Preparation method of 3-iodo-5-bromo-4, 7-diazaindole
A technology of diazepine and indole, applied in the field of pharmaceutical preparations, can solve the problems of low synthesis yield and the like, and achieve the effects of high reaction yield, easy product and short reaction process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
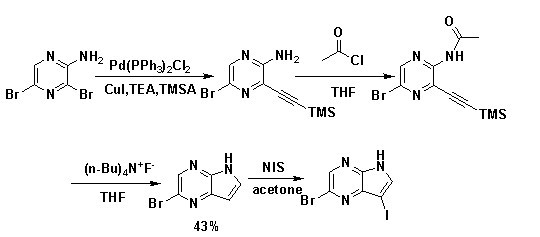
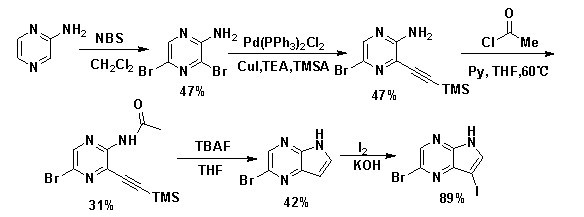
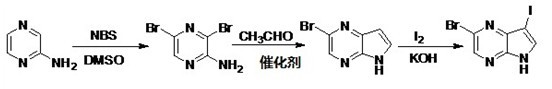
[0025] See attached image 3 , the preparation method of the 3-iodo-5-bromo-4,7-diazaindole of the invention comprises the following steps:
[0026] Step (1) In a flask, add 38.0 g of the raw material 2-aminopyrazine and dissolve with 500 mL of dimethyl sulfoxide (DMSO) and 20 mL of H 2 After O was dissolved, 149.5 g of N-bromosuccinimide (NBS) was added in batches, and the reaction temperature was controlled below 15 °C. After the addition was complete, it was stirred at room temperature, followed by TLC spotting. After the reaction was completed, it was quenched with ice water (300 mL), and the layers were separated after standing. The aqueous phase was extracted with ethyl acetate (200 mL×3), and the organic phases were combined and washed with 5% Na 2 CO 3 solution and H 2 After washing with O, anhydrous Na 2 SO 4 dry. After filtration, the solvent was removed in vacuo to obtain a crude product, which was recrystallized with a small amount of absolute ethanol (EtOH) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com