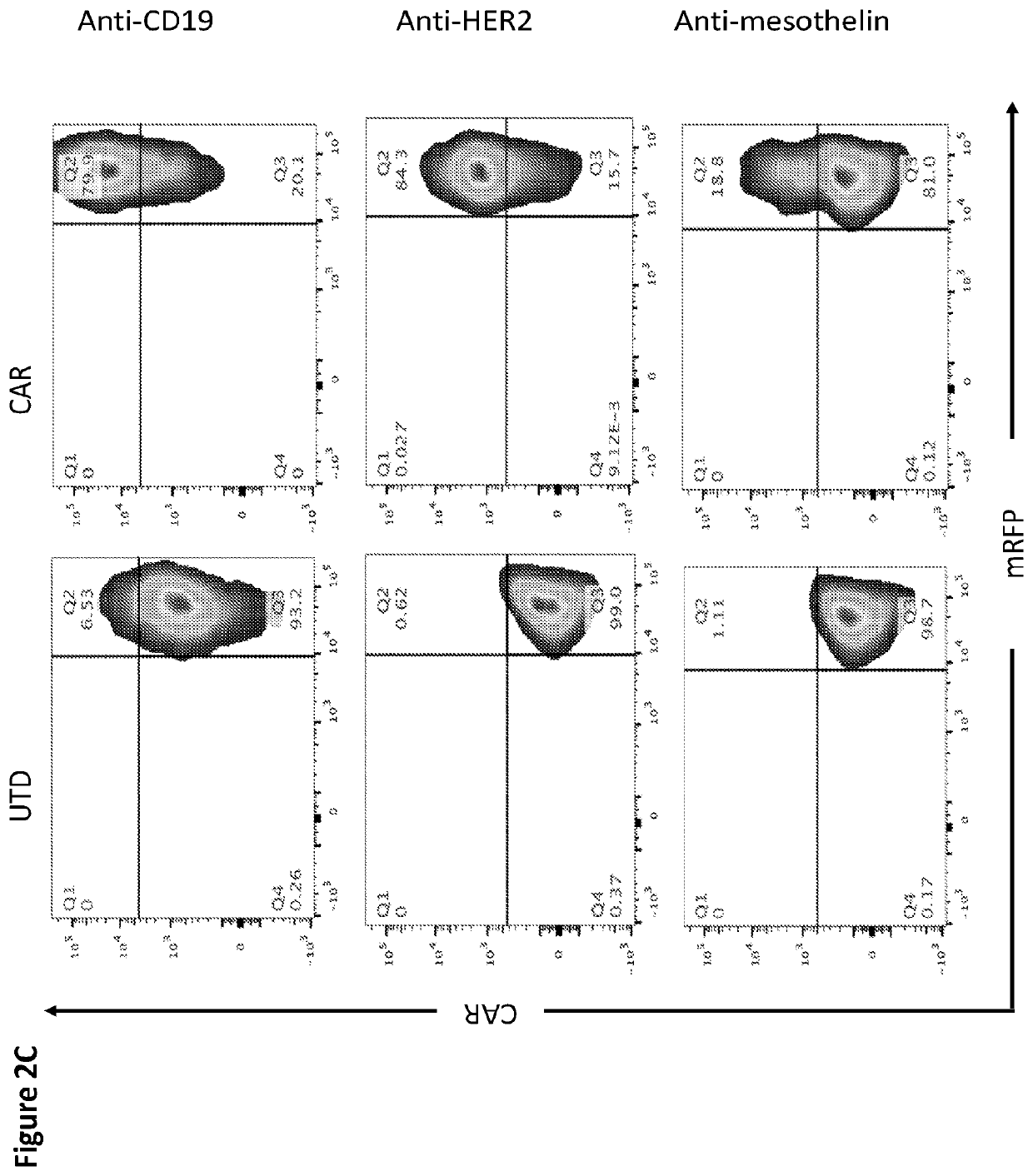
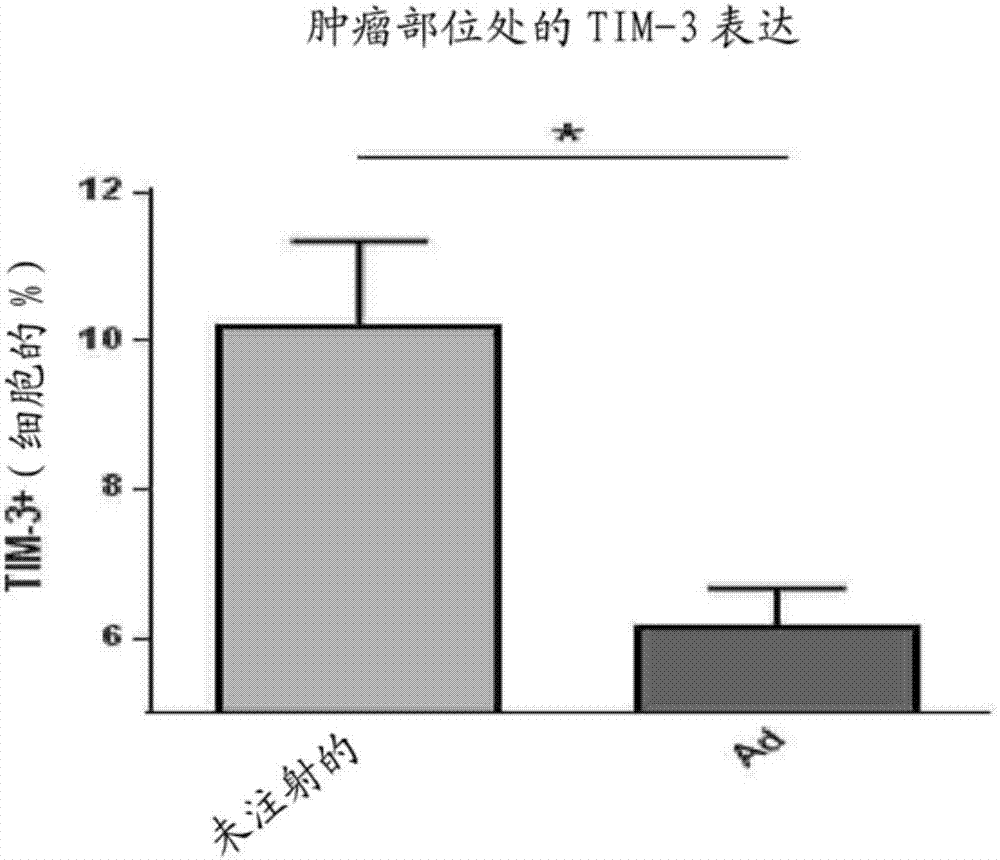
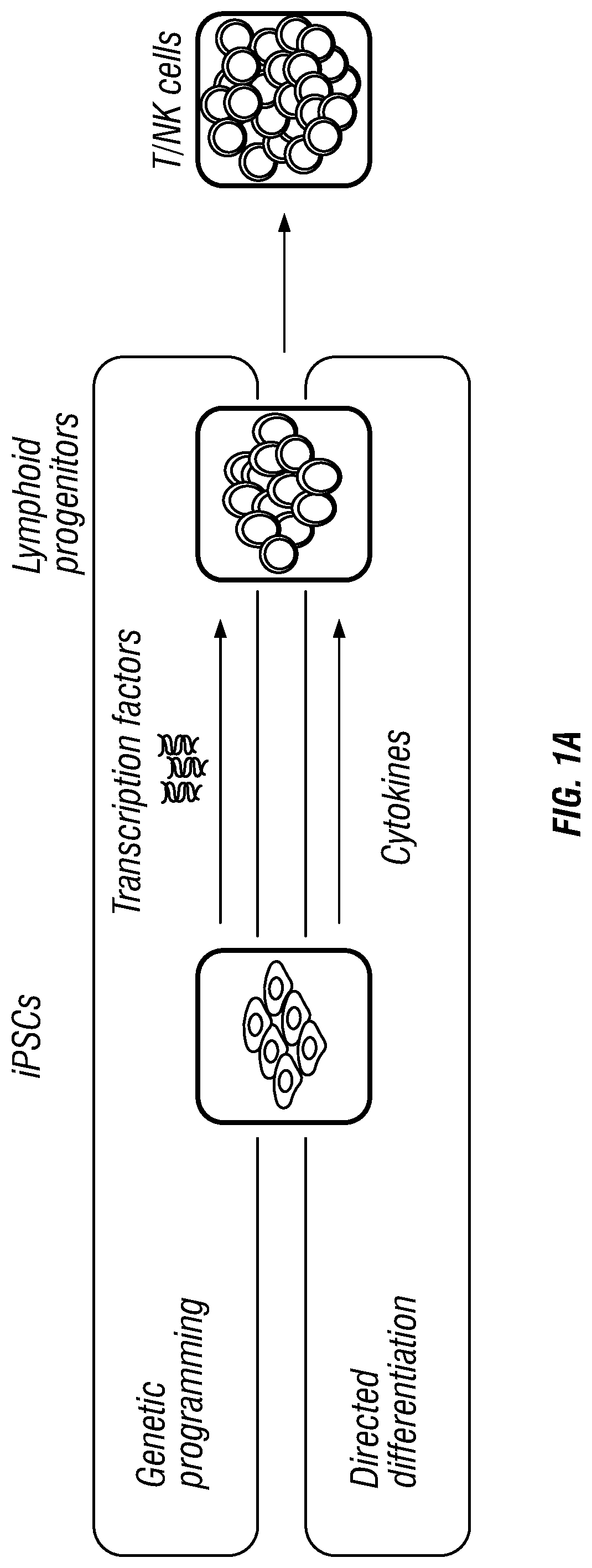
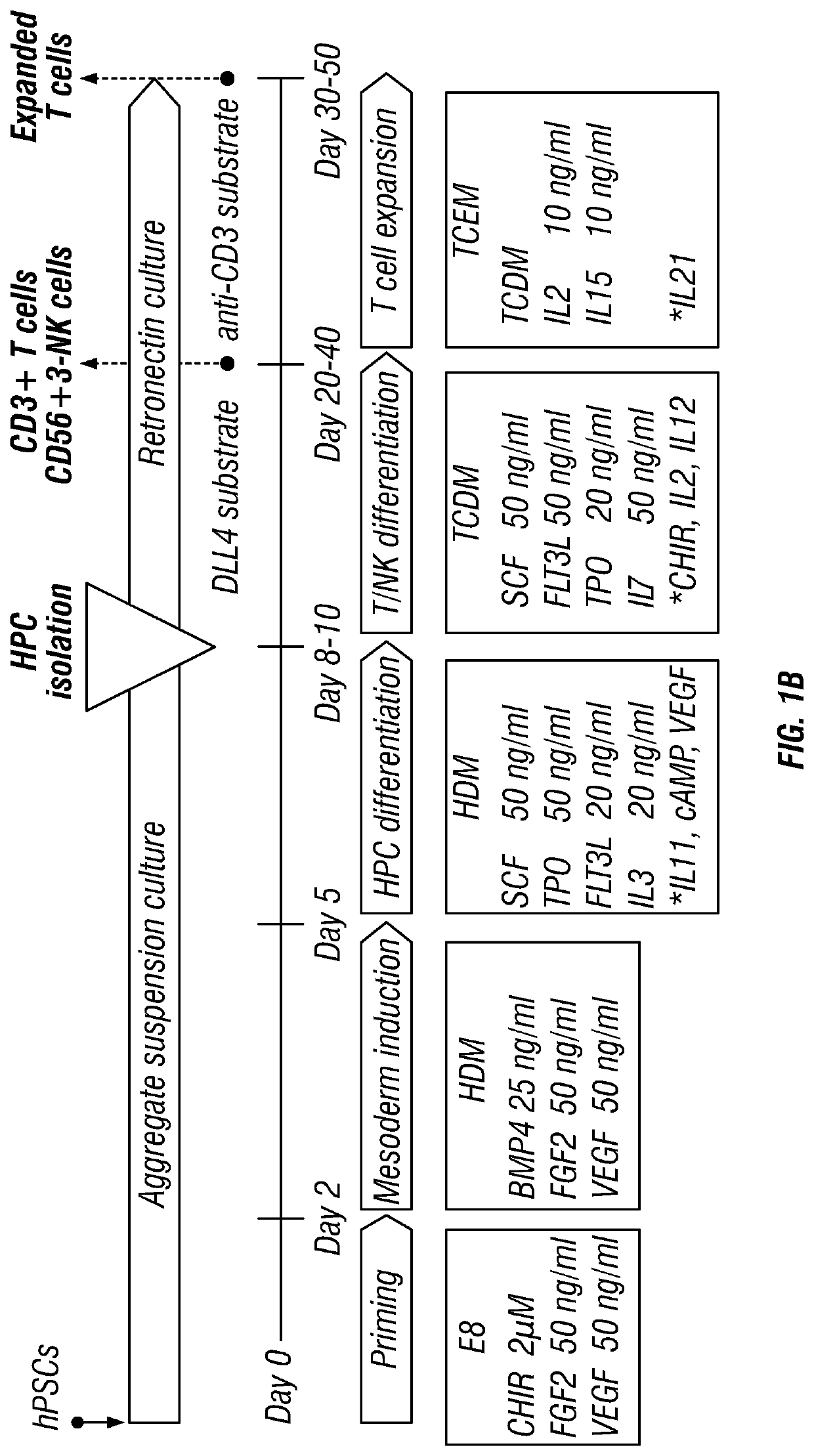
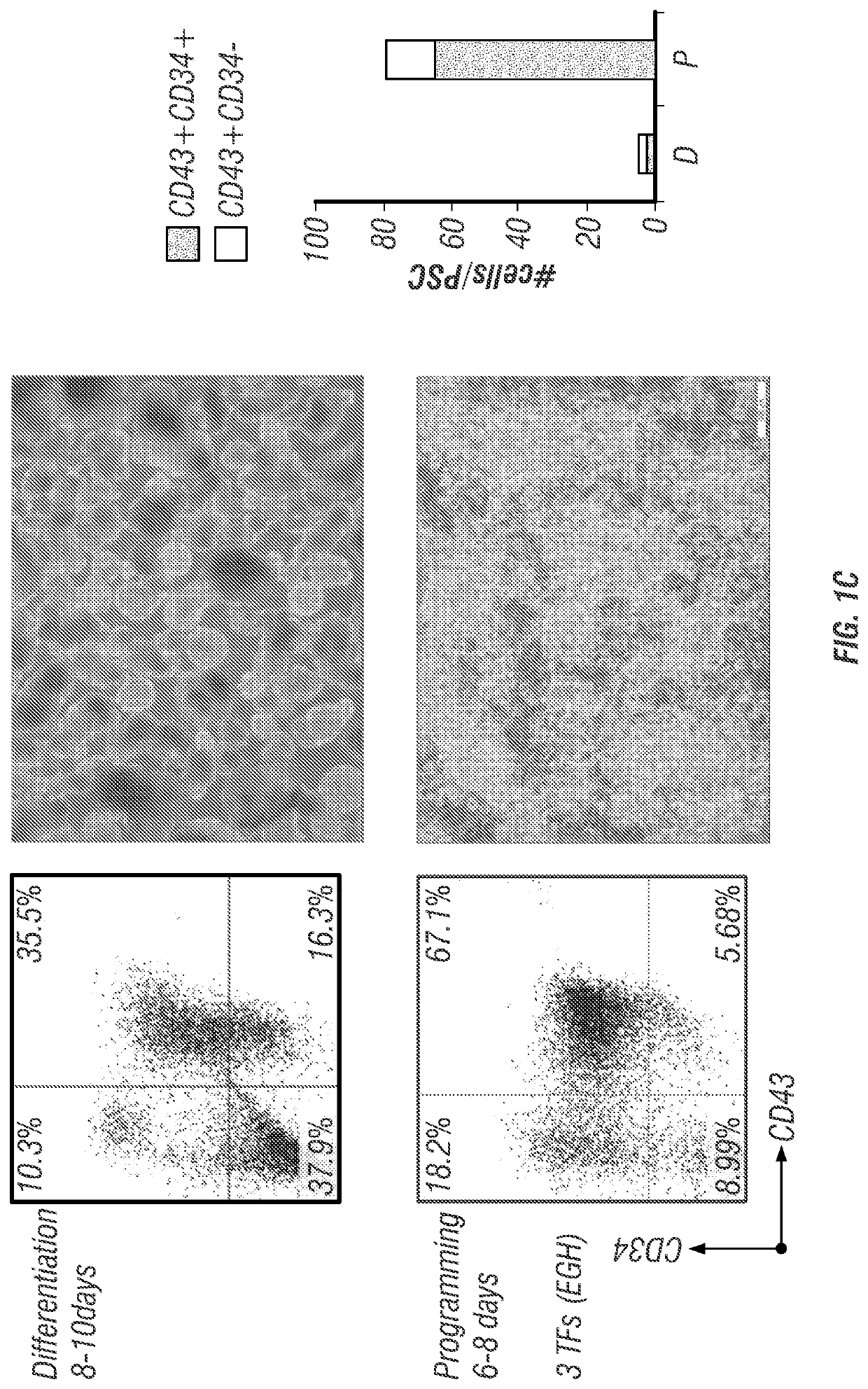
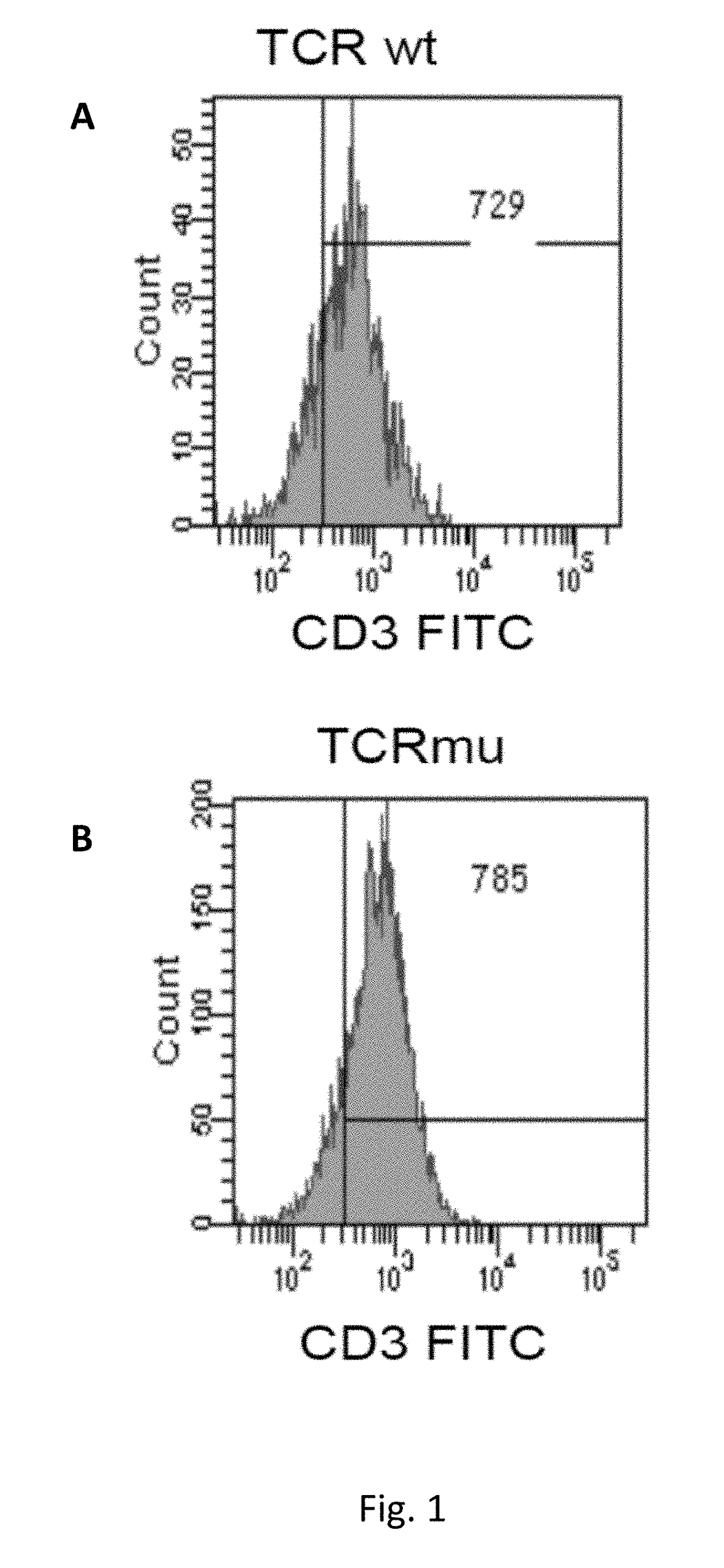
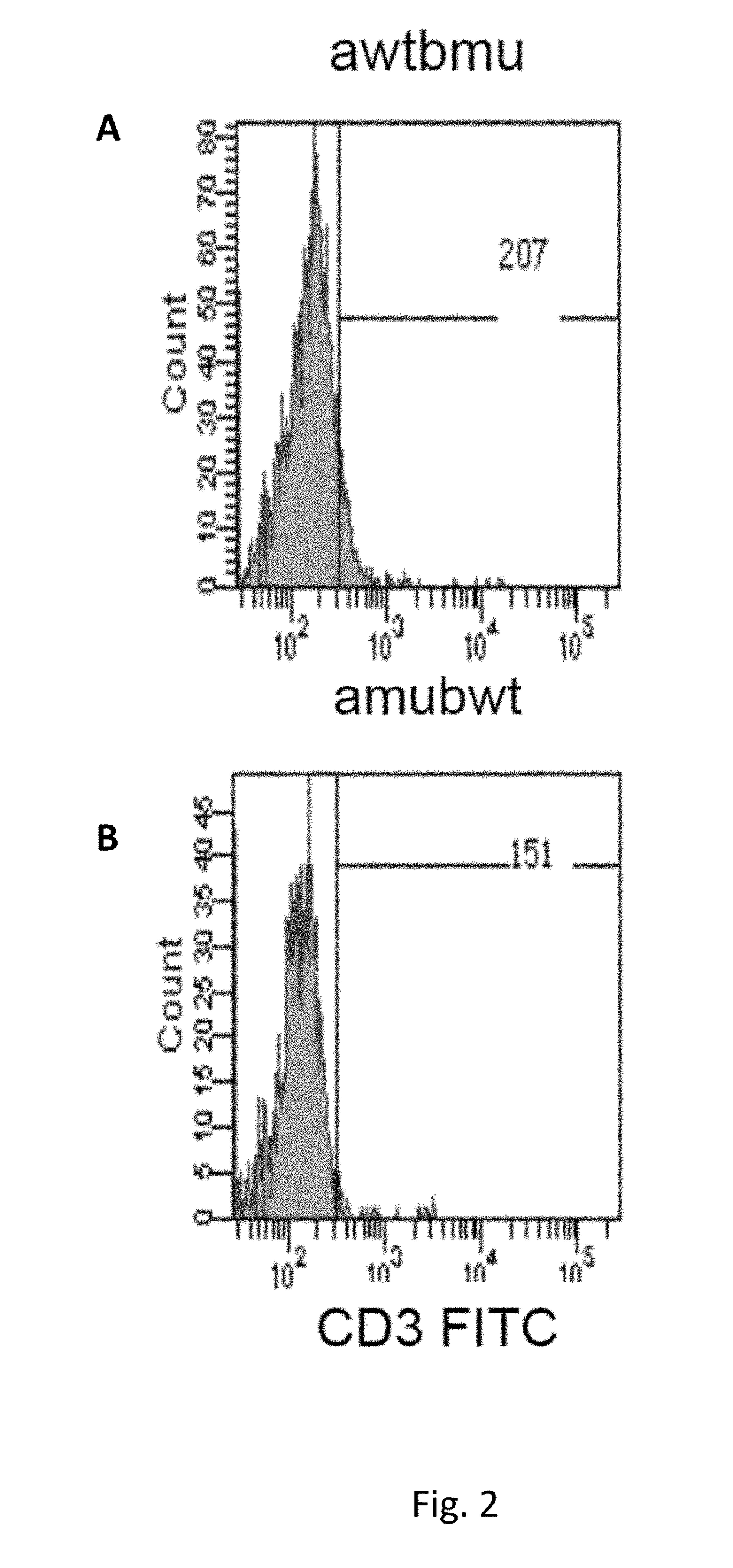
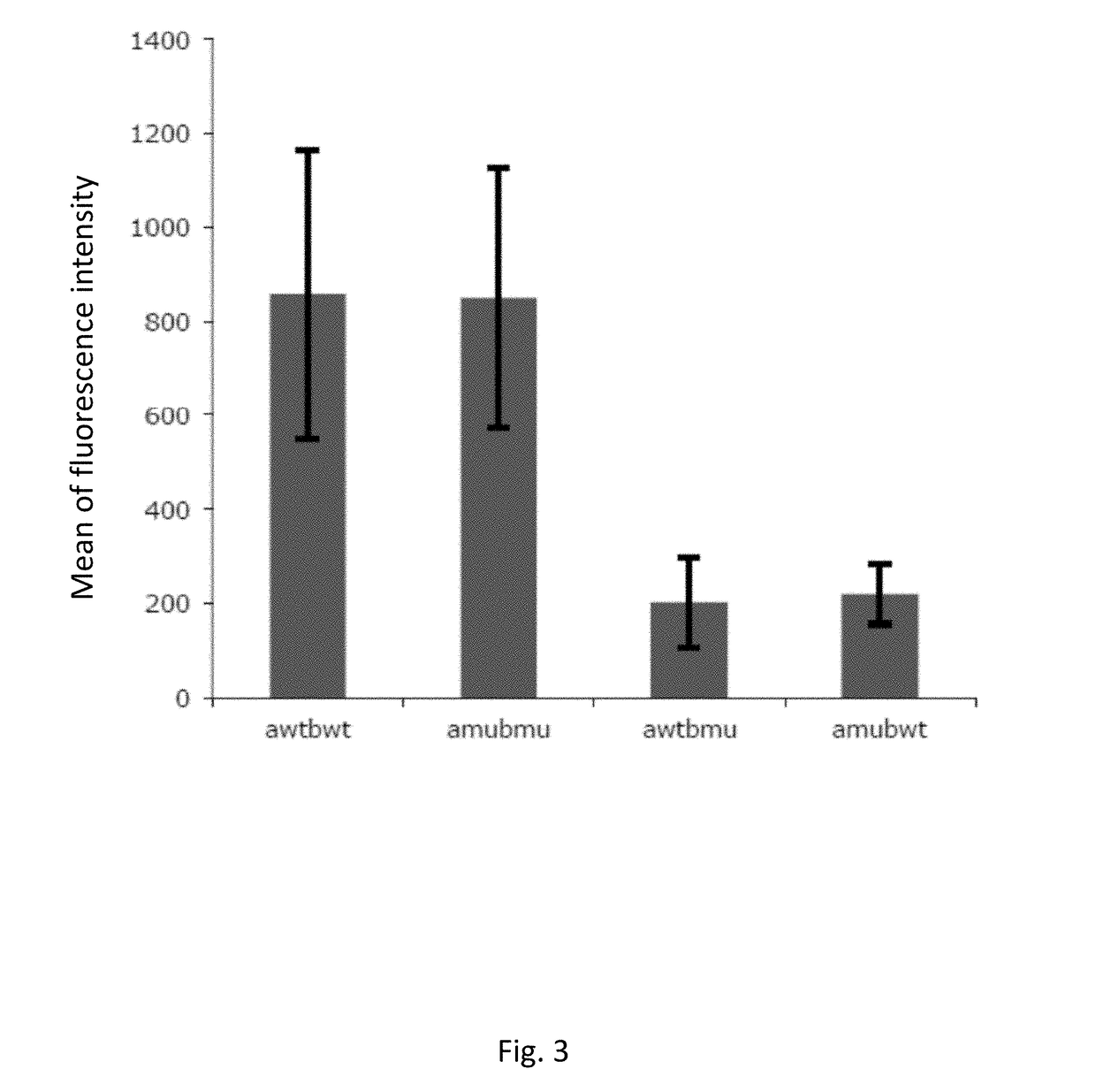
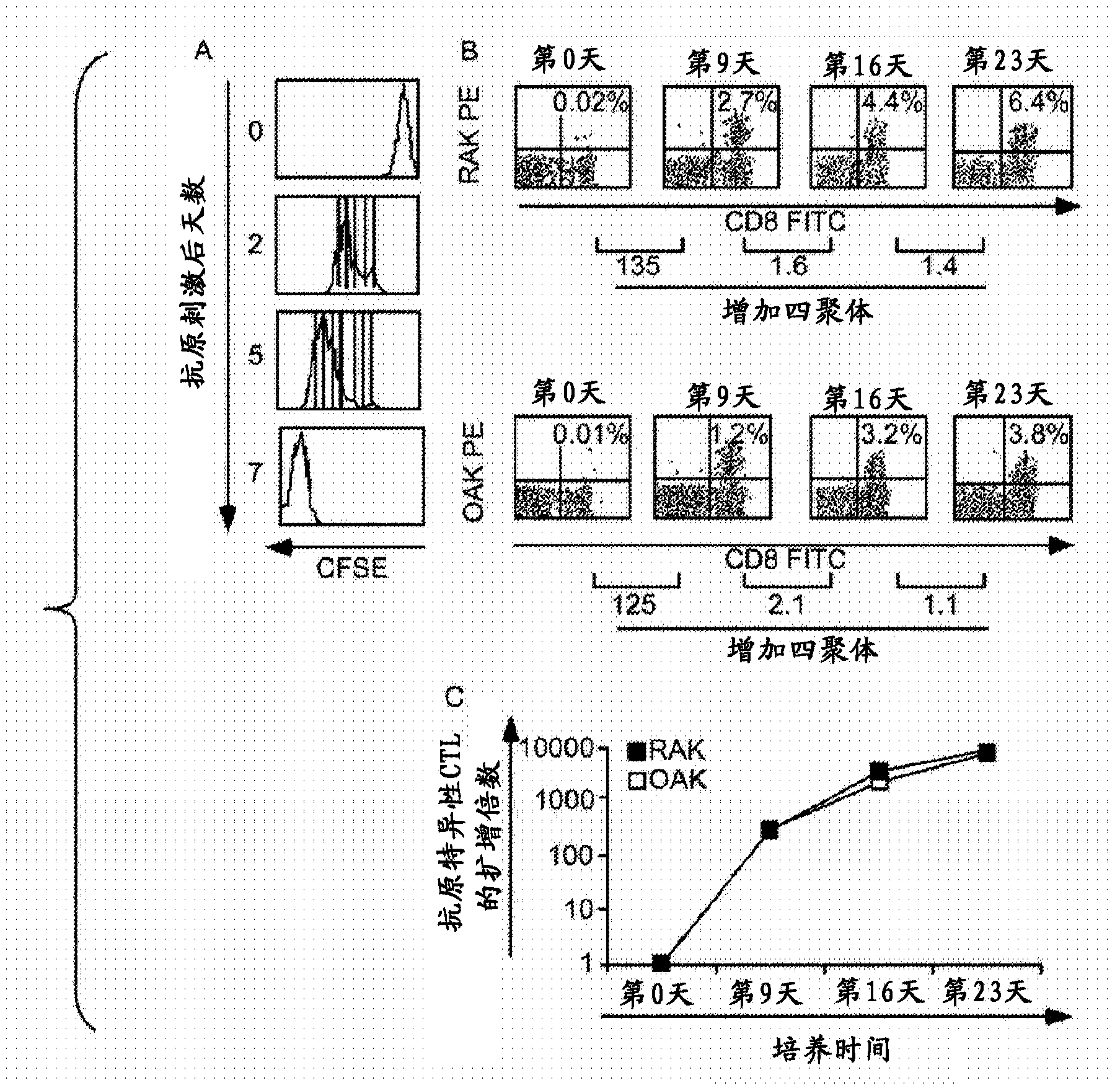
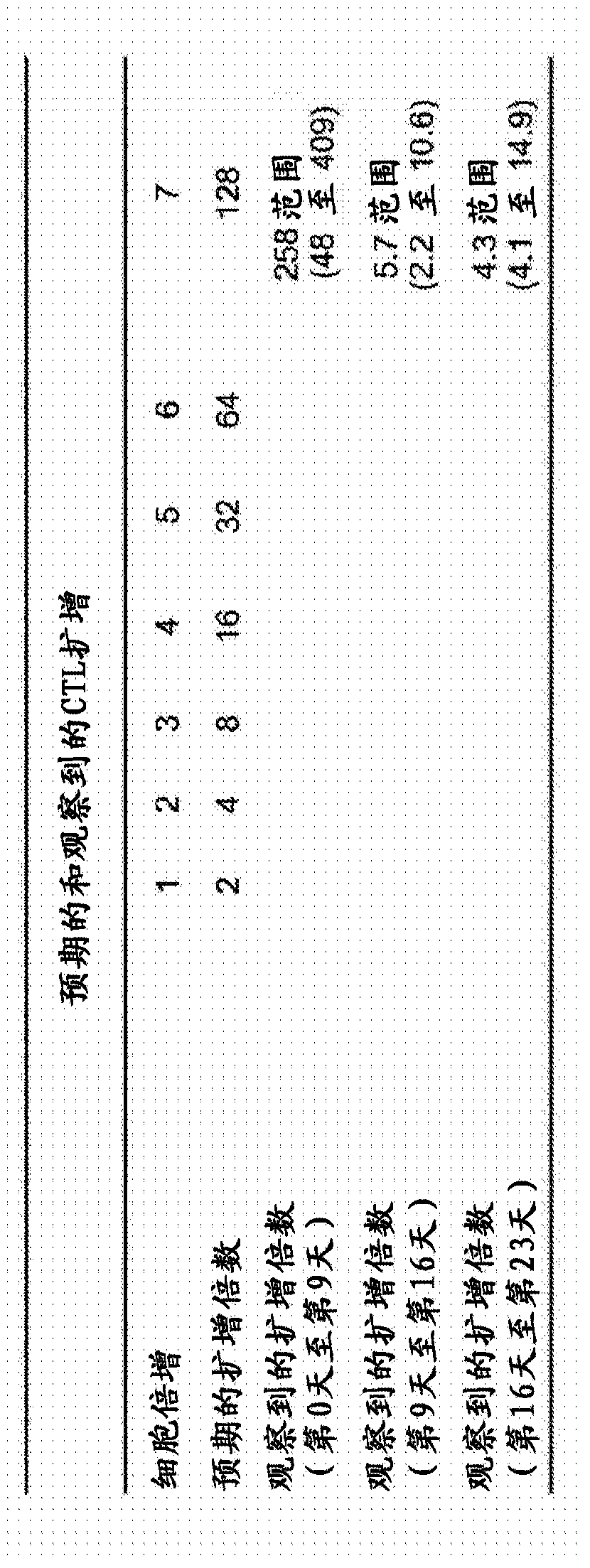
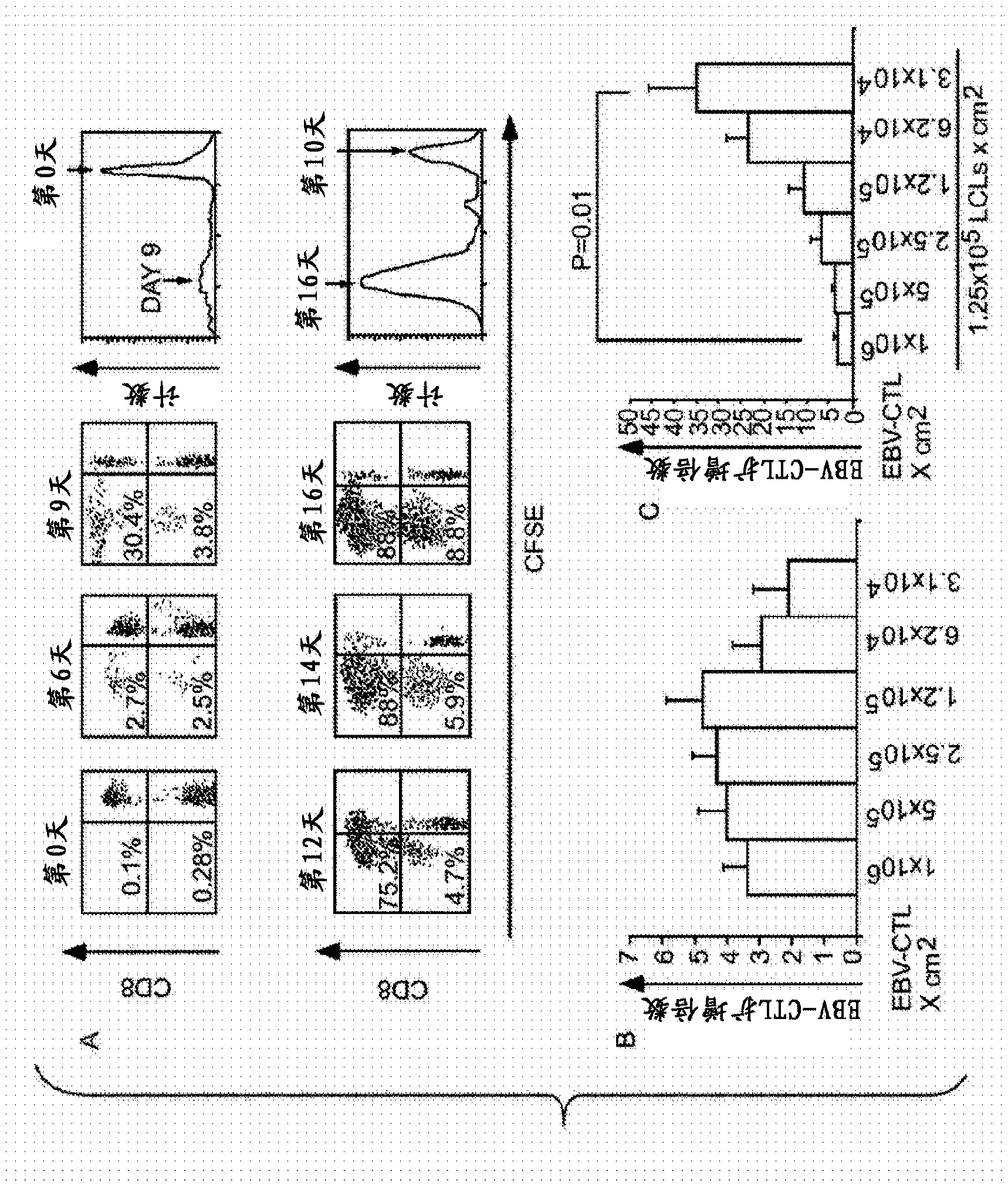
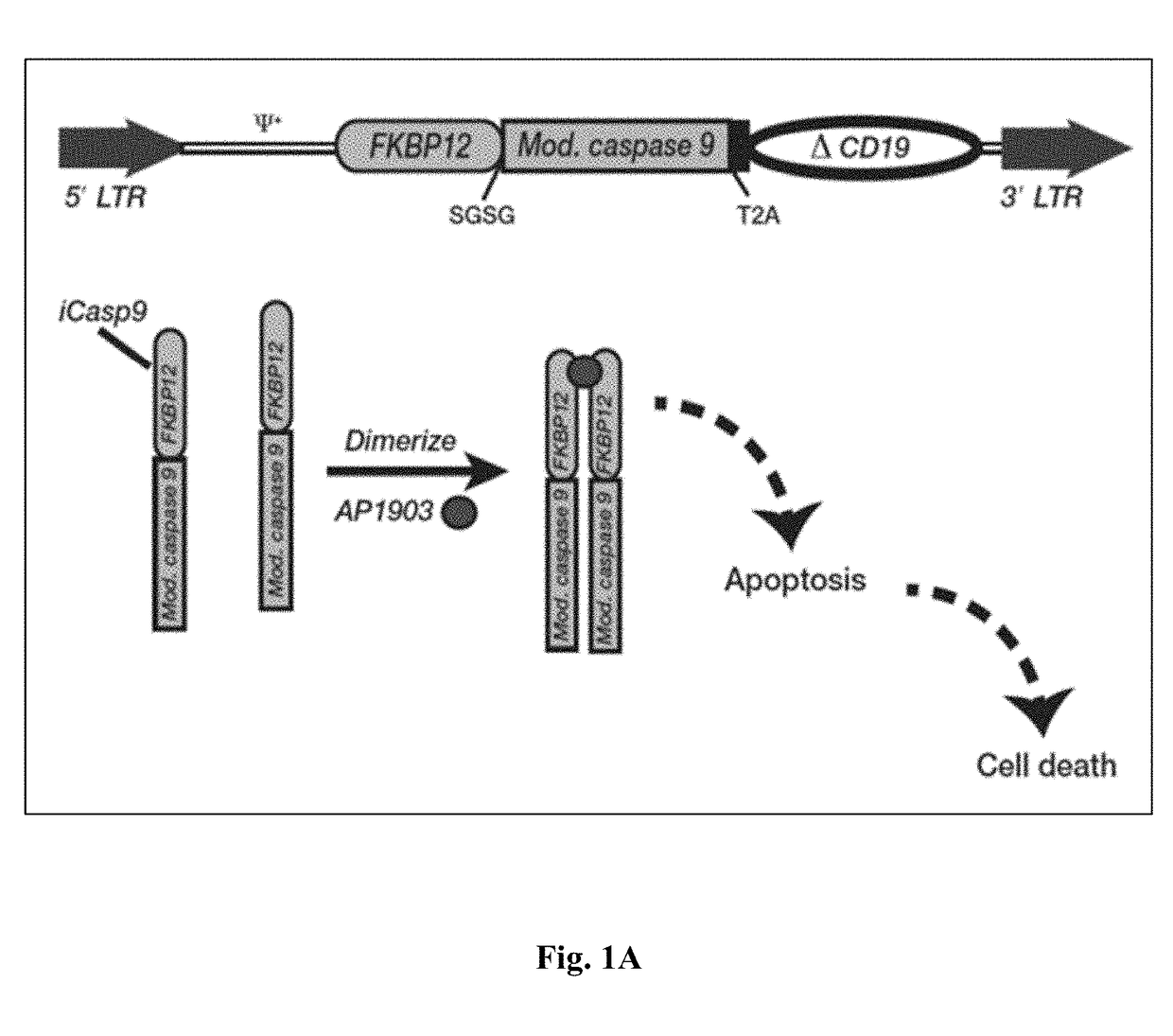
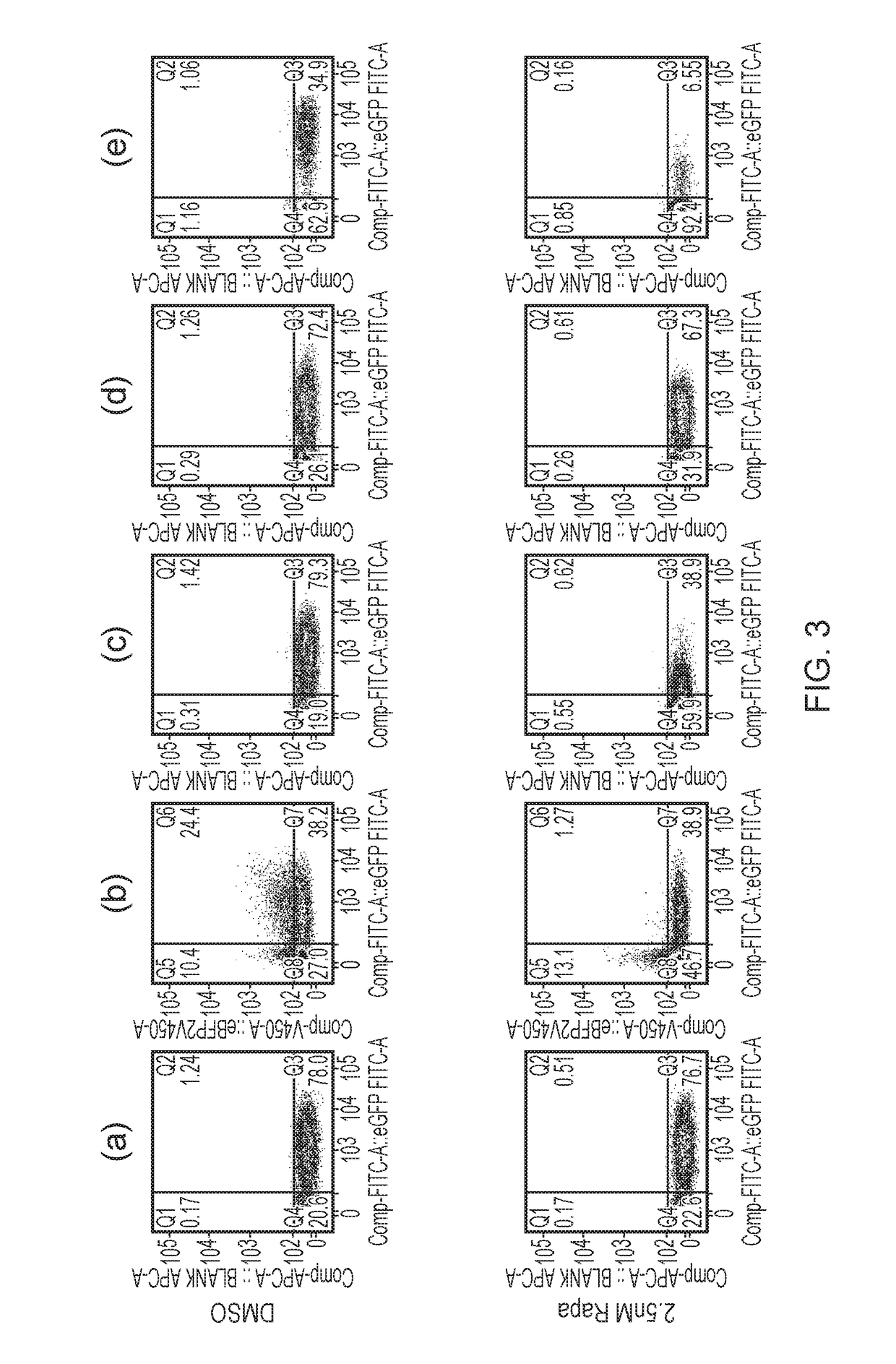
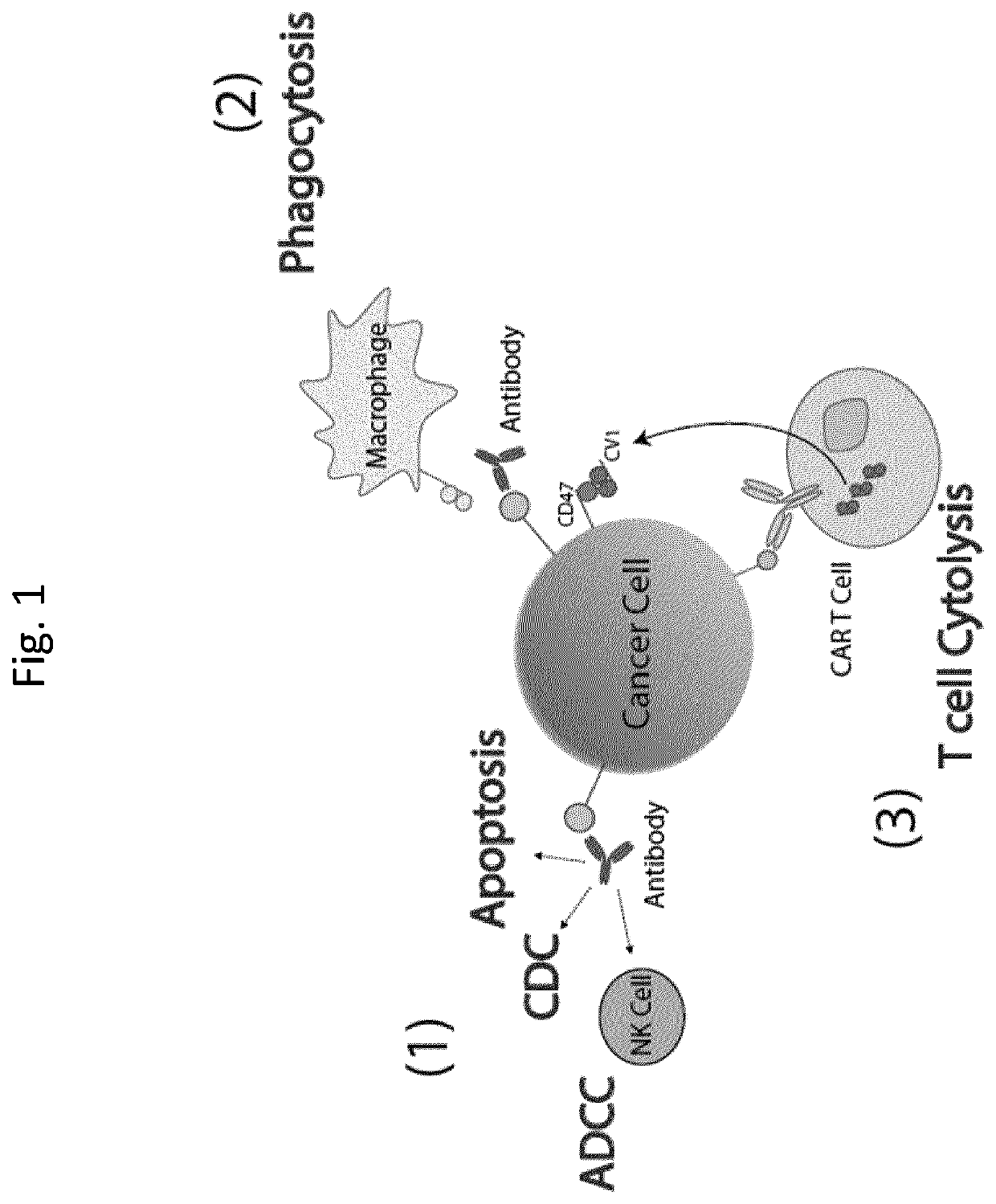
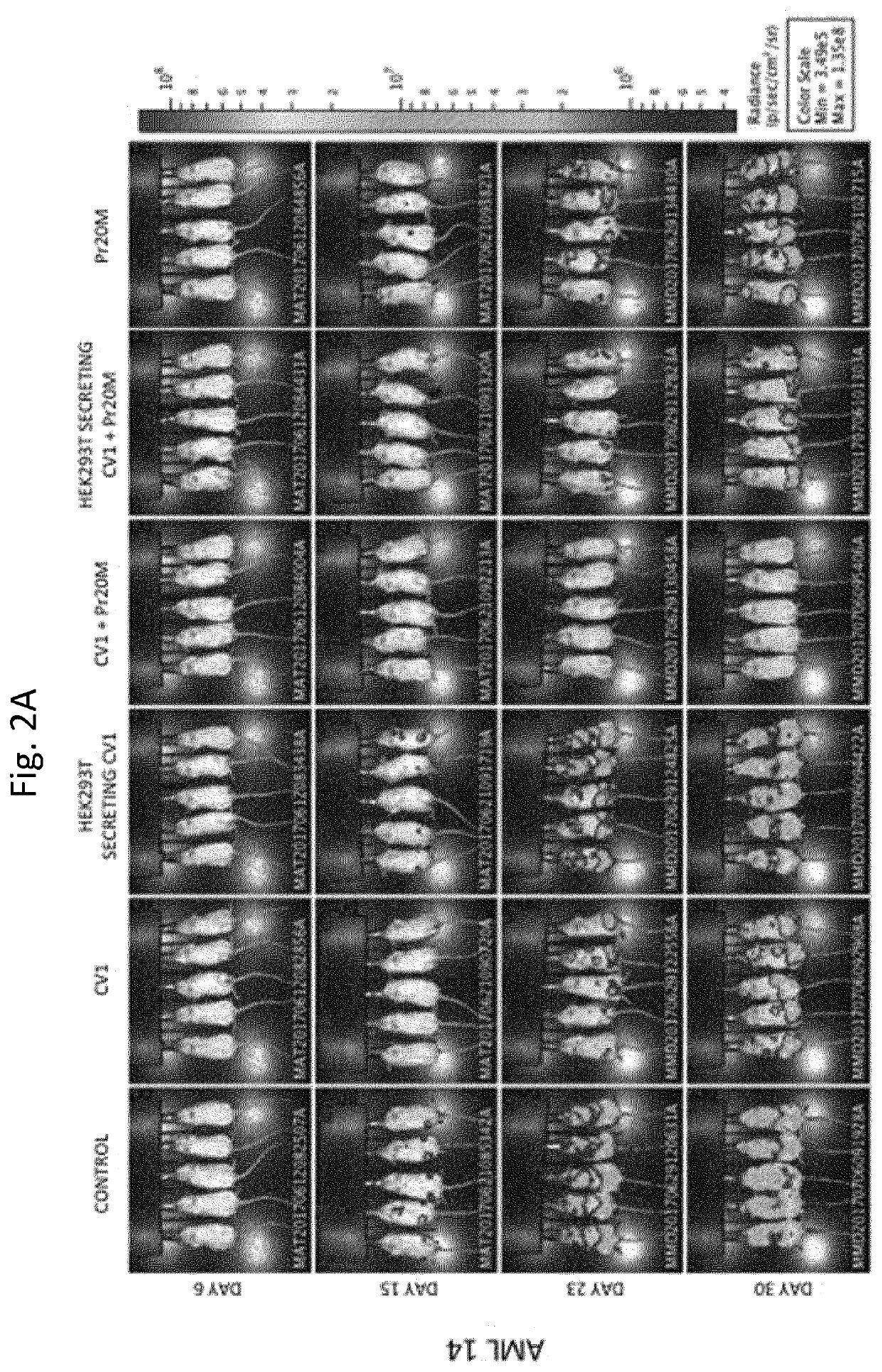
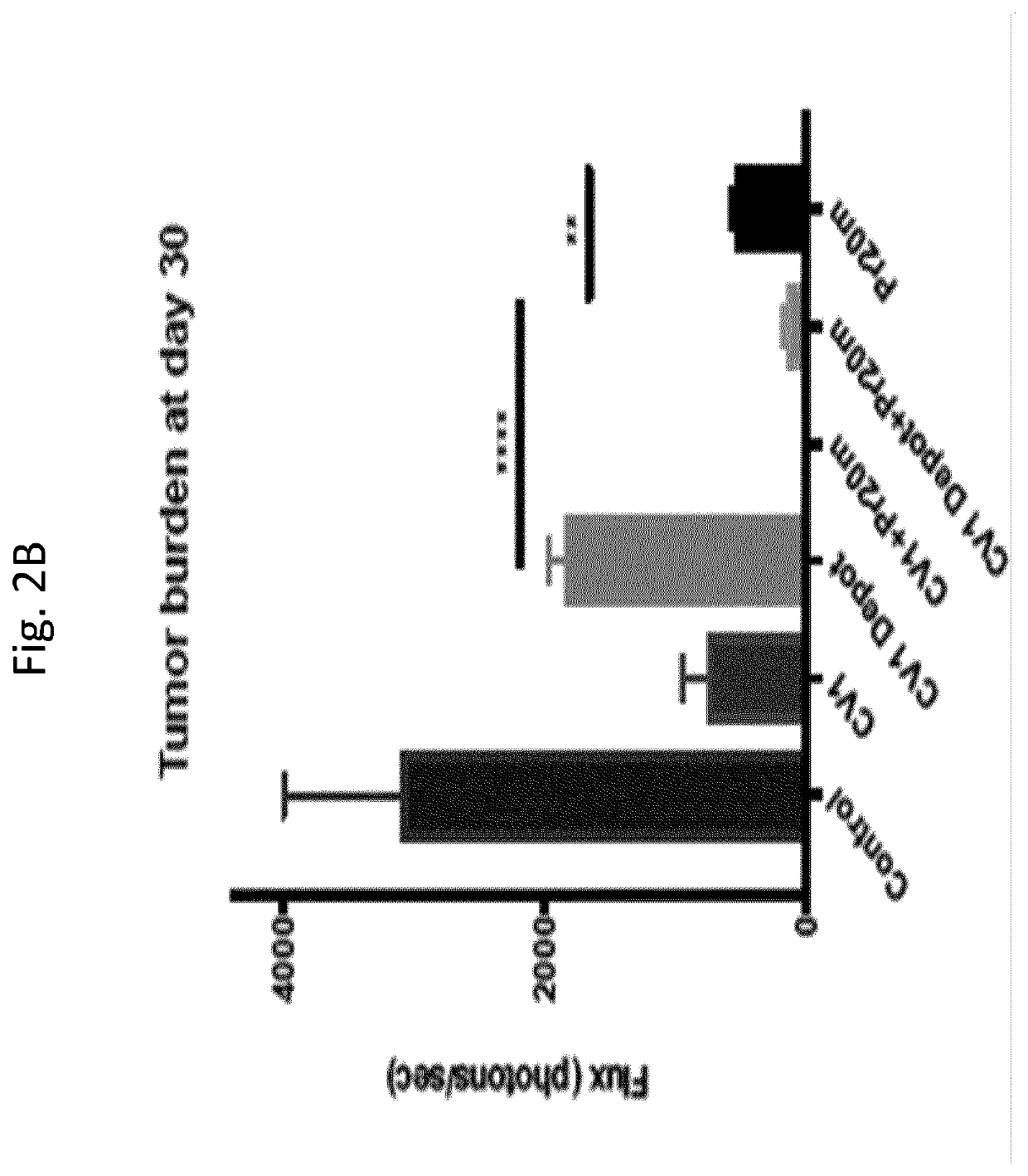
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
97 results about "Adoptive cellular therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Adoptive cell therapy with young T cells
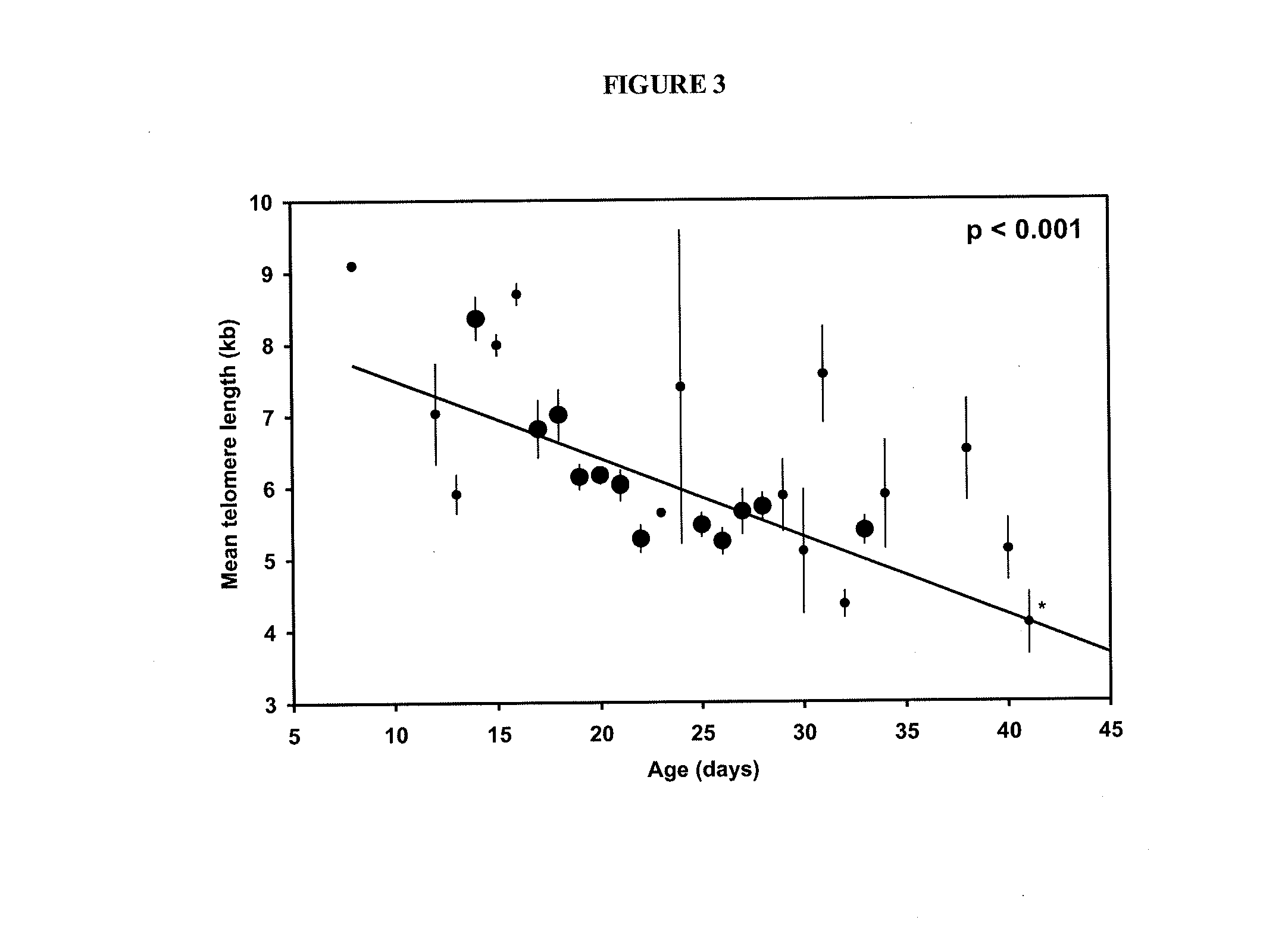
The invention provides a method of promoting regression of a cancer in a mammal comprising (i) culturing autologous T cells; (ii) expanding the cultured T cells; (iii) administering to the mammal nonmyeloablative lymphodepleting chemotherapy; and (iv) after administering nonmyeloablative lymphodepleting chemotherapy, administering to the mammal the expanded T cells, wherein the T cells administered to the mammal are about 19 to about 35 days old and have not been screened for specific tumor reactivity, whereupon the regression of the cancer in the mammal is promoted.
Owner:UNITED STATES OF AMERICA
Adoptive cell therapy with young t cells
The invention provides a method of promoting regression of a cancer in a mammal comprising (i) culturing autologous T cells; (ii) expanding the cultured T cells; (iii) administering to the mammal nonmyeloablative lymphodepleting chemotherapy; and (iv) after administering nonmyeloablative lymphodepleting chemotherapy, administering to the mammal the expanded T cells, wherein the T cells administered to the mammal are about 19 to about 35 days old and have not been screened for specific tumor reactivity, whereupon the regression of the cancer in the mammal is promoted.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Enhanced adoptive cell therapy
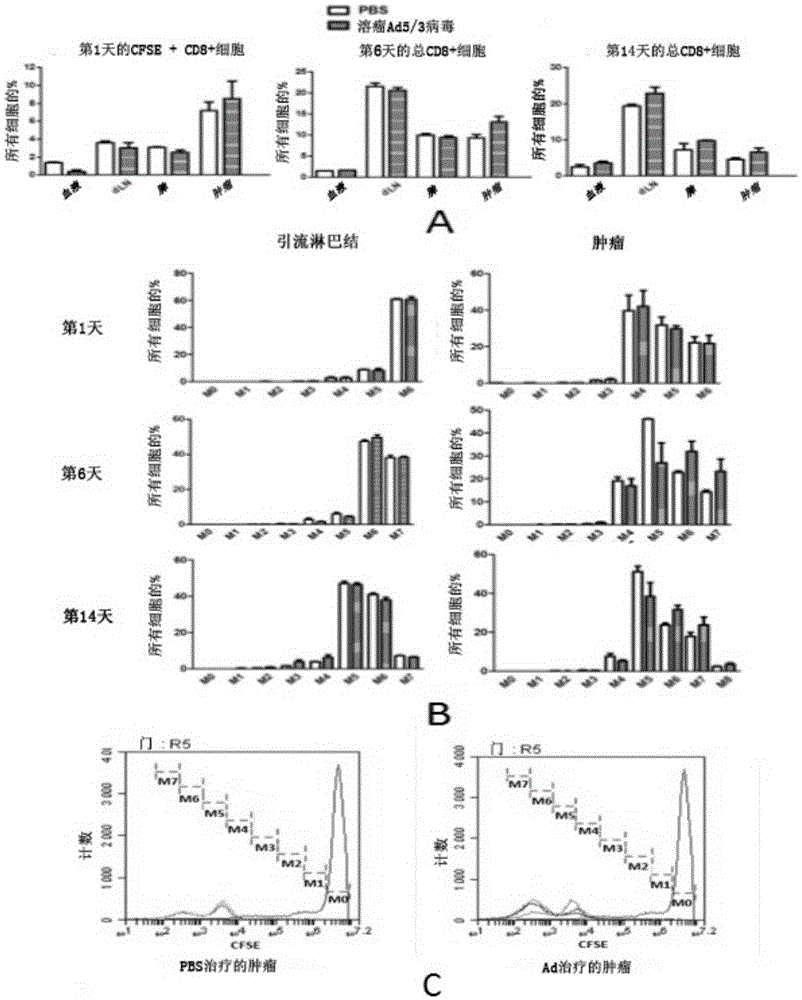
ActiveCN105307671AEnhance the popular effectEasy to handlePeptide/protein ingredientsGenetic material ingredientsAdoptive cellular therapyOncolytic adenovirus
The present invention relates to the fields of life sciences and medicine. Specifically, the invention relates to cancer therapies of humans. More specifically, the present invention relates to oncolytic adenoviral vectors alone or together with therapeutic compositions for therapeutic uses and therapeutic methods for cancer. In one aspect the present invention relates to separate administration of adoptive cell therapeutic composition and oncolytic adenoviral vectors. Furthermore, the present invention relates to a pharmaceutical kit and a pharmaceutical composition, both utilizing oncolytic adenoviral vectors.
Owner:TILT BIOTHERAPEUTICS OY
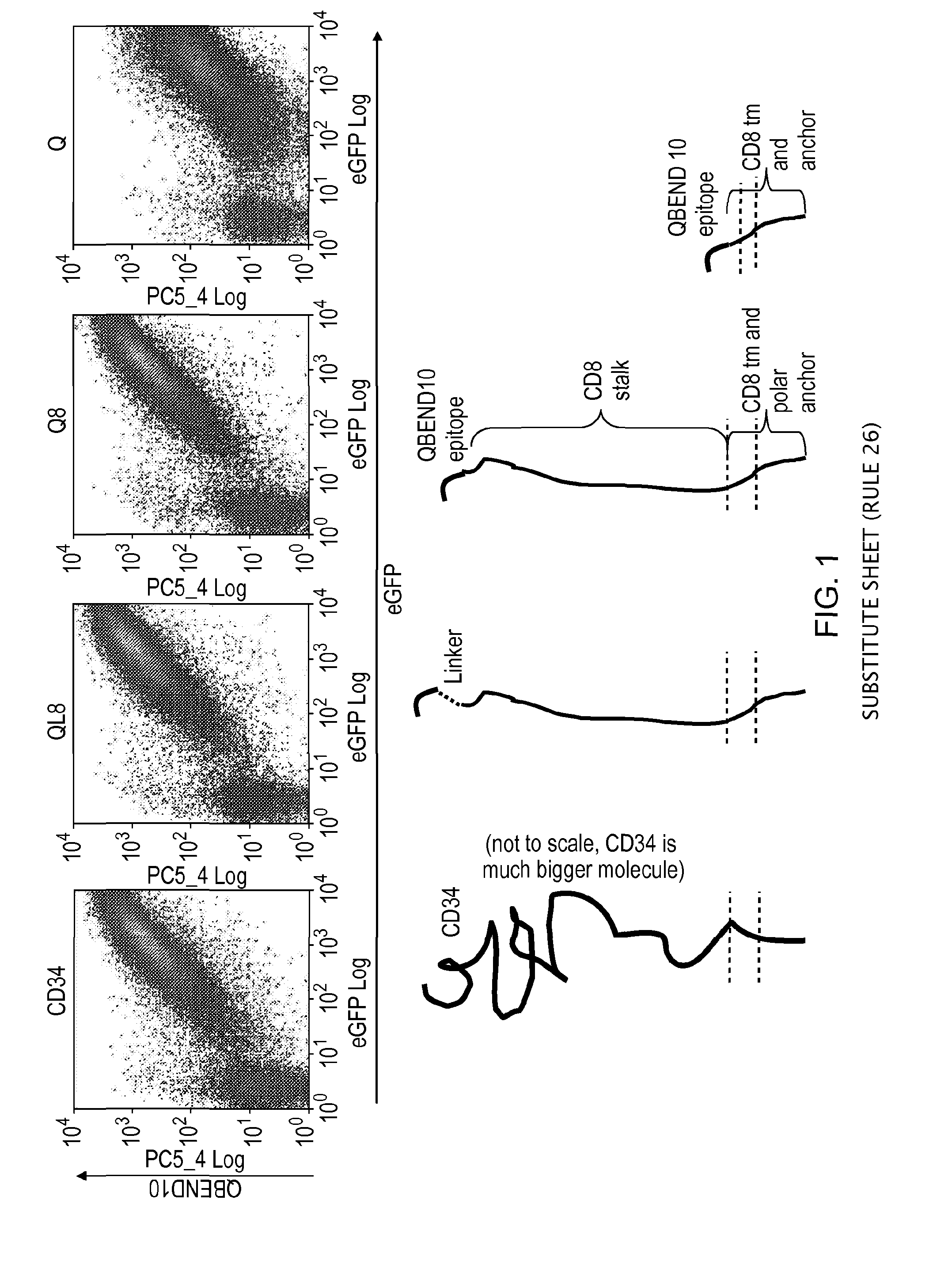
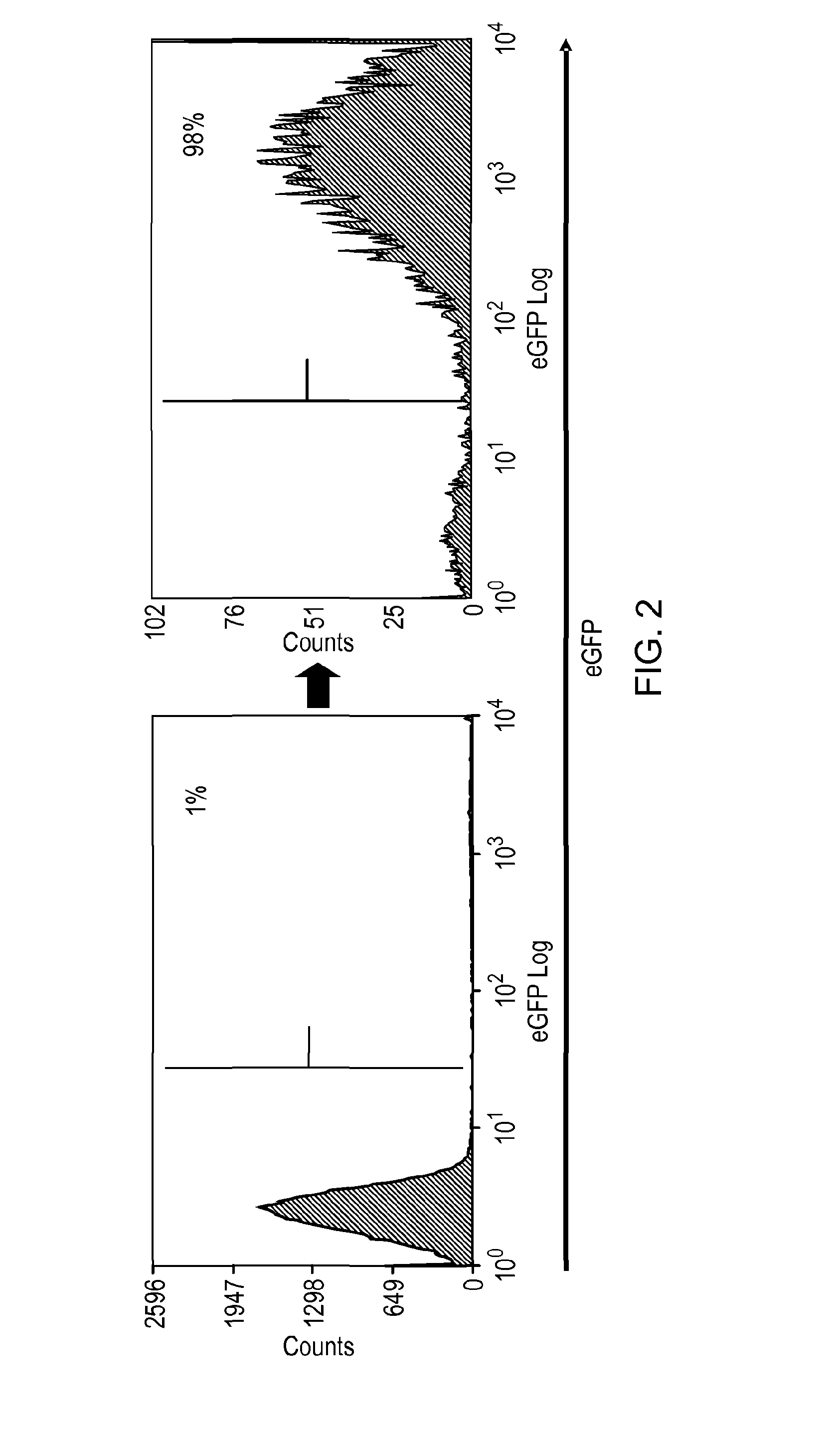
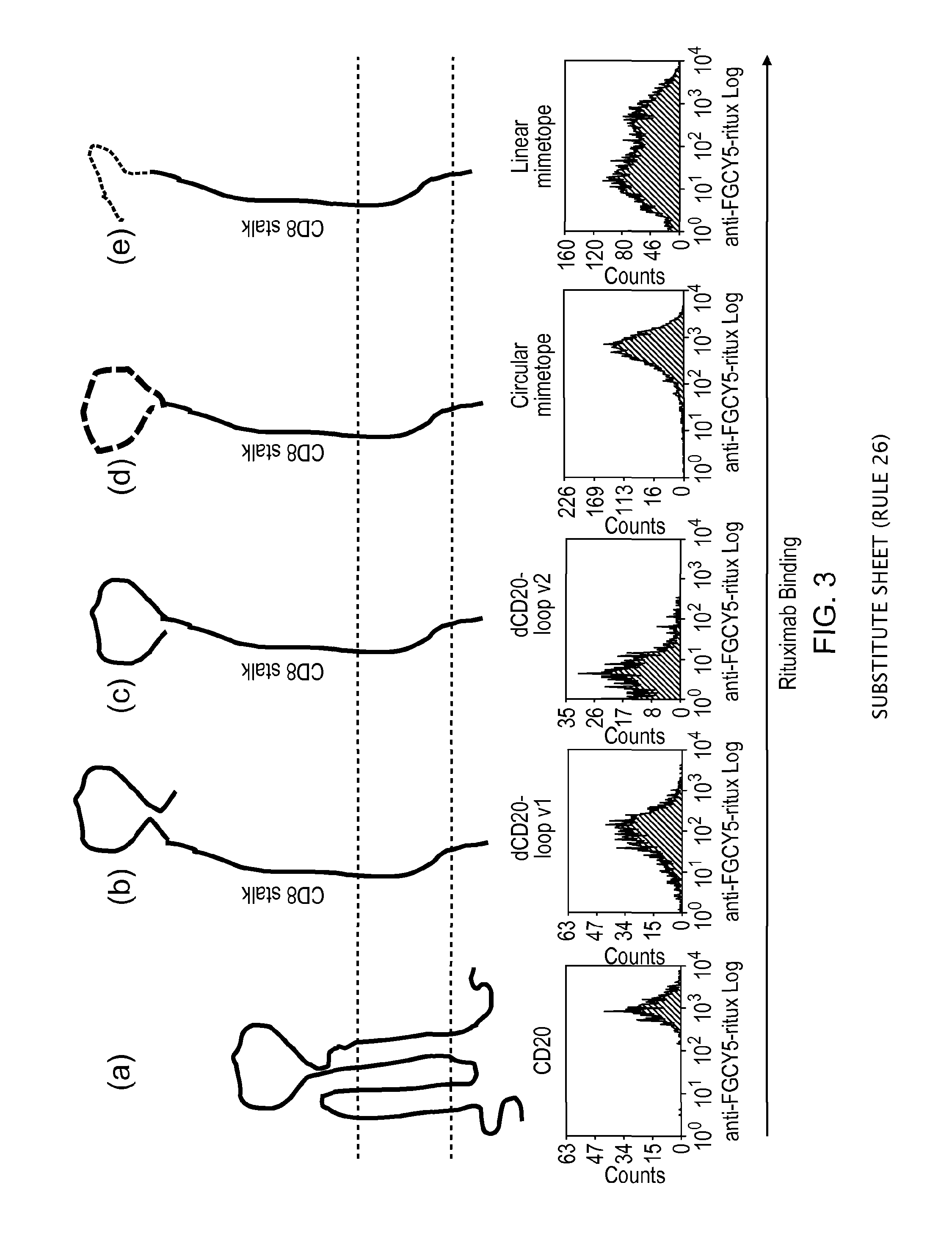
Polypeptide useful in adoptive cell therapy
ActiveUS20150093401A1Easy to packAvoids biological effectAntibody mimetics/scaffoldsGenetic material ingredientsEpitopeAdoptive cellular therapy
The present invention provides a polypeptide having the formula: St-R1-S1-Q-S2-R2 wherein St is a stalk sequence which, when the polypeptide is expressed at the surface of a target cell, causes the R and Q epitopes to be projected from the cell surface; R1 and R2 are a Rituximab-binding epitopes each having the an amino acid sequence selected from the group consisting of SEQ ID No. 1, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 and 16 or a variant thereof which retains Rituximab-binding activity; S1 and S2 are optional spacer sequences, which may be the same or different; and Q is a QBEnd1O-binding epitope having the amino acid sequence shown as SEQ ID No. 2 or a variant thereof which QBEnd1O-binding activity. The invention also provides a nucleic acid sequence encoding such a polypeptide and uses thereof in adoptive cell transfer.
Owner:UCL BUSINESS PLC
Methods and compositions for transducing lymphocytes and regulated expansion thereof
ActiveUS20170296678A1Increase proliferative activityReduce riskVirusesAntibody mimetics/scaffoldsAdoptive cellular therapyNatural Killer Cell Inhibitory Receptors
The present disclosure provides methods for genetically modifying lymphocytes and methods for performing adoptive cellular therapy that include transducing T cells and / or NK cells without prior ex vivo stimulation. The methods typically include engineered signaling polypeptides that can include a lymphoproliferative element, and / or a chimeric antigen receptor (CAR), for example a microenvironment restricted CAR. Additional elements of such engineered signaling polypeptides are provided herein, as well as vectors, such as retroviral vectors, packaging cell lines and methods of making the same. Furthermore, recombinant retroviruses and methods of making the same are provided. Numerous controls are provided, including riboswitches that are controlled, for example in vivo, by nucleoside analogues.
Owner:EXUMA BIOTECH CORP
Adoptive cell therapy with specific regulatory lymphocytes
InactiveUS20140356318A1BiocidePeptide/protein ingredientsTolerance inductionAdoptive cellular therapy
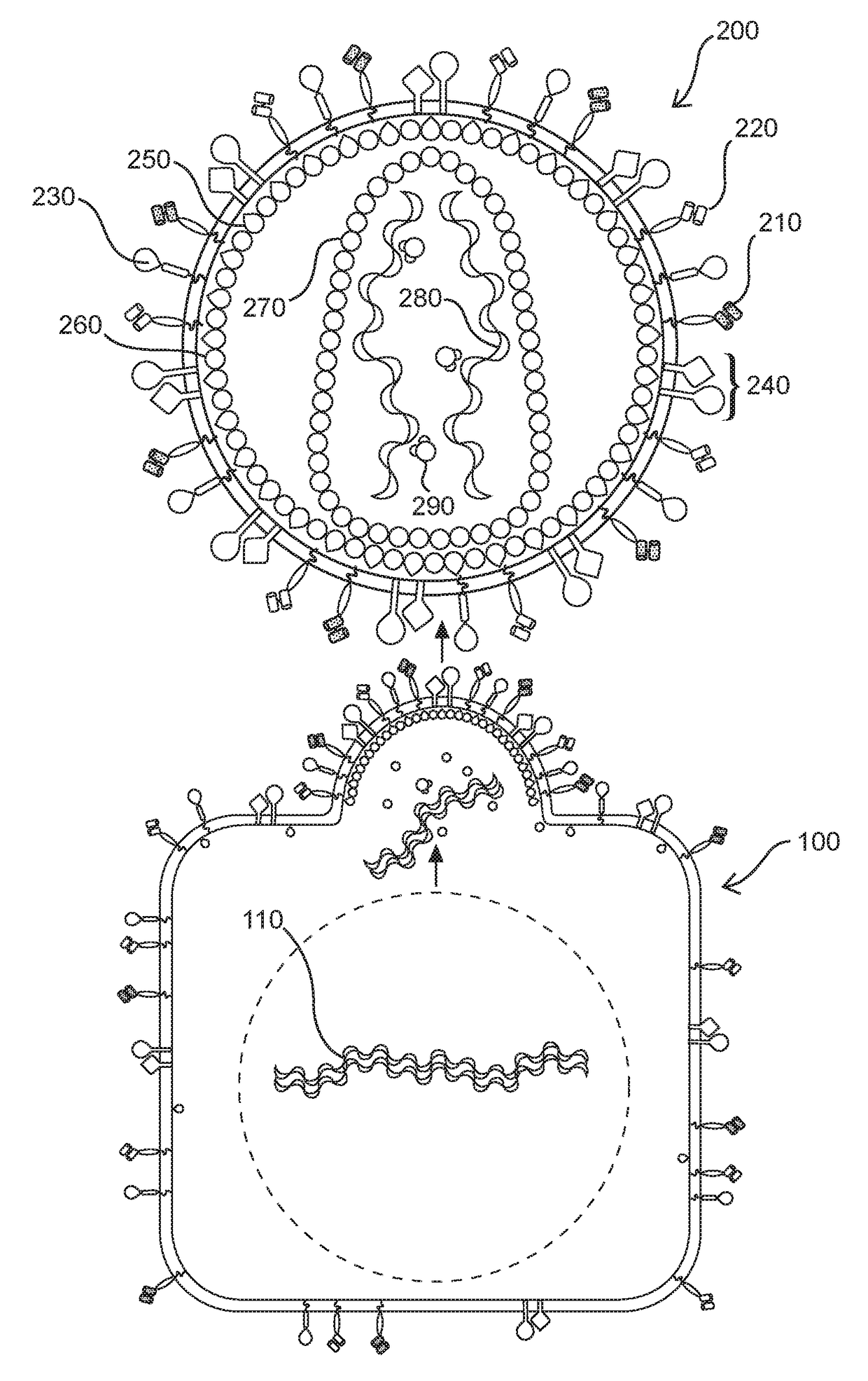
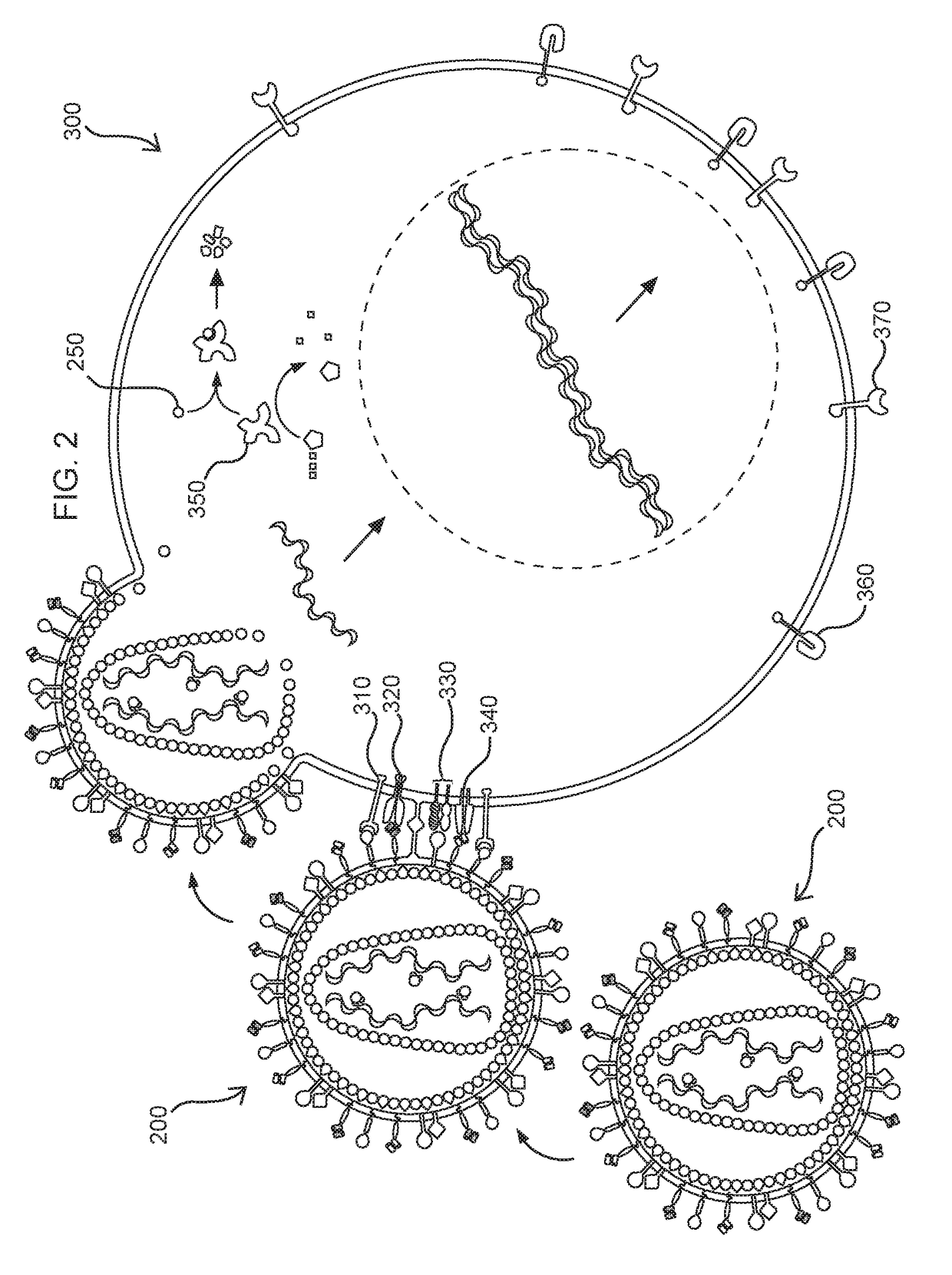
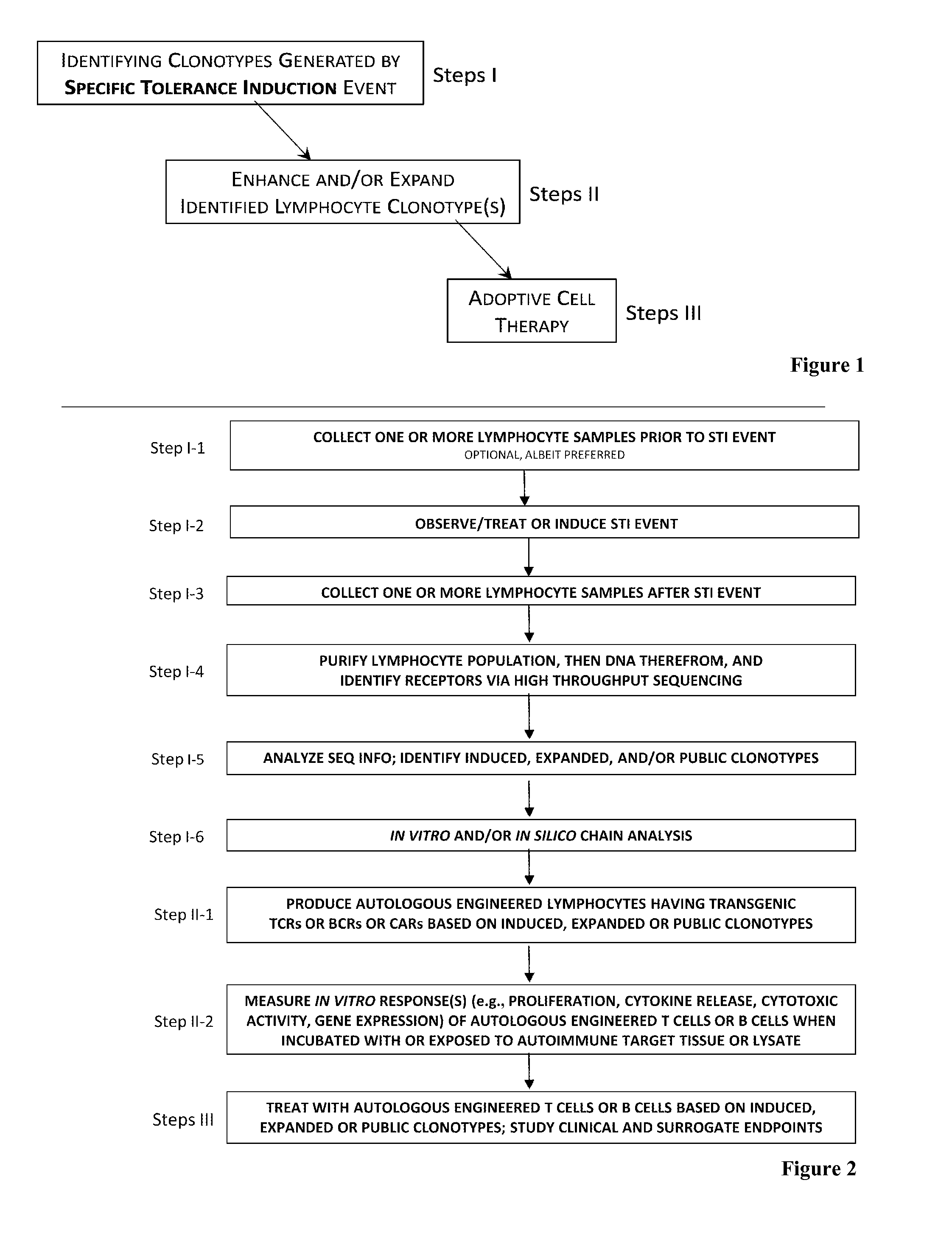
The present invention comprises a method of treatment of an autoimmune disease involving a specific tolerance induction (“STI”) event, wherein the method includes: collecting a first sample from the patient prior to the STI event; detecting an STI event or performing a procedure that correlates in time to an STI event; collecting a second sample from the patient after the STI event; preparing lymphocytes from the first and second samples; preparing and sequencing DNA or cDNA from the prepared lymphocytes; identifying sequences of prevalent T or B cell receptors (“prevalent receptor sequences”) among the lymphocytes of the second sample; selecting a regulatory lymphocyte that carries at least one prevalent receptor sequence, which selected regulatory lymphocyte (i) expresses at least one prevalent receptor sequence or (ii) is generated from an autologous or allogeneic naïve lymphocyte, which naïve lymphocyte is engineered and induced to become a regulatory lymphocyte that expresses at least one prevalent receptor sequence; culturing the selected regulatory lymphocyte, thereby generating daughter cells of said regulatory lymphocyte; and administering the daughter cells to the patient.
Owner:BARKEN ISRAEL
Adoptive cell therapy with young t cells
ActiveUS20140030806A1Organic active ingredientsPeptide/protein ingredientsAdoptive cellular therapyTumor response
The invention provides a method of promoting regression of a cancer in a mammal comprising (i) culturing autologous T cells; (ii) expanding the cultured T cells; (iii) administering to the mammal nonmyeloablative lymphodepleting chemotherapy; and (iv) after administering nonmyeloablative lymphodepleting chemotherapy, administering to the mammal the expanded T cells, wherein the T cells administered to the mammal are about 19 to about 35 days old and have not been screened for specific tumor reactivity, whereupon the regression of the cancer in the mammal is promoted.
Owner:UNITED STATES OF AMERICA
Expression of transgenic t cell receptors in lak-t cells
InactiveUS20110020308A1Short timeQuick buildAntibacterial agentsBiocideAdoptive cellular therapyDisease
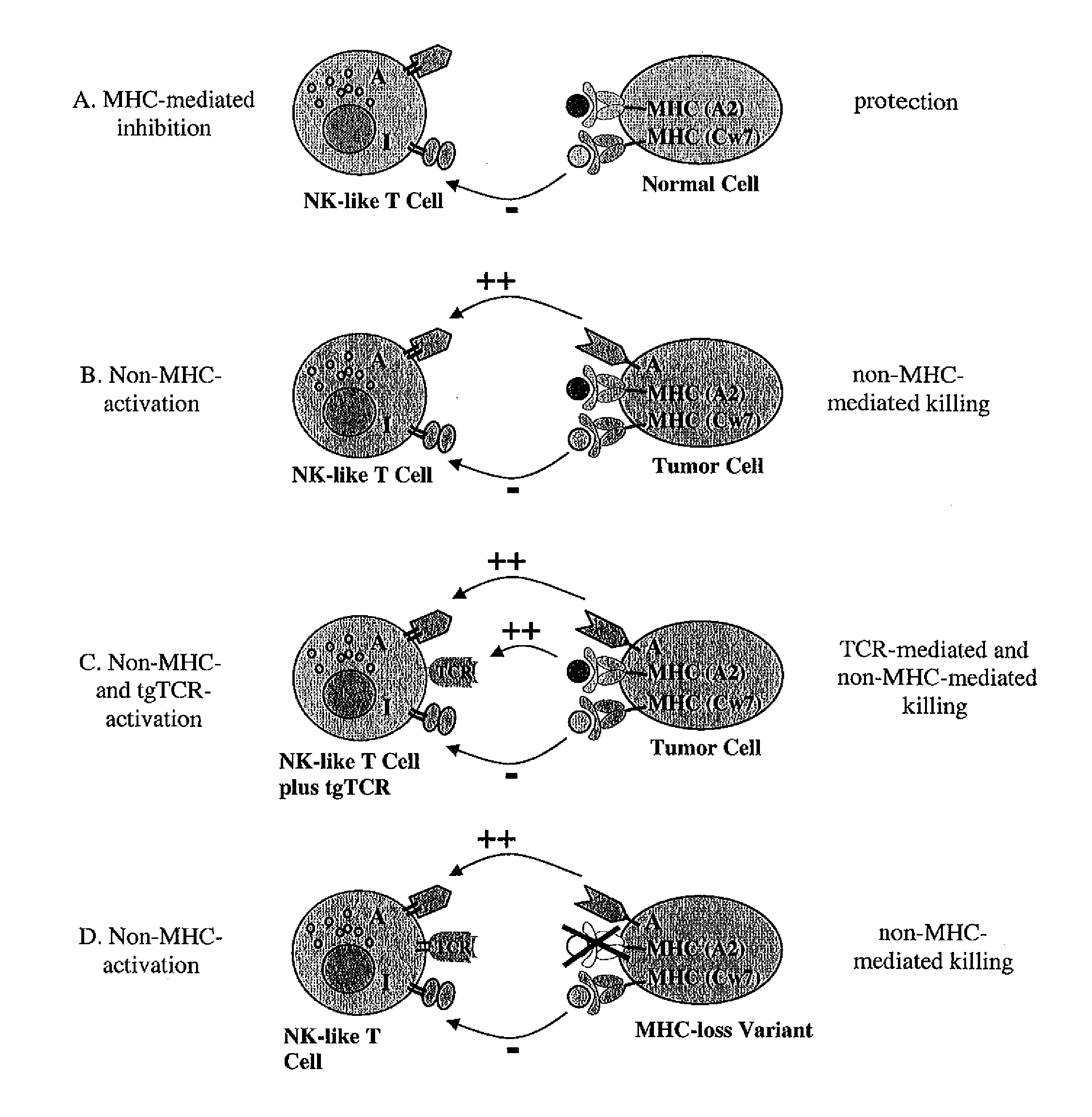
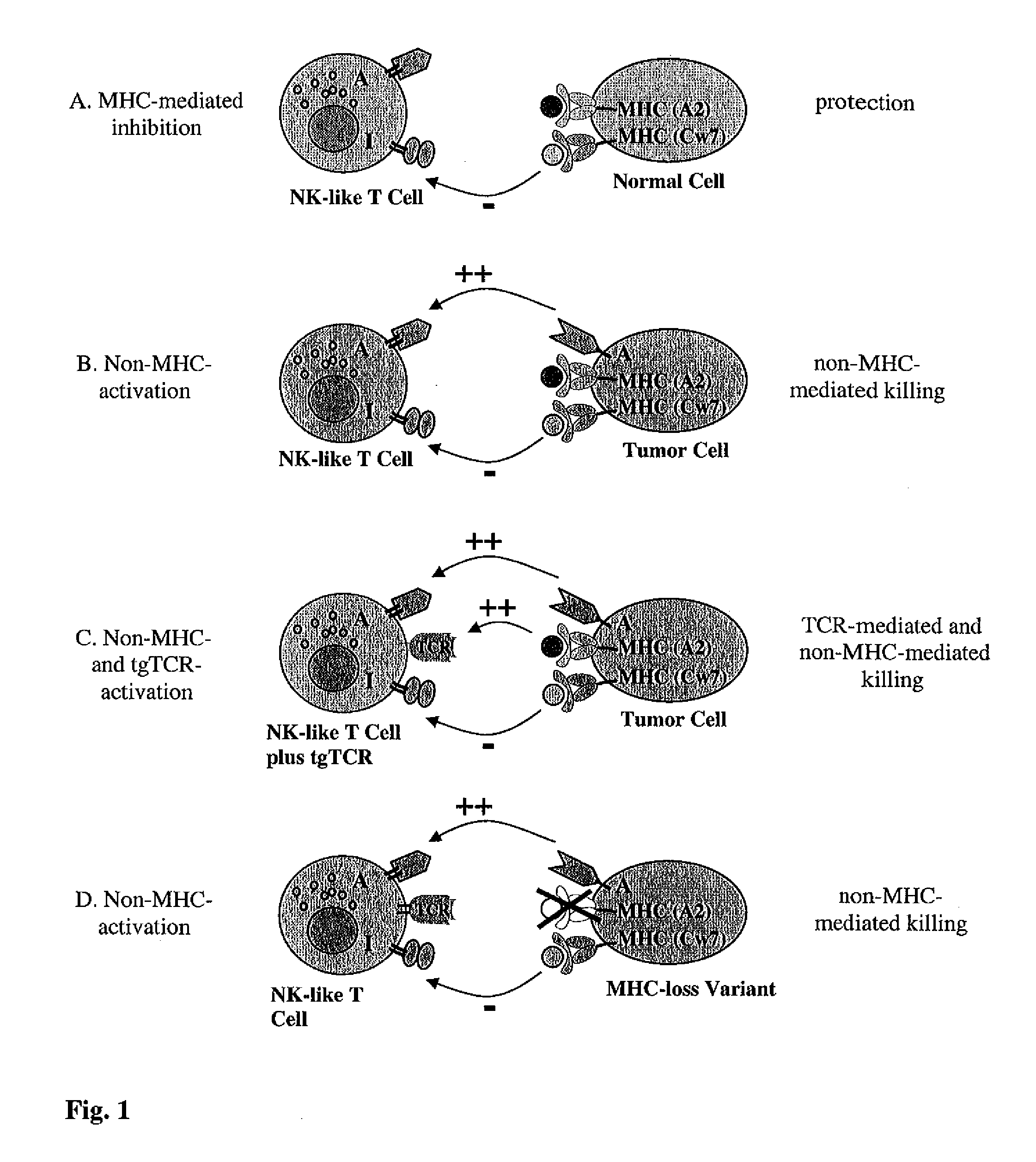
The present invention is directed to LAK-T cells, which have been transformed by a transgenic T cell receptor (tg-TCR). The invention is further directed to a method of generating those transgenic T cells, a pharmaceutical composition comprising said cells and the use of the LAK-T cells or of the pharmaceutical composition in the adoptive cell therapy and for treating hematological malignancies or solid tumors or acute or chronic infections or autoimmune diseases.
Owner:HELMHOLTZ ZENT MUNCHEN DEUTES FORSCHUNGSZENT FUR GESUNDHEIT & UMWELT
Anti CD30 chimeric antigen receptor and its use
ActiveUS10808035B2Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAdoptive cellular therapyCancer cell
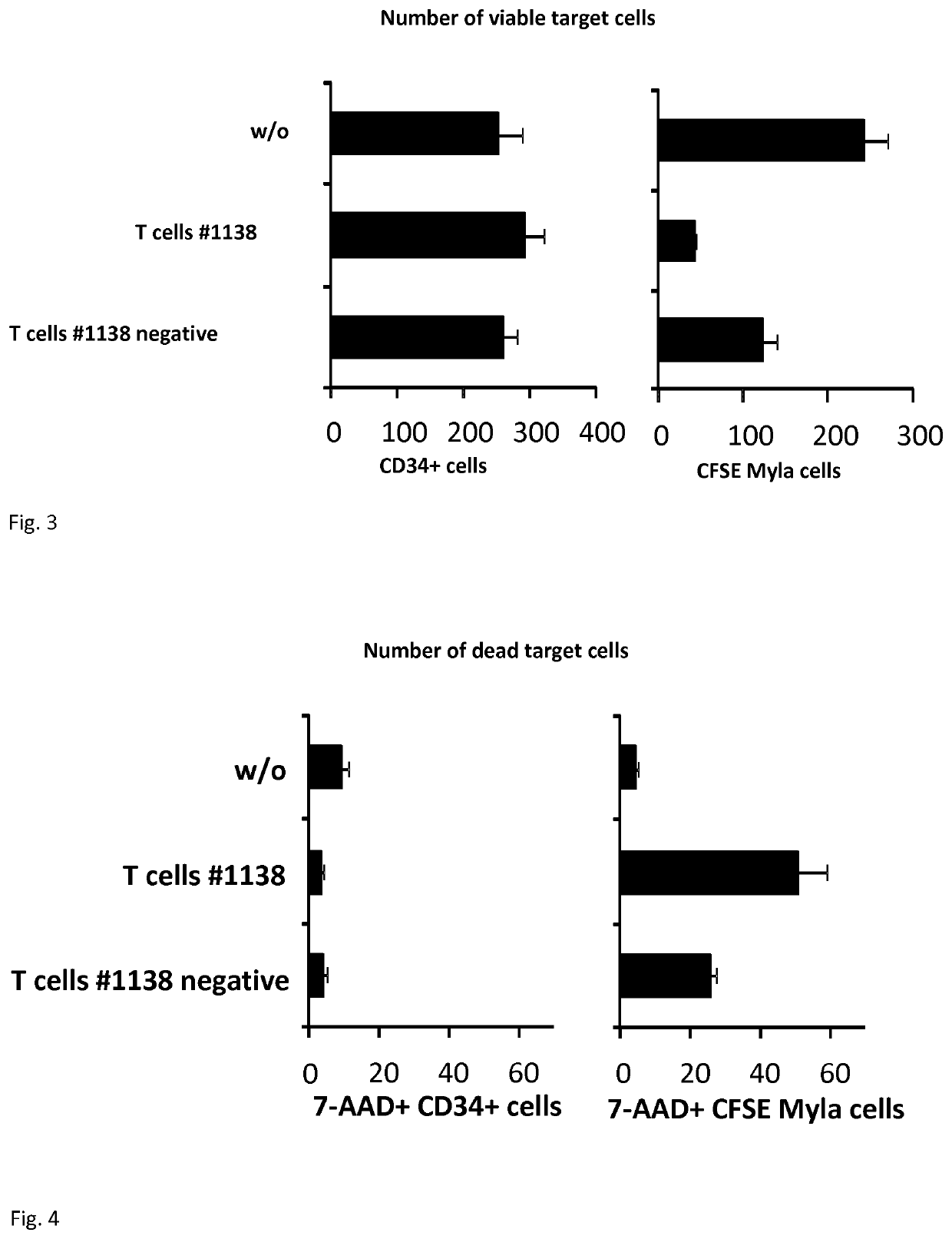
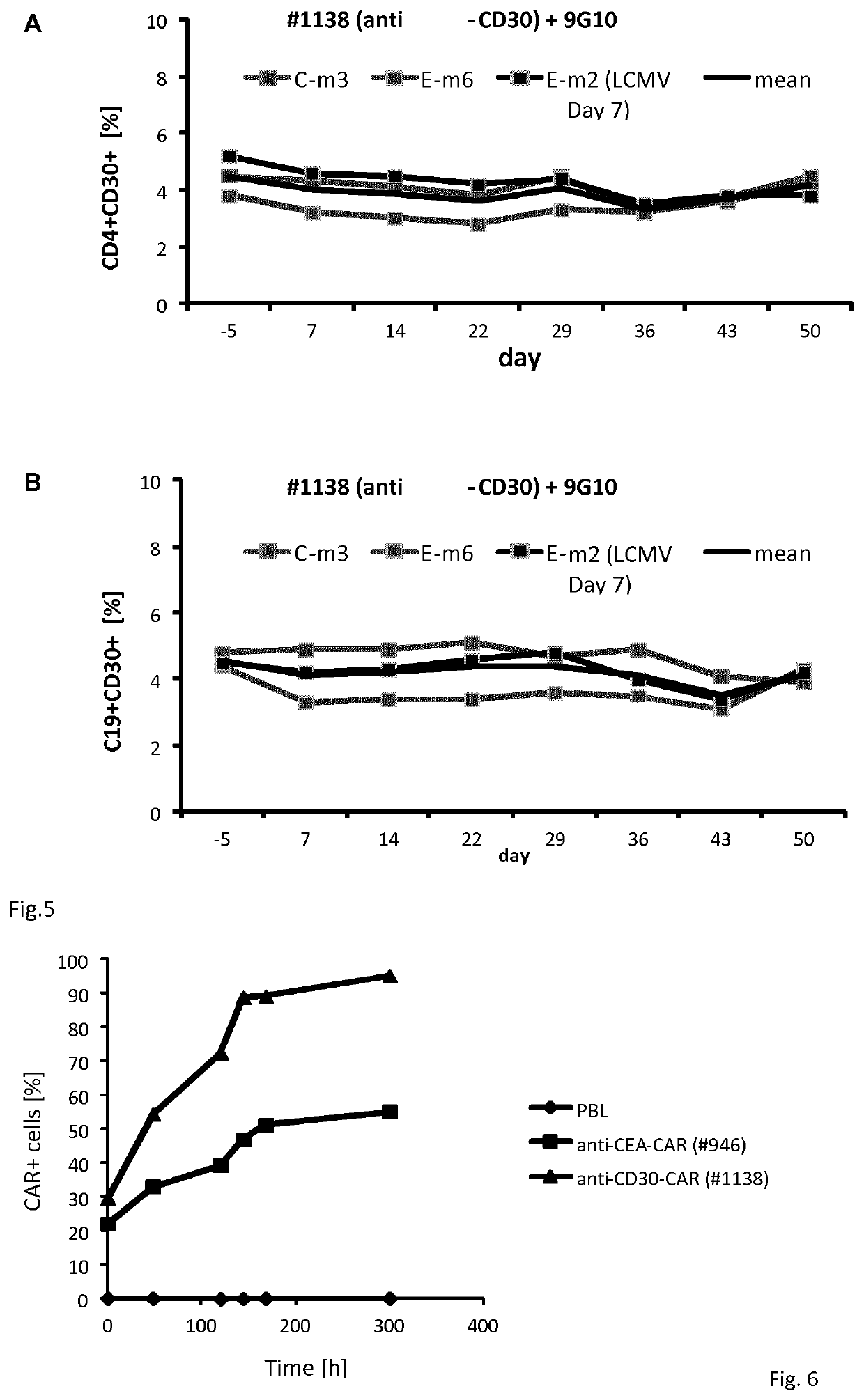
In a first aspect, the present disclosure relates to genetically modified T-cells having a chimeric antigen receptor for use in adoptive cell therapy for treating CD30+cancer in a subject need thereof. In particular, the present disclosure relates to a T-cell containing a specific chimeric antigen receptor being toxic to CD30+cancer cells while being non-toxic to CD30+non-cancer cells. In a further aspect, the present disclosure relates to a specific chimeric antigen receptor and the nucleic acid molecule encoding the receptor as well vectors and cells containing the same. Finally, the present disclosure relates to the use of the chimeric antigen receptor for use in improving persistence and amplification of lymphocyte containing the same and the use of specific peptides for improving persistence and amplification of genetically modified lymphocytes expressing the same.
Owner:CHMIELEWSKI MARKUS +2
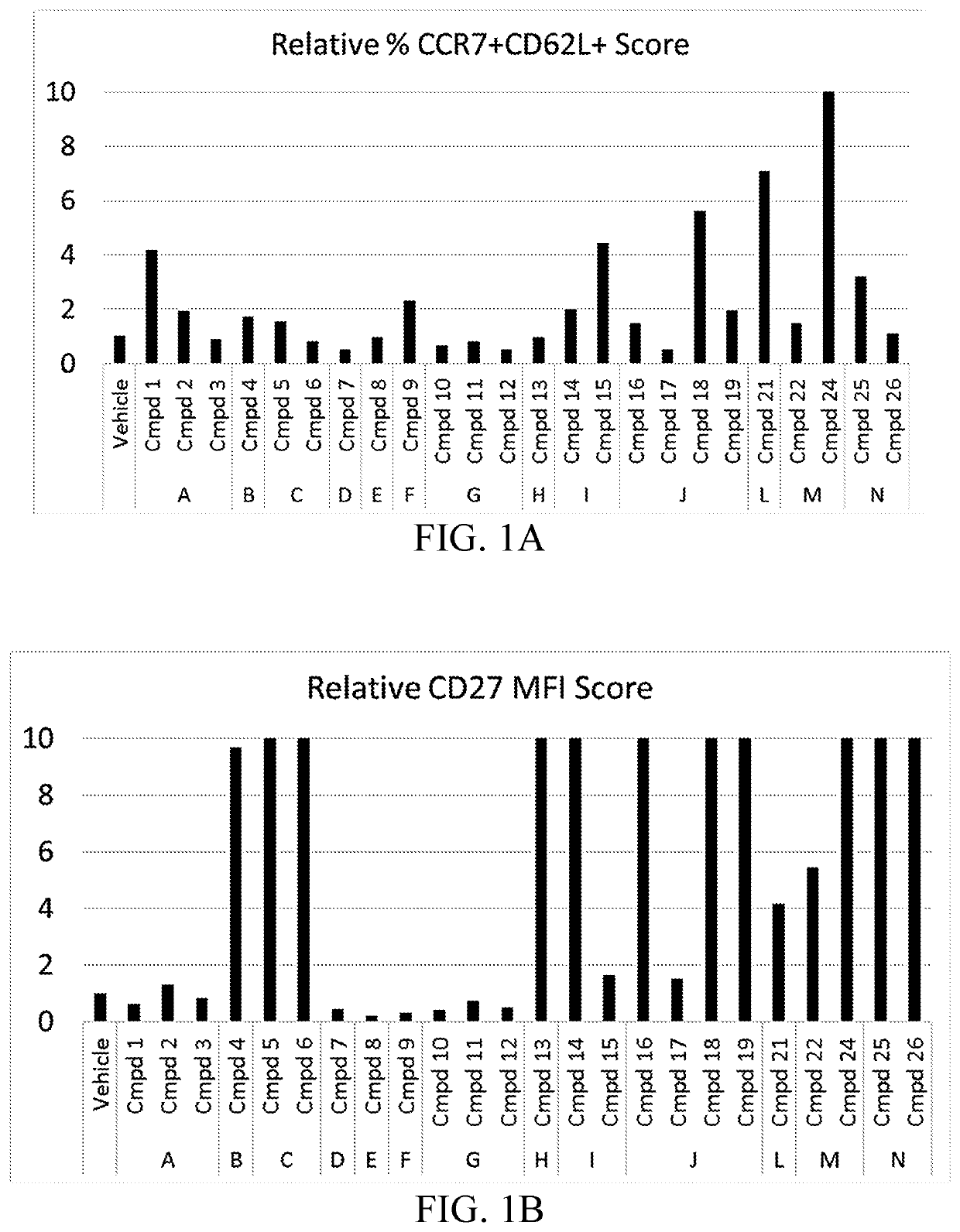
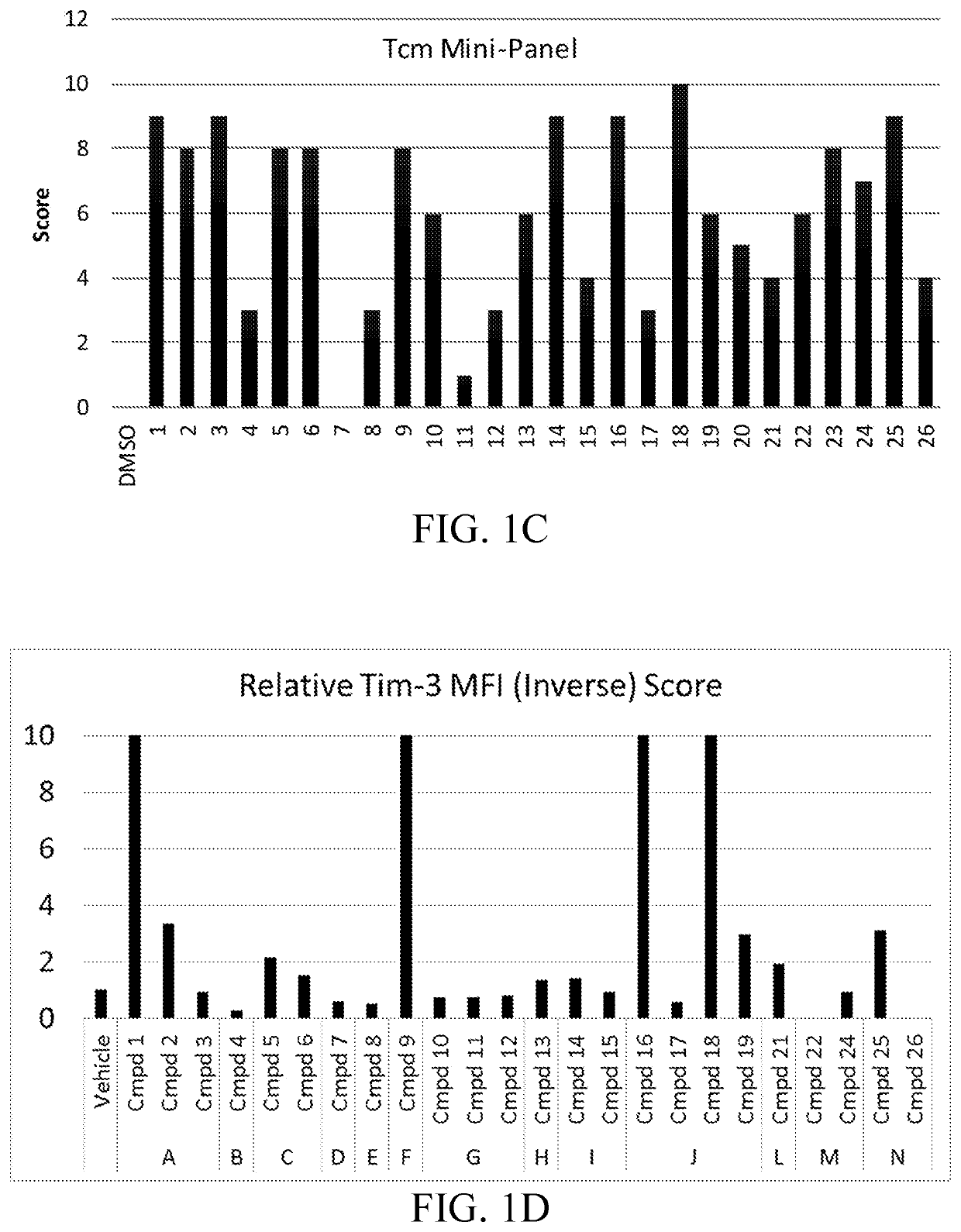
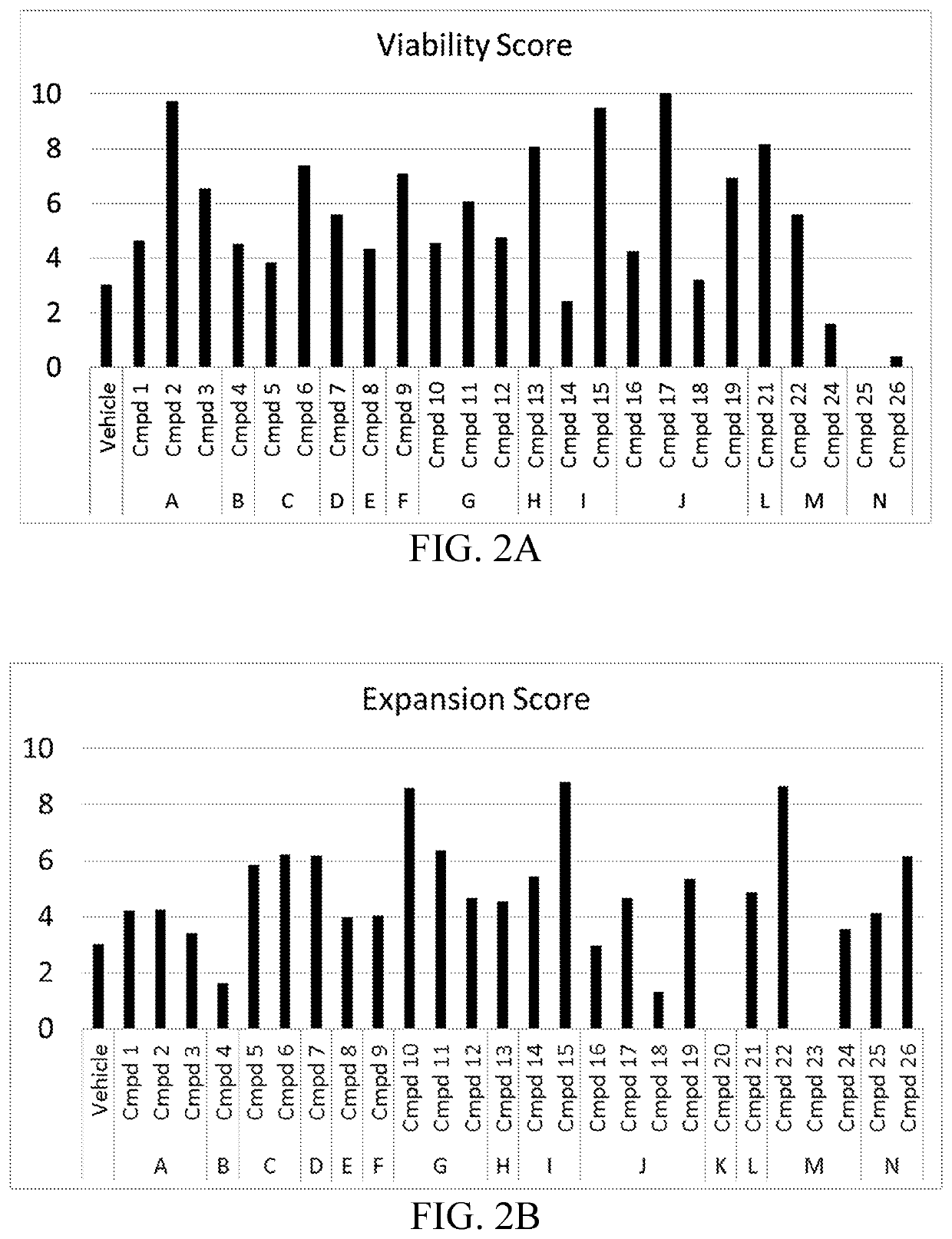
Compositions and methods for immune cell modulation in adoptive immunotherapies
ActiveUS20190125795A1Convenient treatmentPromote resultsOrganic active ingredientsPeptide/protein ingredientsAdoptive cellular therapyCentral Memory T-Cell
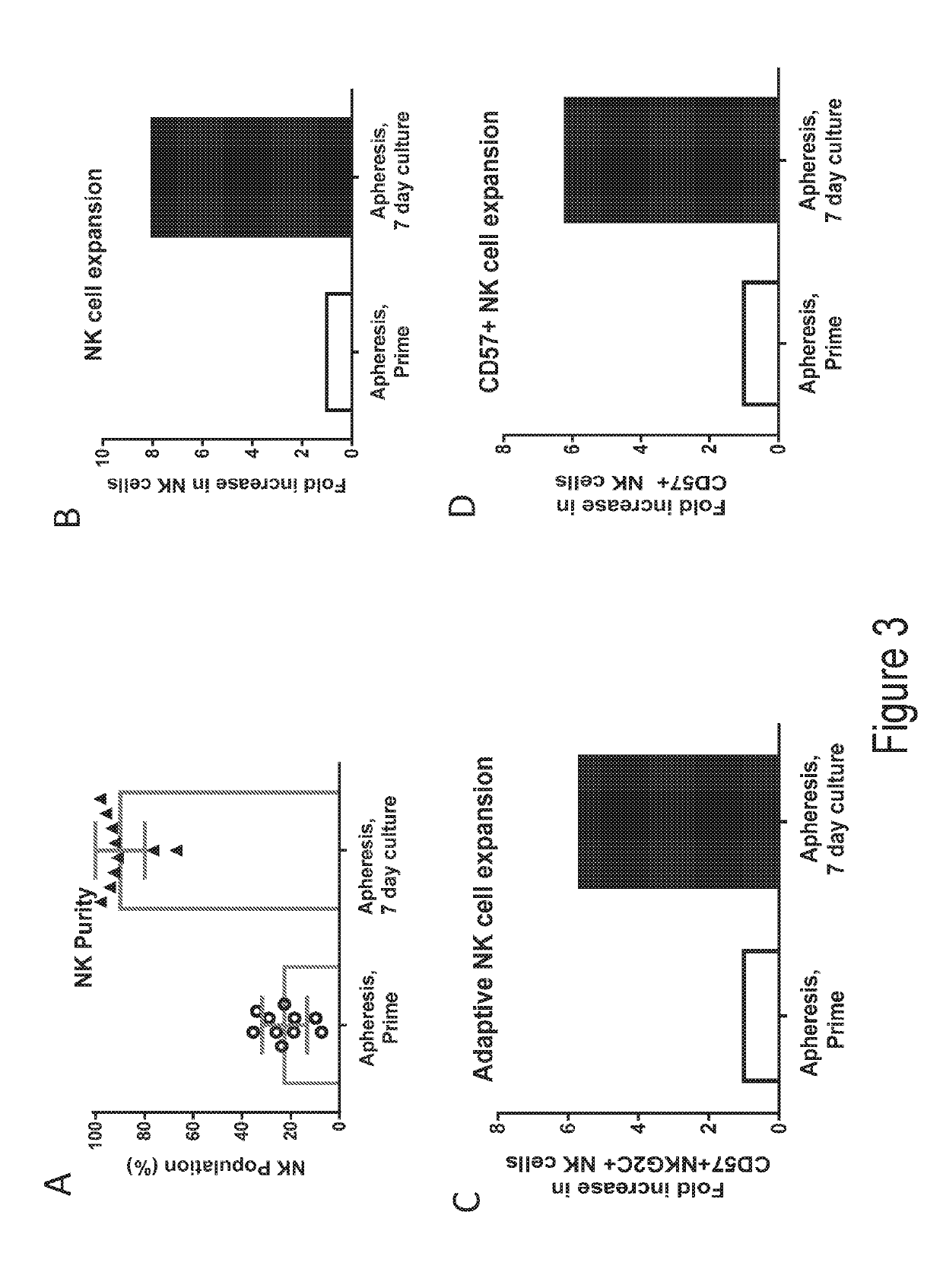
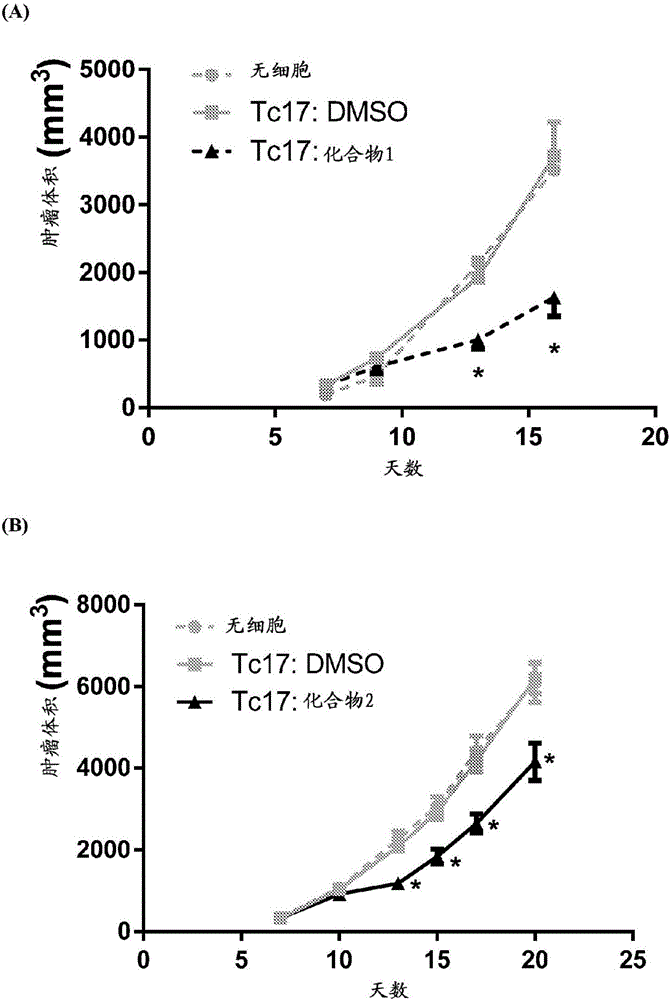
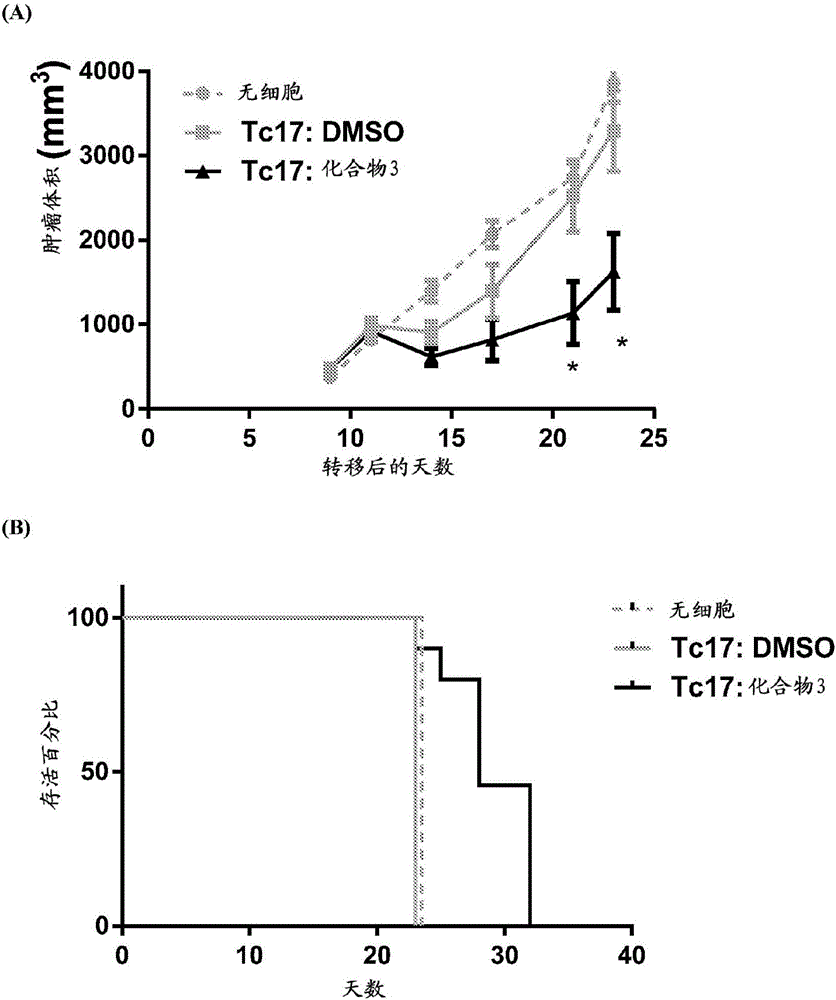
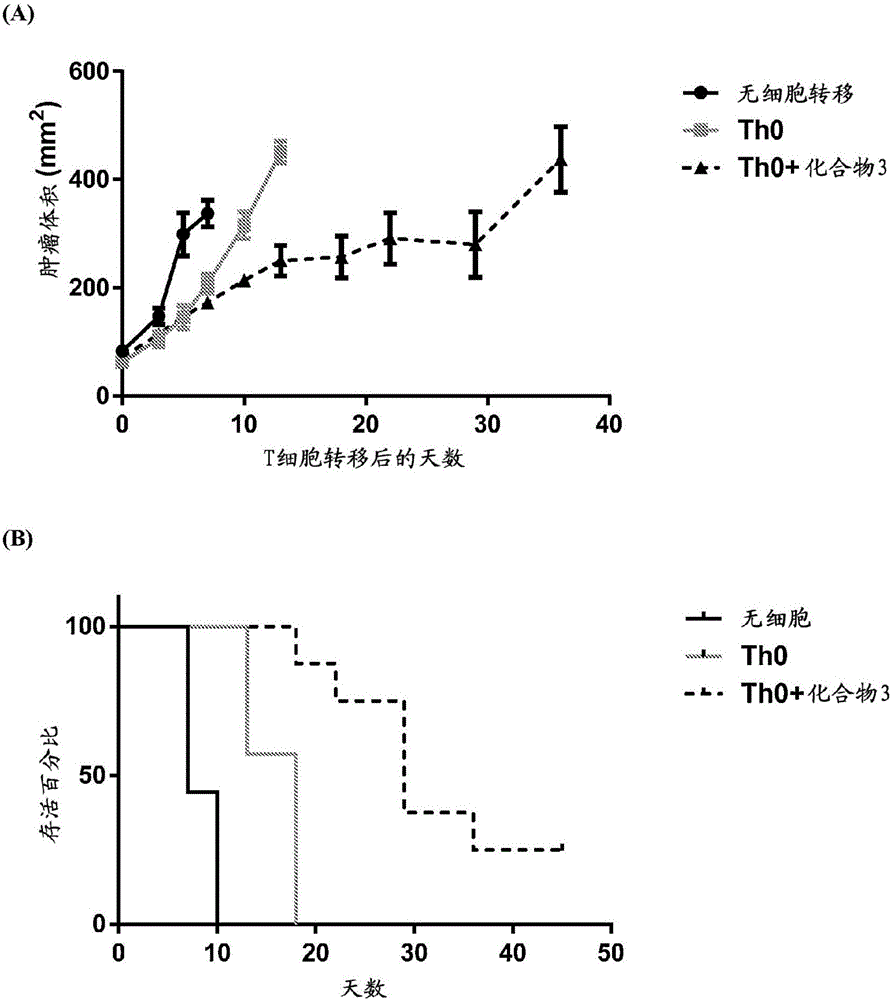
Compounds that either produced a higher proportion or greater absolute number of phenotypically identified nave, stem cell memory, central memory T cells, adaptive NK cells, and type I NKT cells are identified. Compositions and methods for modulating immune cells including T, NK, and NKT cells for adoptive cell therapies with improved efficacy are provided.
Owner:FATE THERAPEUTICS
Adoptive cellular therapy using an agonist of retinoic acid receptor-related orphan receptor gamma & related therapeutic methods
InactiveCN106132422AAntibacterial agentsBacterial antigen ingredientsAdoptive cellular therapyDendritic cell
The invention relates to medical therapy using an agonist of the retinoic acid receptor-related orphan receptor gamma (ROR gamma) and provides adoptive cellular therapies using an agonist of ROR gamma, populations of lymphocyte cells that have been exposed to an agonist of ROR gamma, populations of dendritic cells that have been exposed to an agonist of ROR gamma, pharmaceutical compositions, and methods for enhancing therapeutic effects of lymphocyte cells and / or dendritic cells in a patient by administering an agonist of ROR gamma to a patient.
Owner:LICELLA PTY LTD
Cells expressing recombinant growth factor receptors
ActiveUS20200263130A1Augment lymphocyte expansionInduced proliferationPolypeptide with localisation/targeting motifOrganic active ingredientsAdoptive cellular therapyNatural Killer Cell Inhibitory Receptors
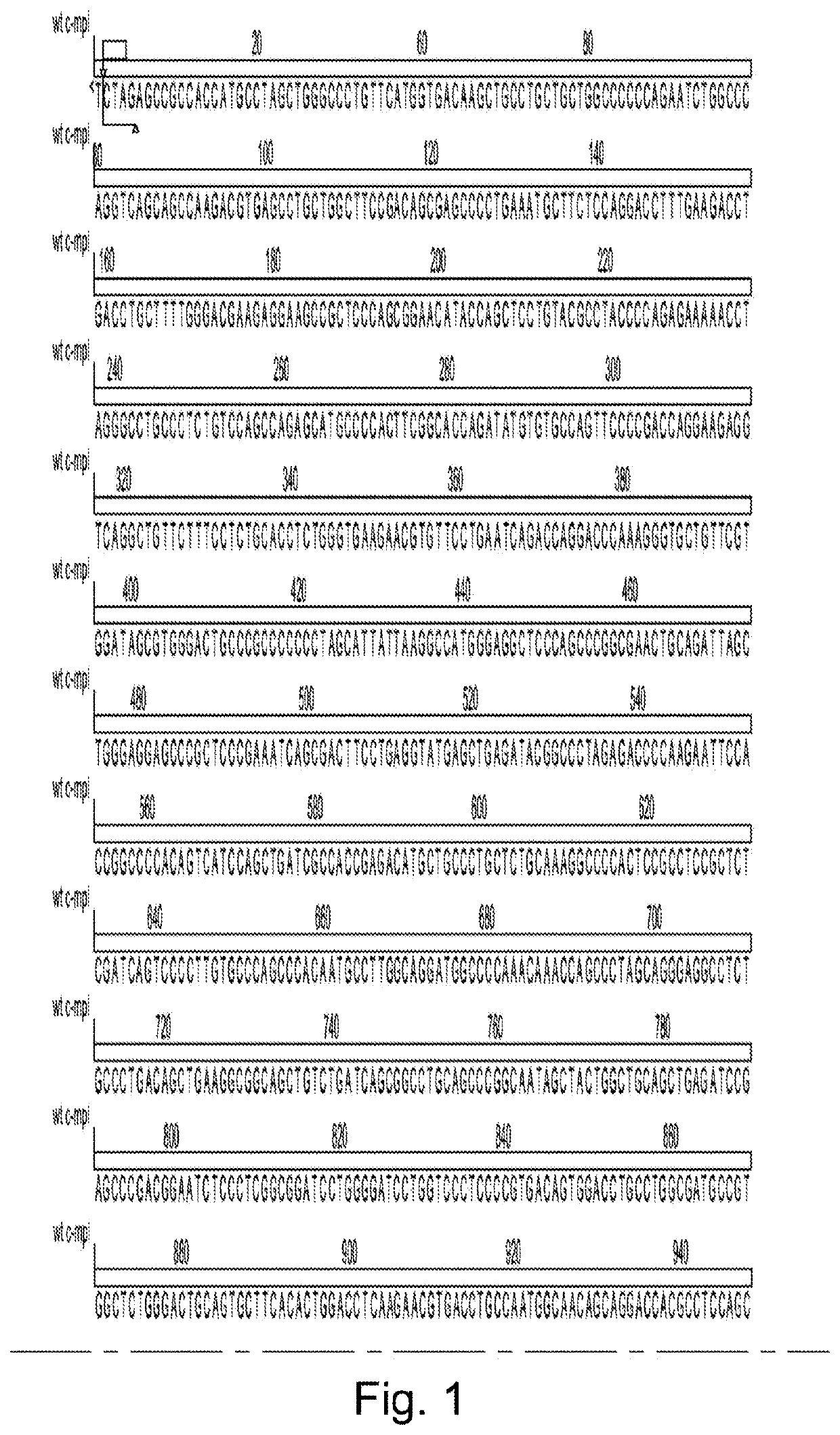
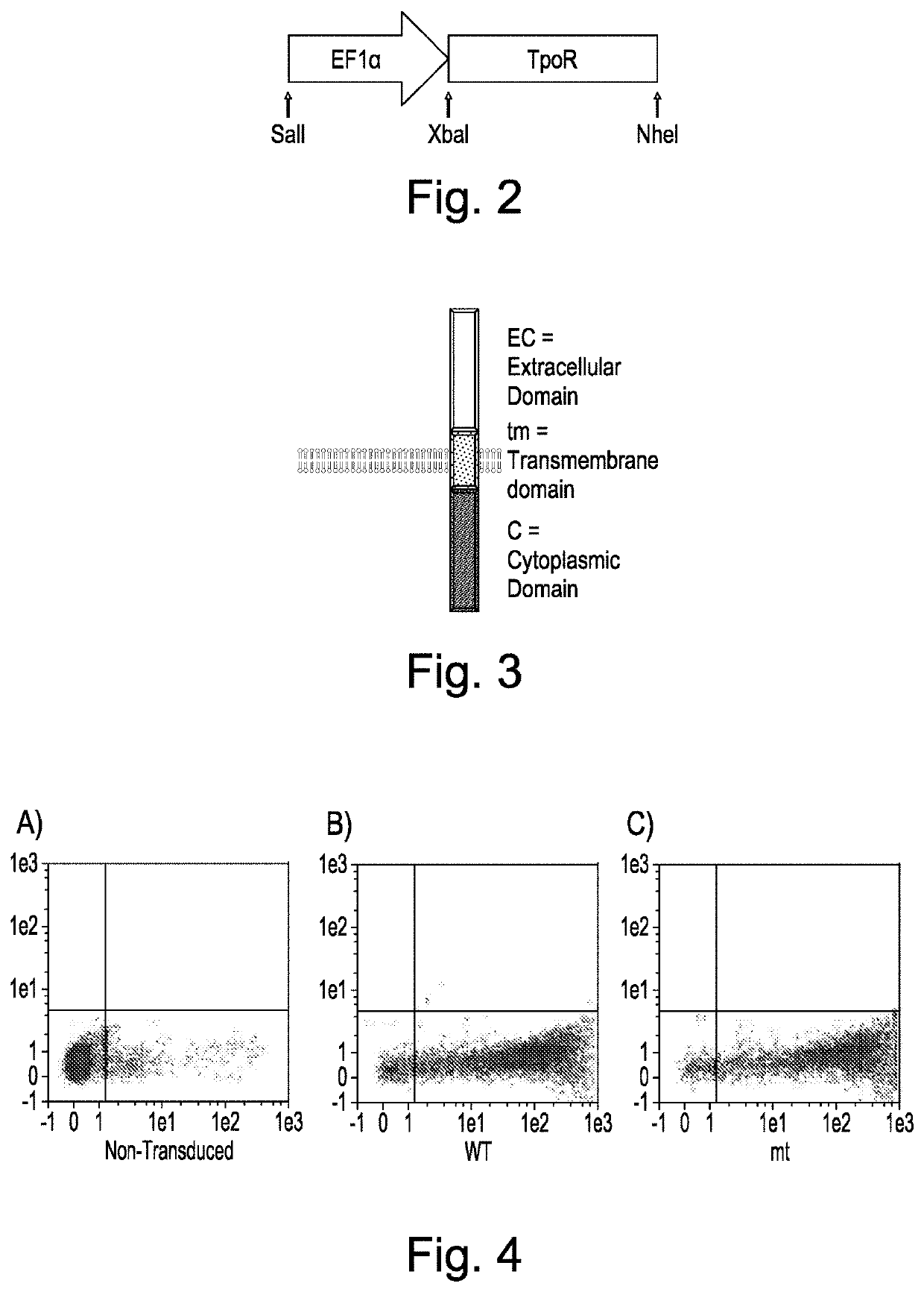
The present invention discloses cell lines and recombinant growth factor receptors useful in adoptive cell therapy (ACT), wherein the recombinant growth factor receptor can act as a molecular switch enabling cells expressing the rGFR protein to be expanded in-vitro or in- vivo. Thus the invention provides a T or NK cell, comprising a recombinant growth factor receptor (rGFR) comprising: (i) an extracellular (EC) domain; (ii) a thrombopoietin receptor transmembrane (TM) domain; and (iii) a growth factor receptor intracellular (IC) domain.
Owner:INSTIL BIO UK LTD
Immune cell compositions and methods of use
InactiveUS20200010803A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAdoptive cellular therapyPharmaceutical drug
Disclosed herein are immunostimulatory cells recombinantly engineered for adoptive cellular therapy. Additionally provided are pharmaceutical compositions comprising such immunostimulatory cells and methods of using such immunostimulatory cells to treat cancer or pathogen infections in a subject in need thereof.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Methods of Selecting T cell Line and Donor Thereof for Adoptive Cellular Therapy
Disclosed herein are methods of selecting an allogeneic T cell line for therapeutic administration to a patient having or suspected of having a pathogen or cancer. Also disclosed are methods of selecting a donor from whom to derive an allogeneic T cell line for therapeutic administration to a patient having or suspected of having a pathogen or cancer.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Compositions and methods for immune cell modulation in adoptive immunotherapies
PendingUS20200181573A1Improve securityOrganic active ingredientsMammal material medical ingredientsAdoptive cellular therapyCentral Memory T-Cell
Compounds that either produced a higher proportion or greater absolute number of phenotypically identified naive, stem cell memory, central memory T cells, adaptive NK cells, and type I NKT cells are identified. Compositions and methods for modulating immune cells including T, NK, and NKT cells for adoptive cell therapies with improved efficacy are provided.
Owner:FATE THERAPEUTICS
Modified monocytes/macrophage expressing chimeric antigen receptors and uses thereof
ActiveUS20200247870A1Peptide/protein ingredientsAntibody mimetics/scaffoldsAdoptive cellular therapyDisease
The present invention includes methods and compositions for treating cancer, whether a solid tumor or a hematologic malignancy. By expressing a chimeric antigen receptor in a monocyte, macrophage or dendritic cell, the modified cell is recruited to the tumor microenvironment where it acts as a potent immune effector by infiltrating the tumor and killing the target cells. One aspect includes a modified cell and pharmaceutical compositions comprising the modified cell for adoptive cell therapy and treating a disease or condition associated with immunosuppression.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Oncolytic adenoviruses coding for bi-specific antibodies and methods and uses related thereto
PendingCN107406859AIncrease useReduce immunosuppressionImmunoglobulins against cell receptors/antigens/surface-determinantsUnknown materialsAdoptive cellular therapyAntiendomysial antibodies
The present invention relates to the fields of life sciences and medicine. Specifically, the invention relates to cancer therapies of humans. More specifically, the present invention relates to an oncolytic adenoviral vector encoding a bispecific monoclonal antibody. Furthermore, the present invention relates to methods and uses utilizing the oncolytic adenoviral vectors, also together with adoptive cell therapies.
Owner:TILT BIOTHERAPEUTICS OY
Antigen-specific immune effector cells
PendingUS20200123501A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAdoptive cellular therapyPluripotential stem cell
Provided herein are methods for the production of antigen-specific effector T cells and NK cells from pluripotent stem cells which express a chimeric antigen receptor (CAR). Further provided herein are methods for the adoptive cell therapy by administering the effector T cells and / or NK cells provided herein.
Owner:FUJIFILM CELLULAR DYNAMICS INC
T cell receptors
InactiveUS20190030071A1Improved pairing propertyMinimizing and eliminatingImmunoglobulin superfamilyPeptide/protein ingredientsInfected cellAdoptive cellular therapy
The present invention relates to modified T cell receptors (TCRs) and to their use in adoptive cell therapy (ACT), in particular for the transfer of T lymphocytes. The TCRs are mutated in the transmembrane regions of the alpha and beta chains with mutations favoring the correct TCR chain pairing. The correct pairing of the transferred exogenous alpha and beta TCR chains improves the functional activity and safety of the genetically modified T cells for the therapy of tumours and infectious diseases. The invention also relates to T cell receptor alpha or beta chain, to a recombinant TCR, a TCR complex, a nucleic acid coding for the TCR alpha or beta chain, to relative recombinant expression vector, host cells, pharmaceutical composition and to a method of detecting a hematological malignant cell, a solid tumor cell or an infected cell.
Owner:IMMURES
Improved methods of cell culture for adoptive cell therapy
ActiveCN104411819AShorten the timeLow costPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAdoptive cellular therapyAntigen receptors
Owner:WILSON WOLF MFG
Small molecules for dual function positron emission tomography (PET) and cell suicide switches
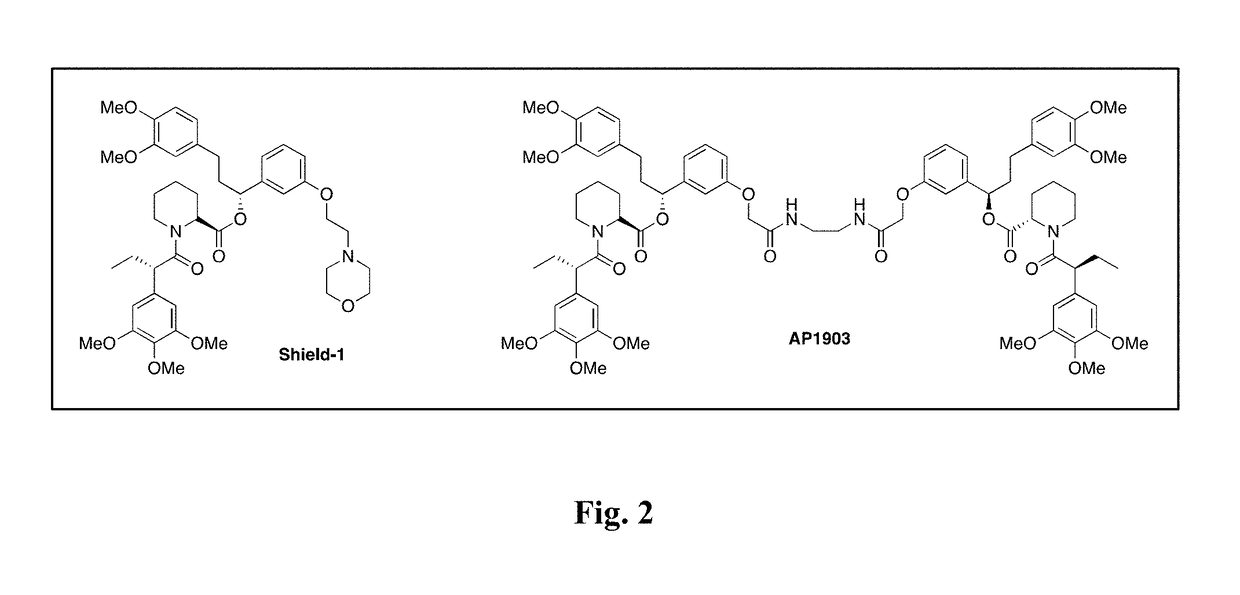
The present invention includes an engineered cell comprising a chimeric antigen receptor (CAR) further comprising a nucleic acid molecule comprising a suicide gene comprising a ligand binding domain and a suicide domain wherein the ligand binding domain is capable of binding to radiolabeled tracer or a small molecule suicide switch. This invention also includes methods for inducing apoptosis of an engineered cell, methods for assessing the efficacy or toxicity of an adoptive cell therapy in a subject, methods for detecting the quantity of engineered T cells in a subject, methods for monitoring an immunotherapy treatment in a subject and methods of imaging engineered T cells in a subject. In some embodiments, the imaging is performed via Positron Emission Topography (PET). This invention further includes a chemical inducer of dimerization (CID), wherein the CID is a Bis-Trimethoprim (Bis-TMP).
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Chimeric protein
ActiveUS10098911B2Excellent bioavailability and volume of distributionOrganic active ingredientsAntibody mimetics/scaffoldsAdoptive cellular therapyADAMTS Proteins
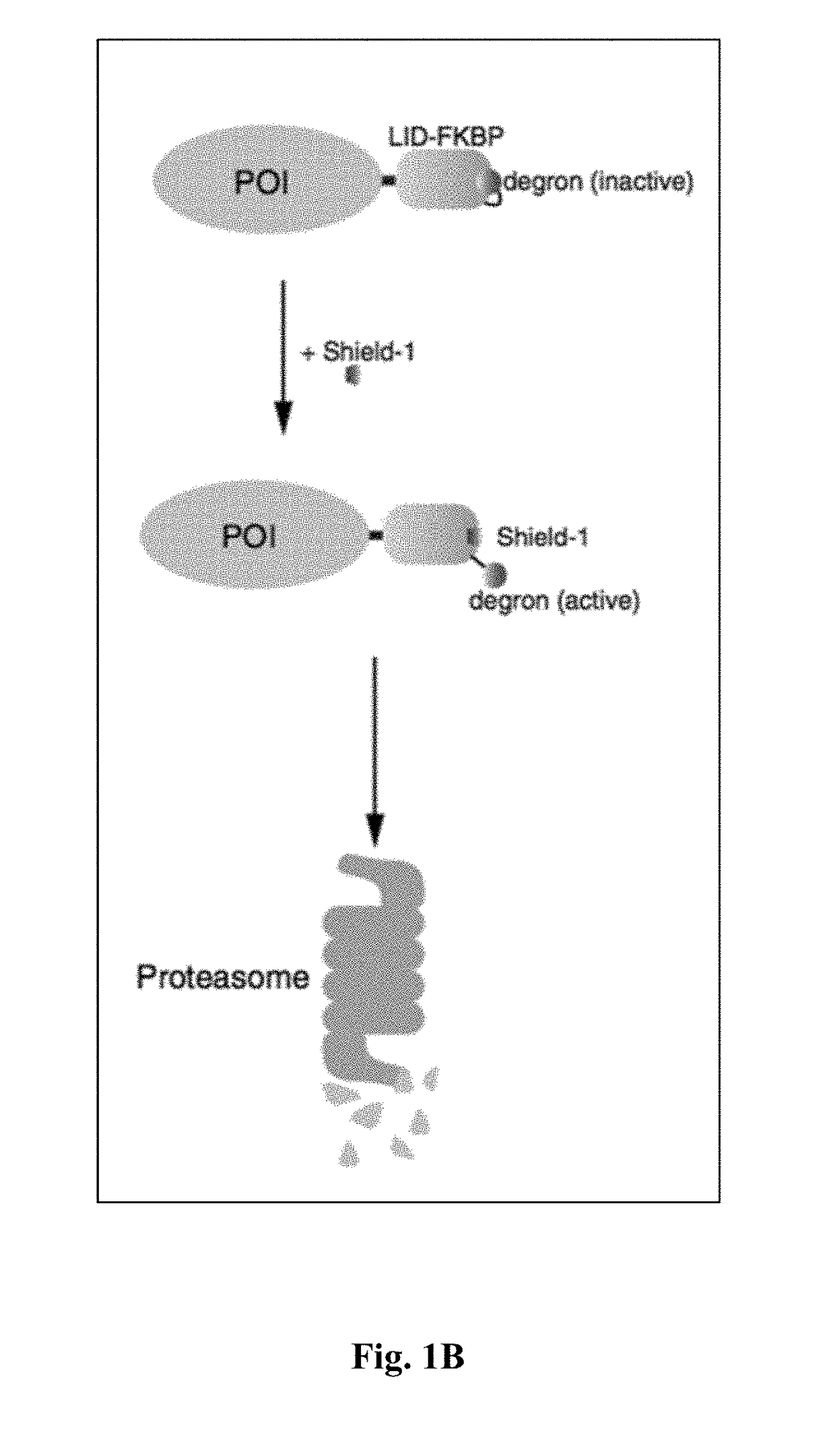
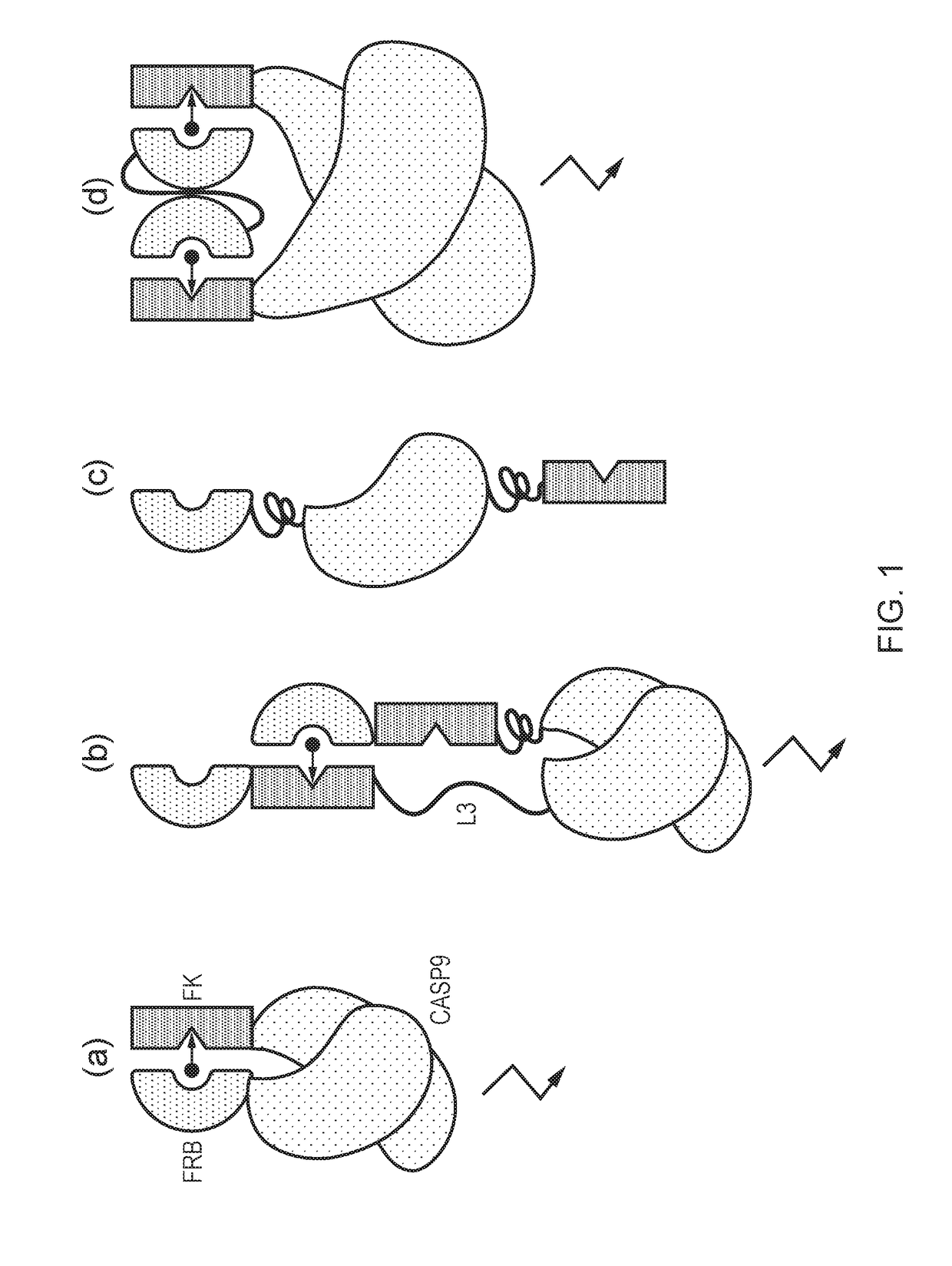
The present invention provides a chimeric protein having the formula: Casp-Ht1-Ht2 wherein Casp is a caspase domain; Ht1 is a first heterodimerization domain; and Ht2 is a second heterodimerization domain and wherein, in the presence of a chemical inducer of dimerization (CID), an identical pair of the chimeric proteins interact such that Ht1 from one chimeric protein heterodimerizes with Ht2 from the other chimeric protein, causing homodimerization of the two caspase domains. The invention also provides a cell comprising such a protein and its use in adoptive cell therapy.
Owner:AUTOLUS LIMIED
Compositions and methods for adoptive cell therapy for cancer
PendingUS20210393692A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAdoptive cellular therapyAntigen receptors
Provided herein are compositions and methods for adoptive cell therapy comprising engineered immune cells that express a tumor antigen-targeted chimeric antigen receptor and a SIRPα polypeptide.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
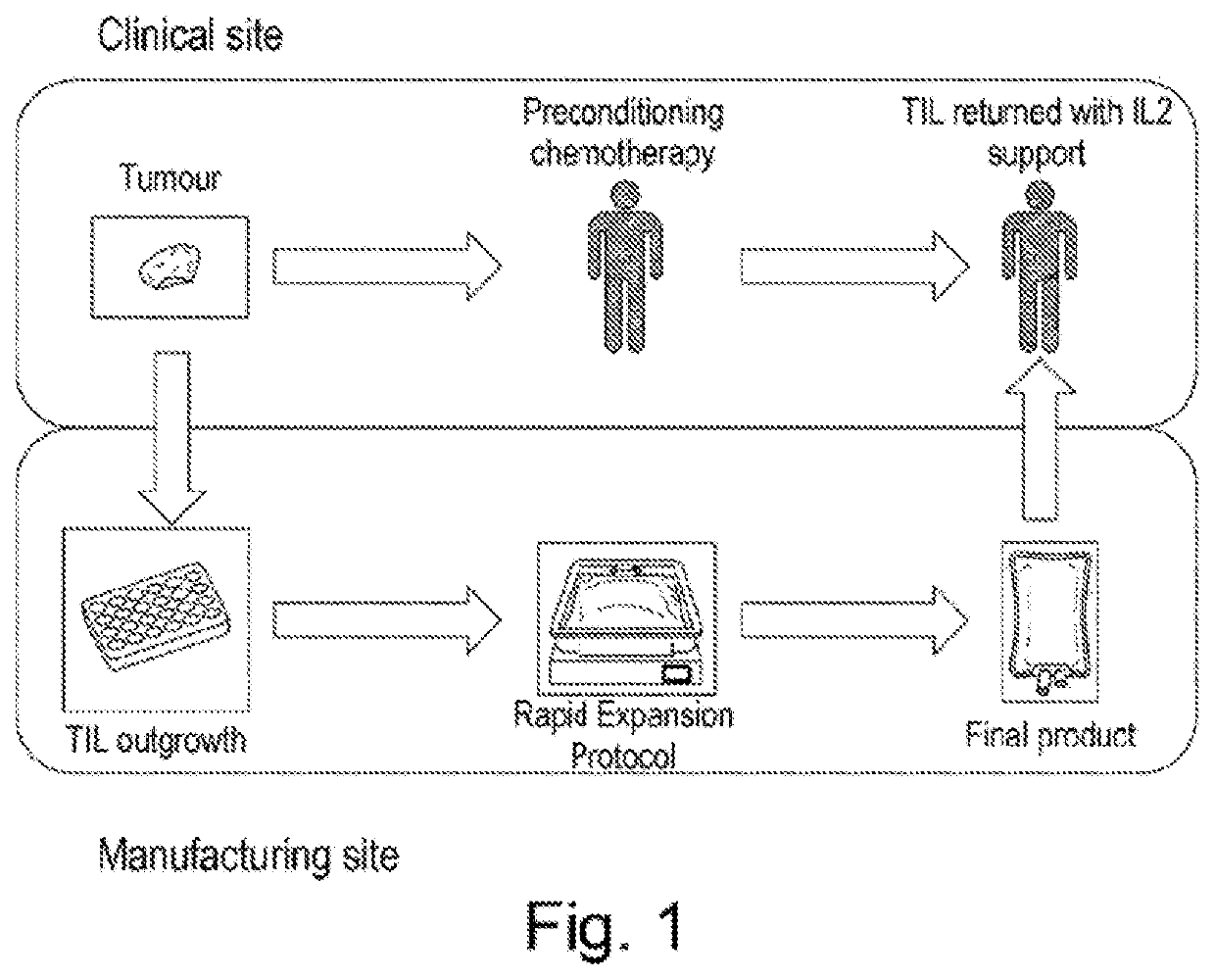
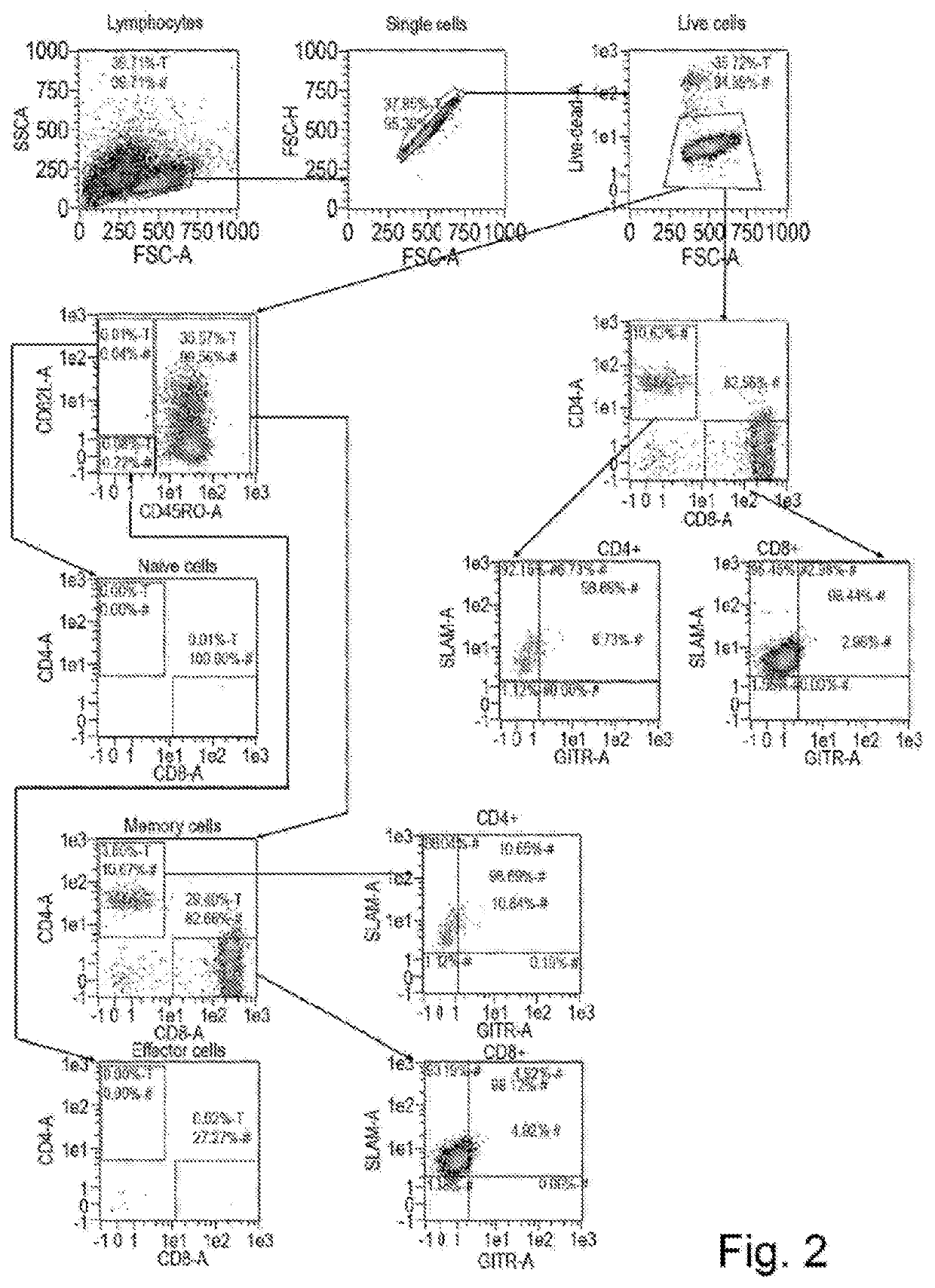
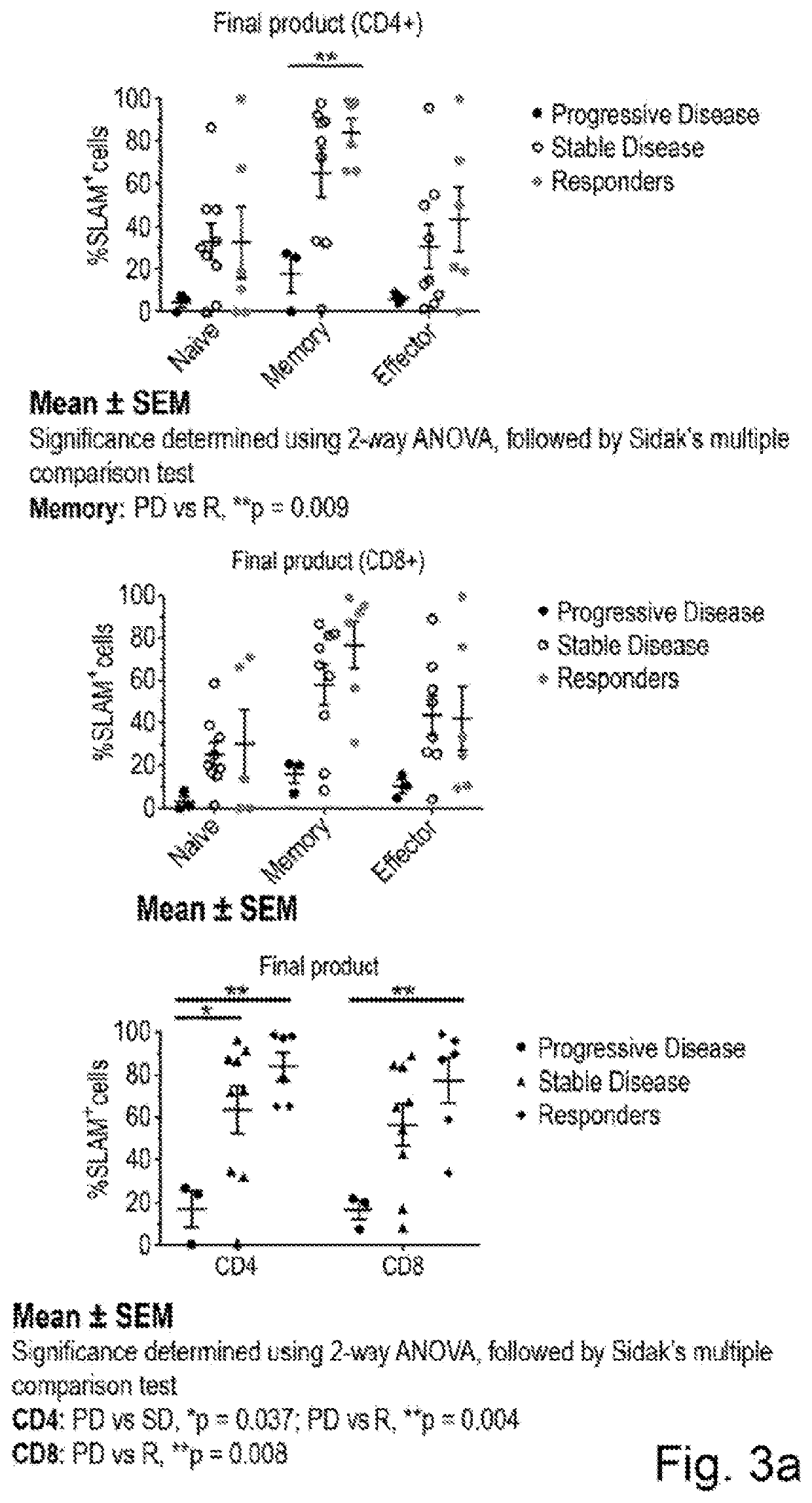
Biomarker predictive of tumour infiltrating lymphocyte therapy and uses thereof
PendingUS20210000872A1Increase tumour reactivityImproved clinical response rateGenetically modified cellsBiological material analysisCancer researchTumour infiltrating lymphocyte
The present invention concerns a biomarker useful in adoptive cell therapy. The biomarker in question is CDI50, otherwise termed SLAM or SLAMF1. Herein Applicants demonstrate that expression of CD 150 on tumour infiltrating lymphocytes infusion products correlates with the response rate seen in those patients. High CDI50 expression is found on patients who go on to have a complete response and low expression on patients who do not respond to therapy. The invention relates to the use of the biomarker to predict response rate or stratify patients for treatment. It also covers exploitation of this receptor in adoptive cell therapy regimens in general, including but not limited to over expression of the receptor in T-cell populations or isolation of cells expressing CD 150 in an effort to increase efficacy.
Owner:INSTIL BIO UK LTD
Methods and compositions for transducing lymphocytes and regulated expansion thereof
ActiveUS10596274B2Reduce riskAvoid environmental exposureVirusesAntibody mimetics/scaffoldsAdoptive cellular therapyNatural Killer Cell Inhibitory Receptors
The present disclosure provides methods for genetically modifying lymphocytes and methods for performing adoptive cellular therapy that include transducing T cells and / or NK cells without prior ex vivo stimulation. The methods typically include engineered signaling polypeptides that can include a lymphoproliferative element, and / or a chimeric antigen receptor (CAR), for example a microenvironment restricted CAR. Additional elements of such engineered signaling polypeptides are provided herein, as well as vectors, such as retroviral vectors, packaging cell lines and methods of making the same. Furthermore, recombinant retroviruses and methods of making the same are provided. Numerous controls are provided, including riboswitches that are controlled, for example in vivo, by nucleoside analogues.
Owner:EXUMA BIOTECH CORP
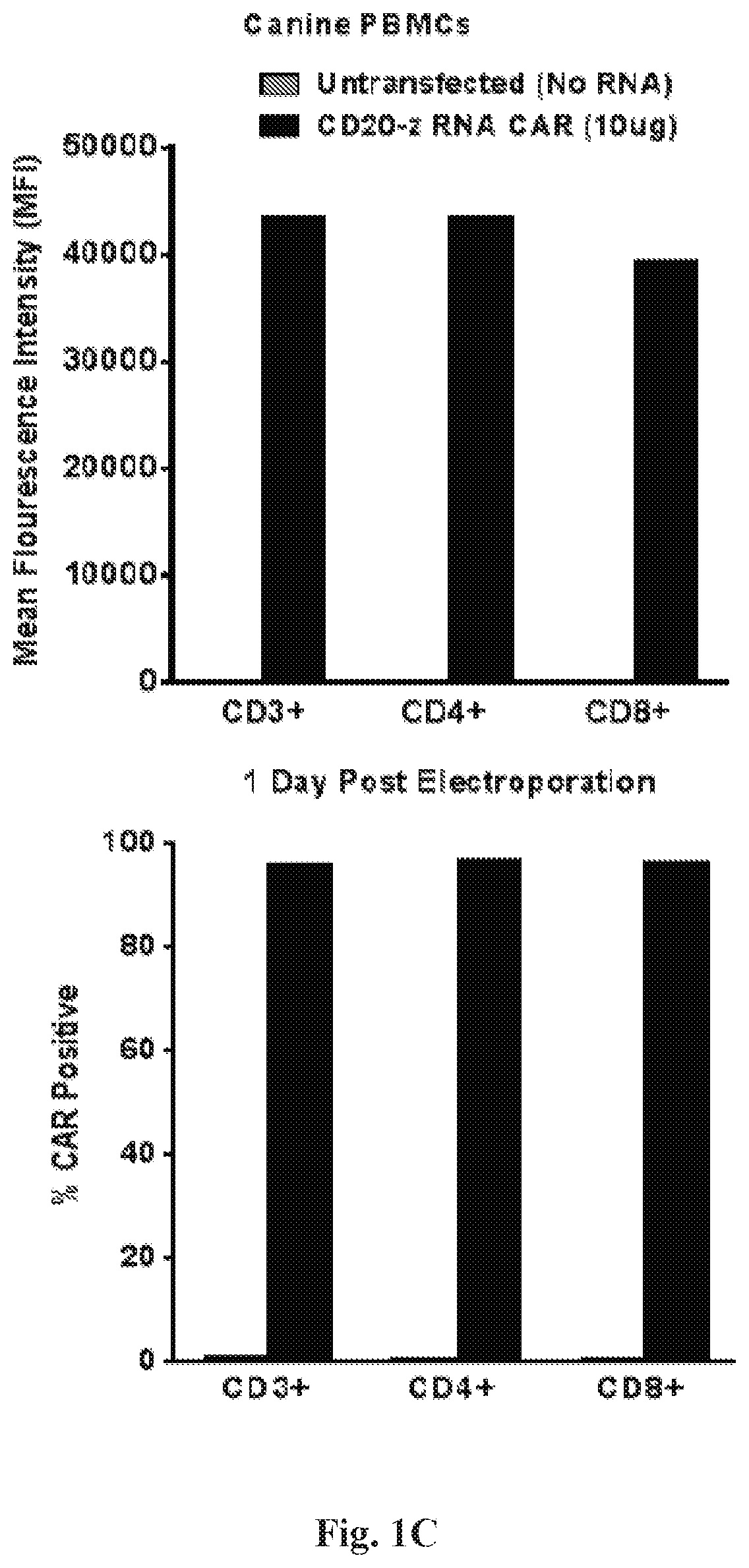
Treatment of a canine cd20 positive disease or condition using a canine cd20-specific chimeric antigen receptor
ActiveUS20190350972A1Stimulate immune responsePolypeptide with localisation/targeting motifImmunoglobulin superfamilyCD20Adoptive cellular therapy
The present invention relates to compositions and methods for the treatment of a canine CD20 positive disease or condition using a canine CD20-specific chimeric antigen receptor. One aspect includes a modified canine T cells and pharmaceutical compositions comprising the modified cells for adoptive cell therapy and treating a disease or condition associated with enhanced immunity in canine.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Compositions and methods for immune cell modulation in adoptive immunotherapies
PendingUS20200179451A1Improved cell therapyConvenient treatmentOrganic active ingredientsMammal material medical ingredientsAdoptive cellular therapyCentral Memory T-Cell
Compounds that either produce a higher proportion or greater absolute number of phenotypically identified naive, stem cell memory, central memory T cells, adaptive NK cells, and type I NKT cells are identified. Compositions and methods for modulating immune cells including T, NK, and NKT cells for adoptive cell therapies, such as those providing improvements in one or more therapeutic outcomes, are provided.
Owner:FATE THERAPEUTICS +1
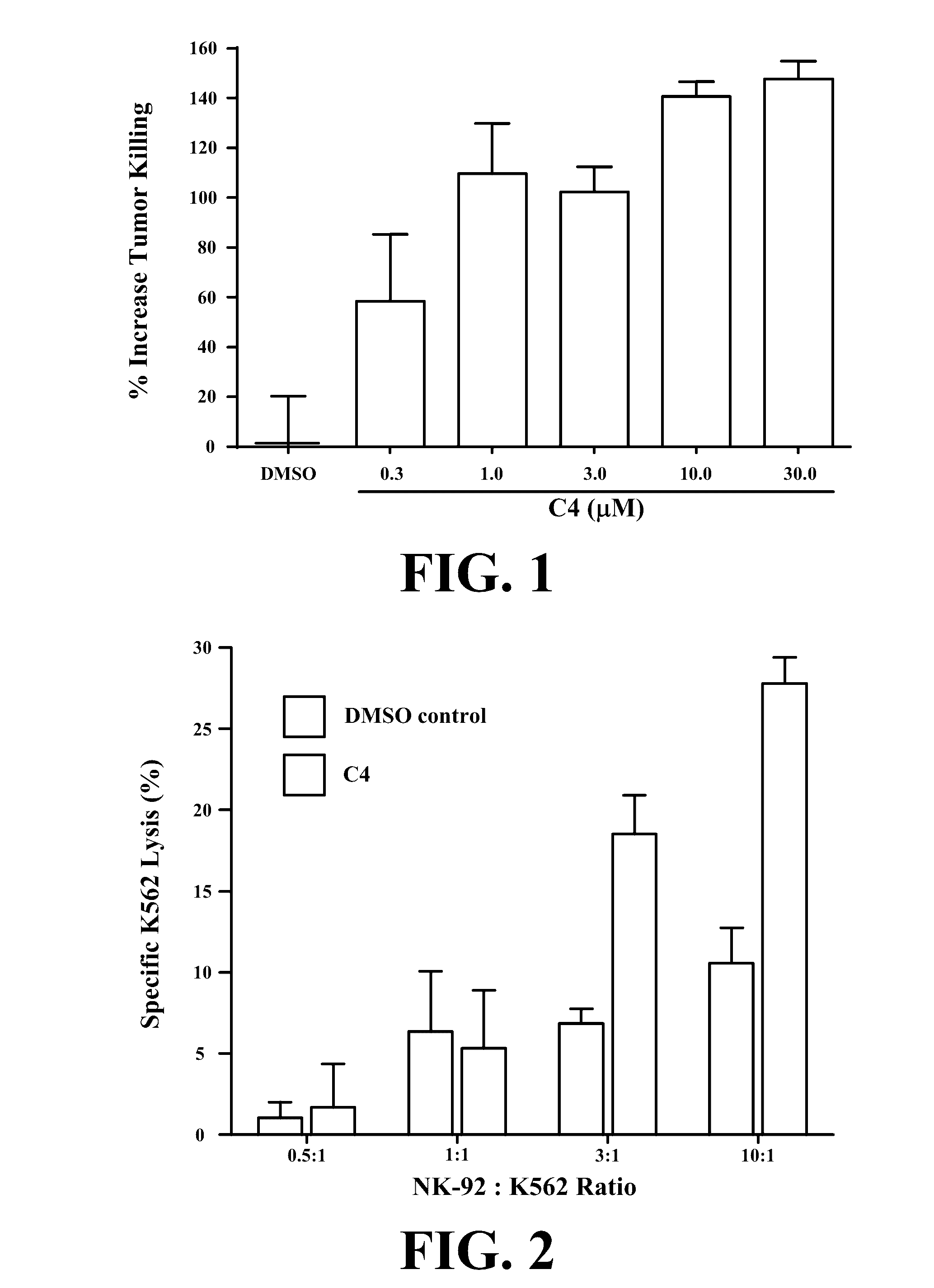
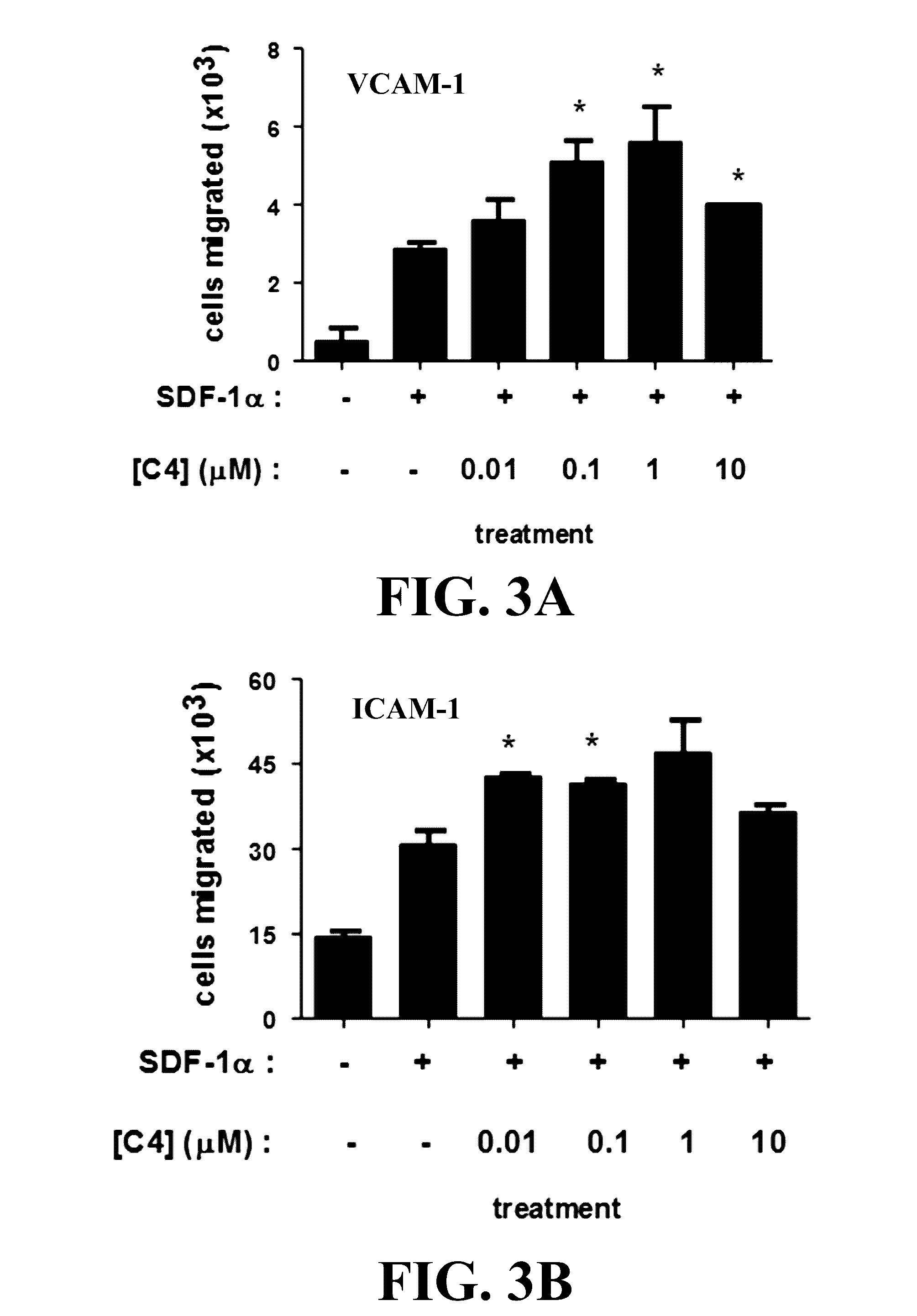
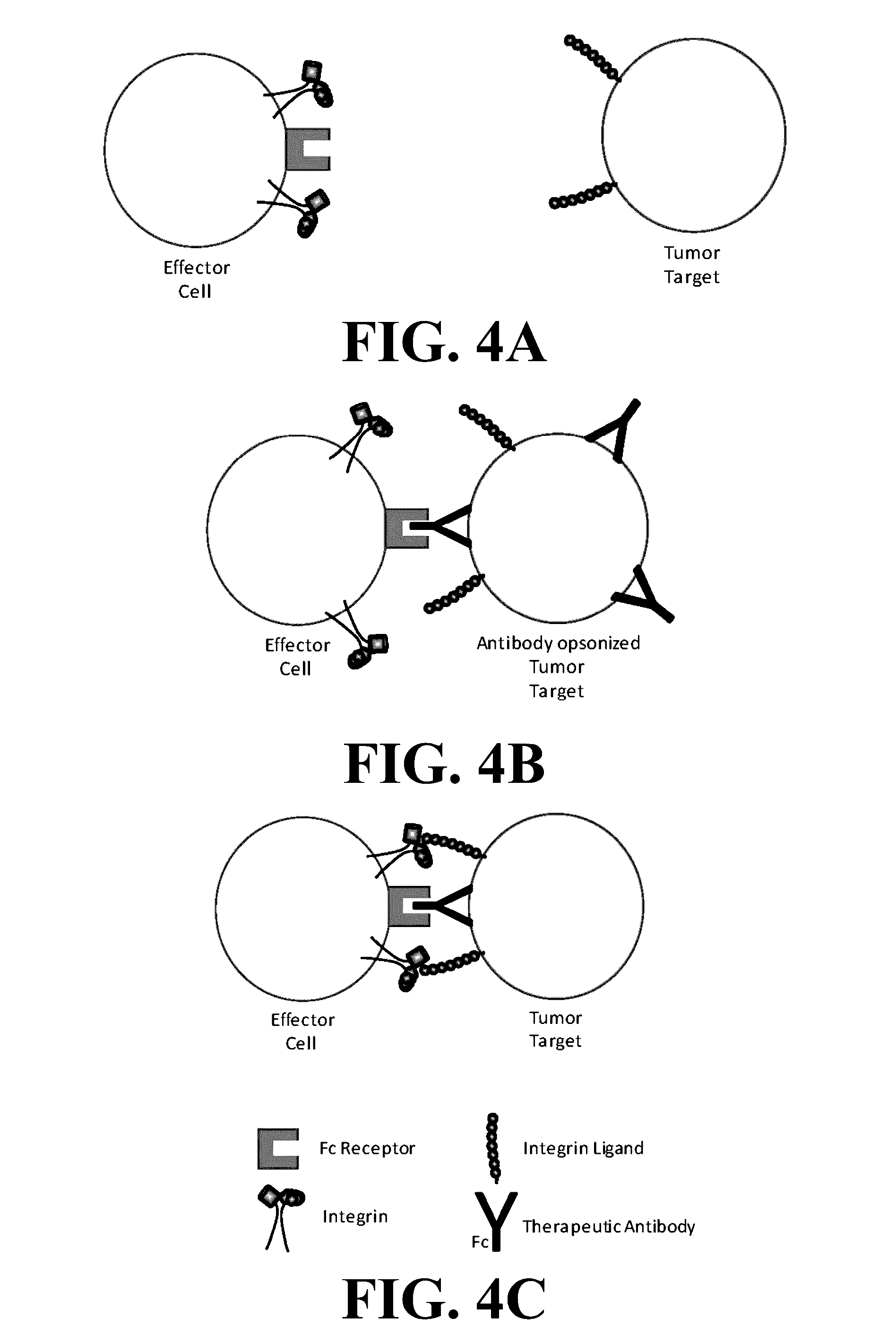
Novel compositions and methods for immunotherapies comprising small molecule integrin receptor-ligand agonist adjuvants
ActiveUS20160339098A1Antibody ingredientsCancer antigen ingredientsAdoptive cellular therapyAdjuvant
Small molecule integrin ligand mimetics facilitate integrin-ligand interactions, which may be used to prepare vaccines, adoptive cell therapies, immunotherapies for cancer, and a variety of other conditions. As integrin mediated cell-cell interactions are critical to antigen presentation and effector cell killing, increasing the efficiency of integrin receptor-ligand interactions will stabilize the immune synapse and improve effector functions. Compositions and methods including the mimetics enhance: (1) the priming of vaccines (including, but not limited to, cancer vaccines); (2) cytolytic activity of adoptive cell therapies (including, but not limited to γδT-cells, CTLs, NK, iNKT); (3) immunotherapies (including, but not limited to, negative checkpoint blockage strategies such as anti-CTLA-4 and anti-PD-1); and (4) biologic therapies (including, but not limited, to trastuzumab and rituxamab), whereby the mechanism-of-action includes antibody dependent cellular cytotoxicity (ADCC).
Owner:TEXAS HEART INST +1
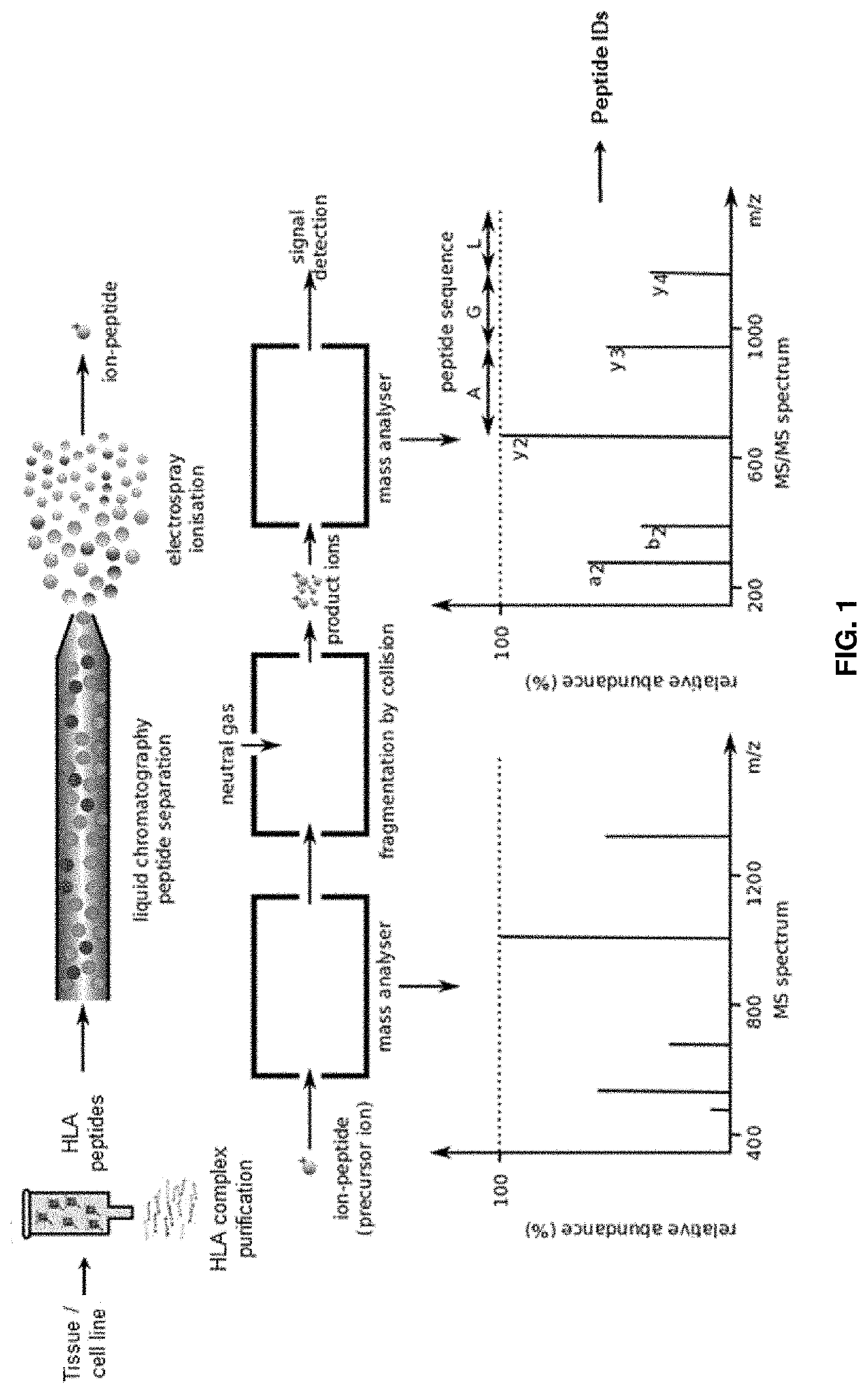
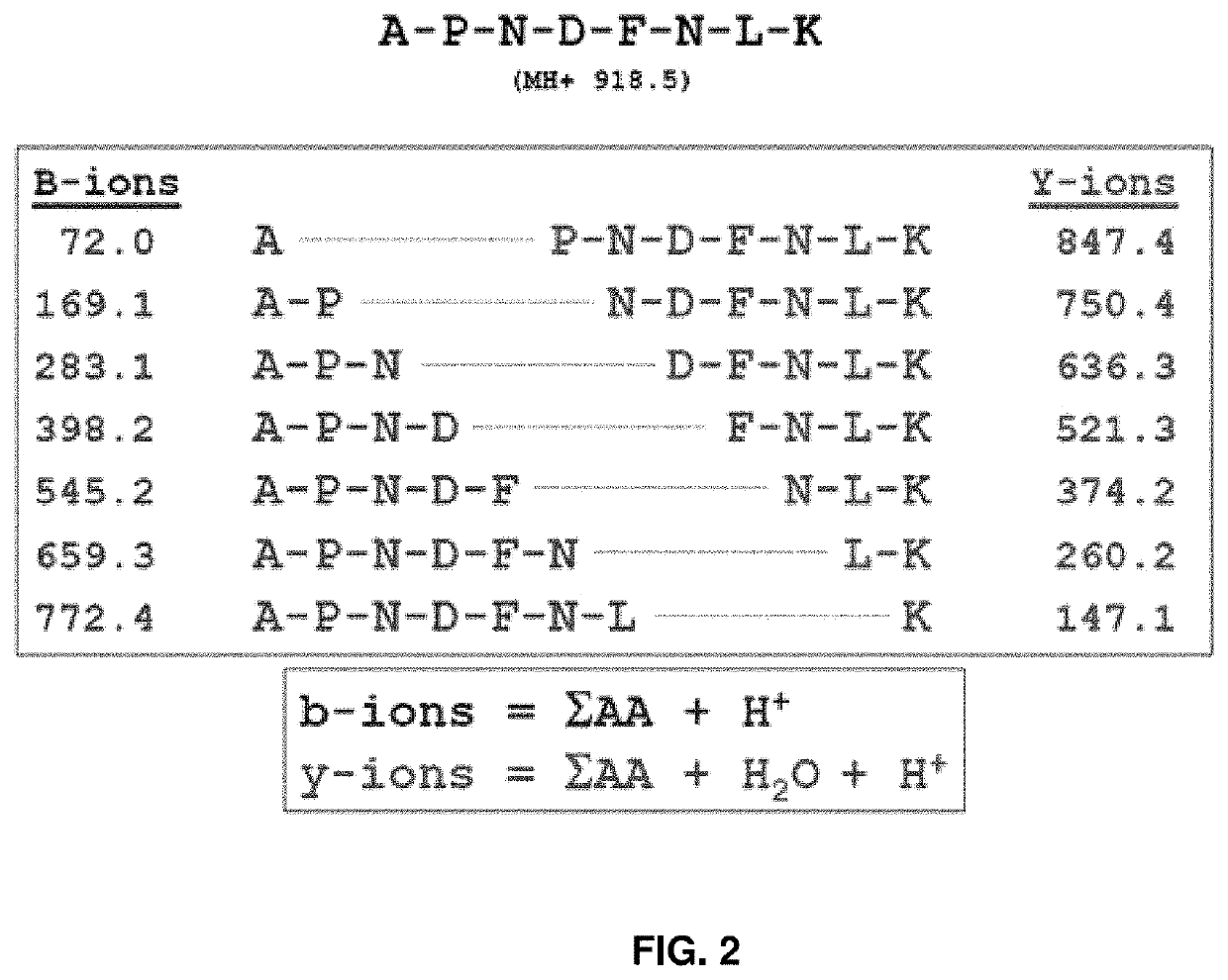
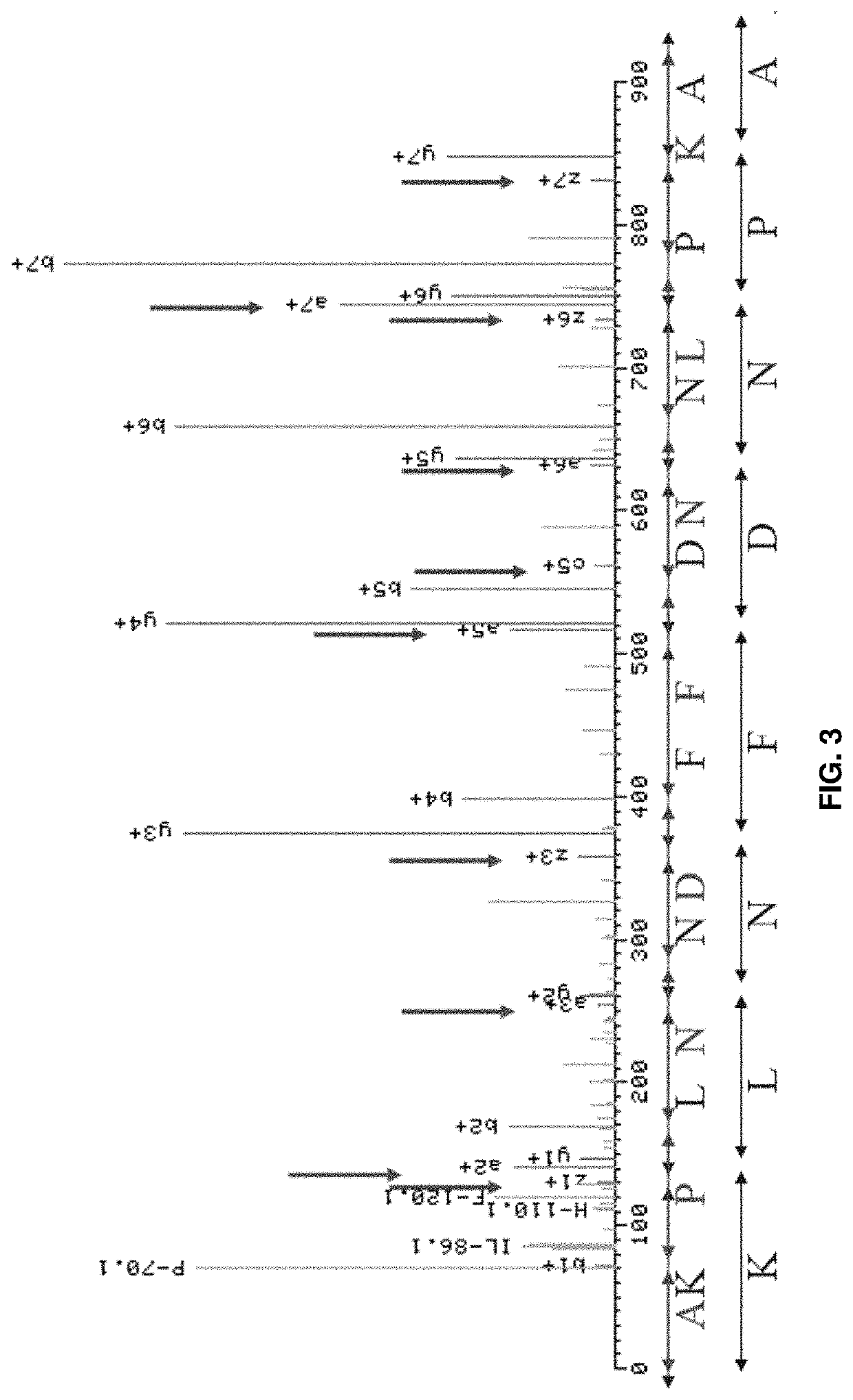
Methods for peptide mass spectrometry fragmentation prediction
PendingUS20210041454A1Improve accuracyGood confidenceComponent separationBiological material analysisAdoptive cellular therapyWhite blood cell
The present disclosure relates to methods of improved identification of peptides, for example, antigenic peptides. In particular, the present disclosure relates to methods of more accurately identifying human leukocyte antigen (HLA) peptides by utilizing classification systems. The disclosure also provides for utilizing the described methods for the field of personalized cancer therapies, such as adoptive cellular therapy (ACT).
Owner:IMMATICS US INC +1
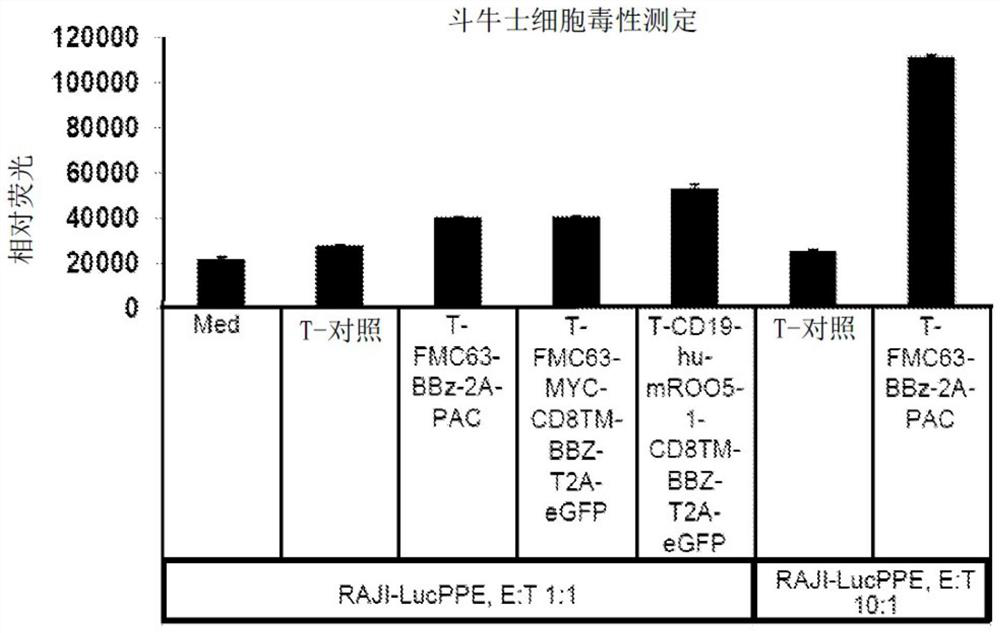
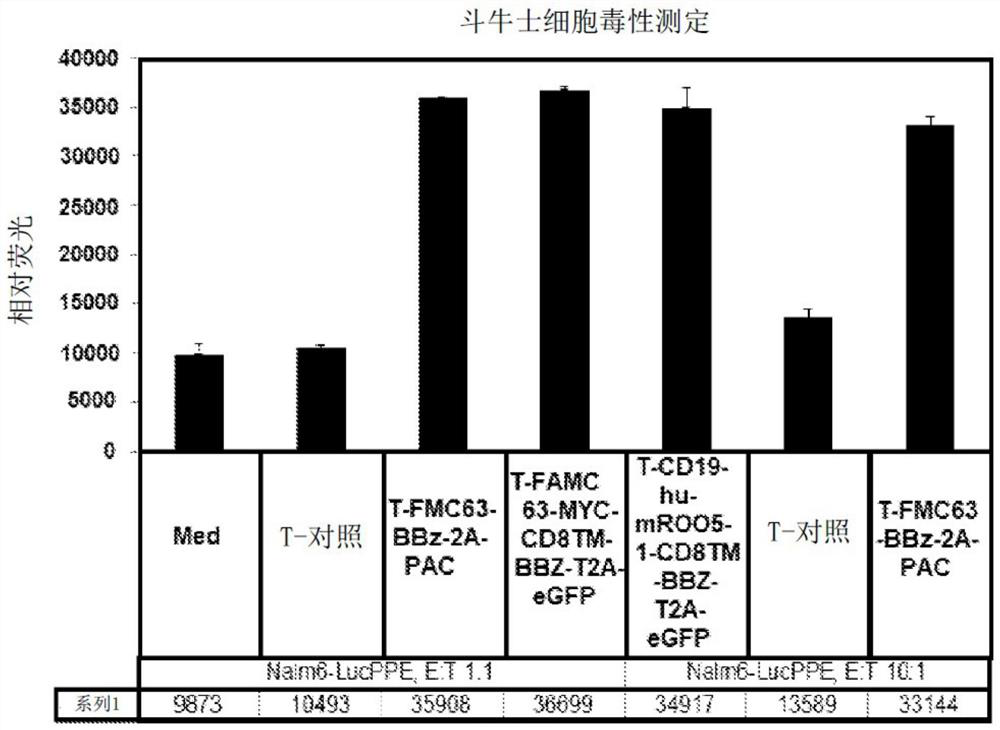
Improving the efficacy and safety of adoptive cellular therapies
PendingCN113286811AOrganic active ingredientsPeptide/protein ingredientsAdoptive cellular therapyDisease
The disclosure relates generally to the generation and use of immune effector cells (e.g., T cells, NK cells) engineered to express an immune receptor (e.g., Chimeric Antigen Receptor, Synthetic Immune Receptor, T Cell Receptor etc.) to treat a disease associated with expression of a target antigen.
Owner:UNIV OF SOUTHERN CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com