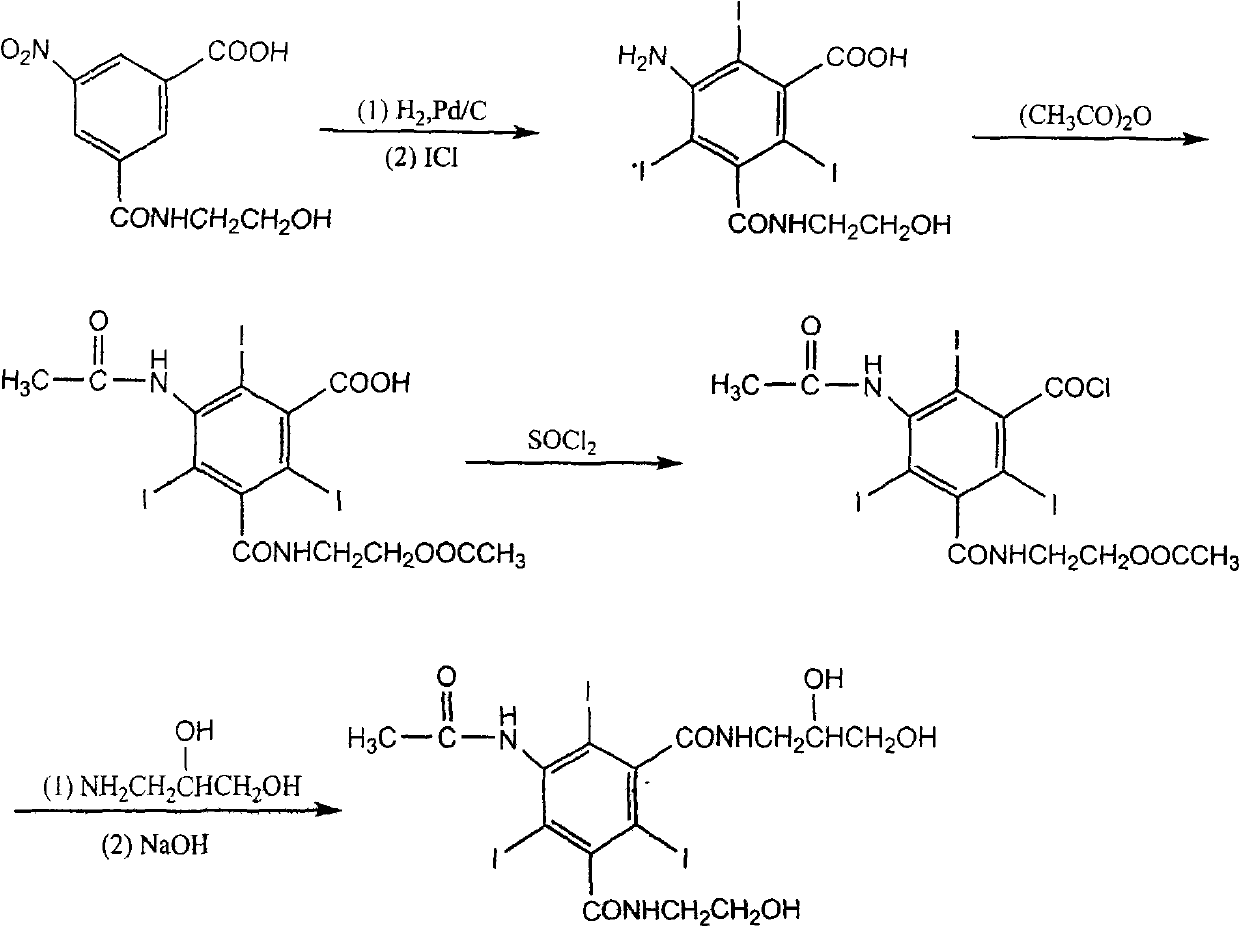
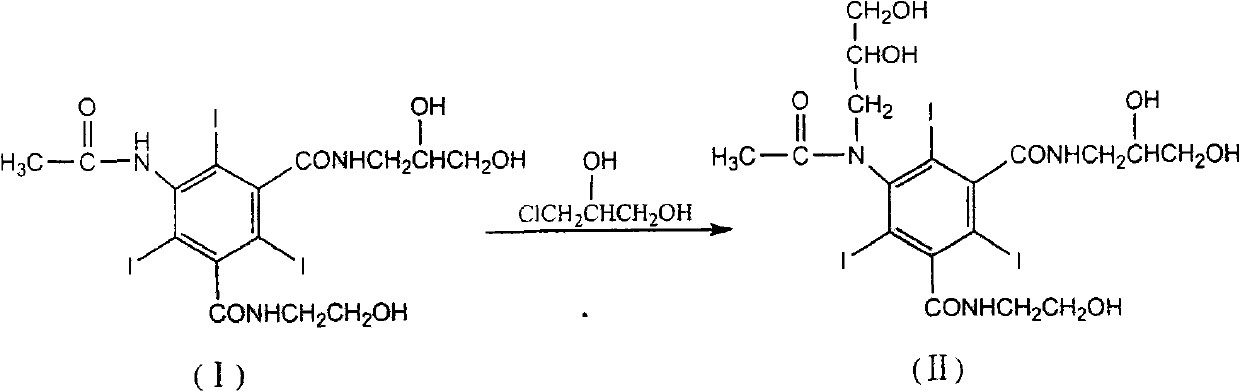
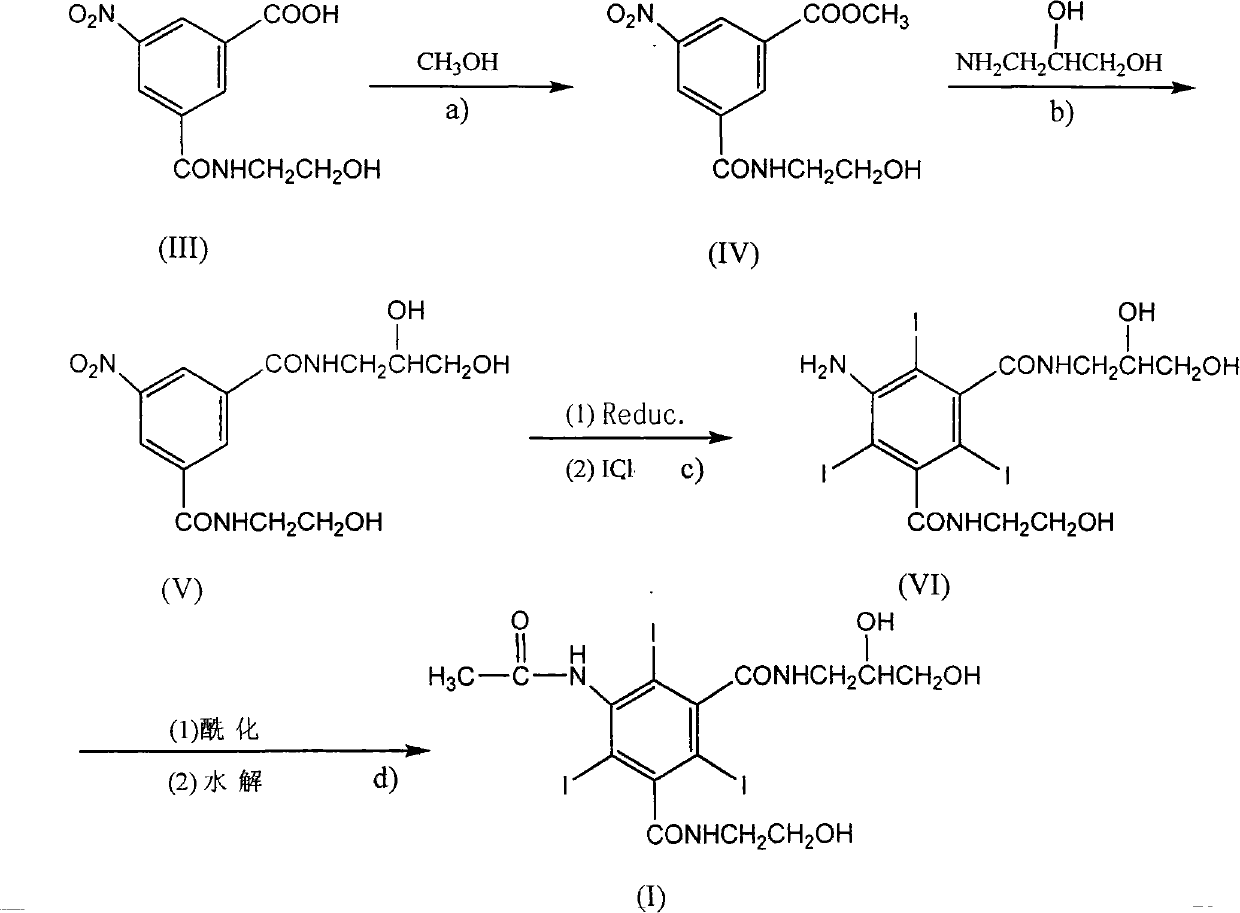
Method for preparing loxilan intermediate
The technology of an intermediate, ioxilan, is applied in the field of preparation of non-ionic X-ray contrast agents, can solve the problems of high price of iodine monochloride, high reaction cost, long reaction steps and the like, and achieves low cost, high product purity, The effect of short reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Synthesis of 5-nitro-N-(2-hydroxyethyl)-m-carboxamidobenzoic acid methyl ester:
[0020] In a flask equipped with a stirrer and a reflux condenser, add 51 g (0.2 mol) of 5-nitro-N-(2-hydroxyethyl)-m-carboxamidobenzoic acid and 200 ml of methanol at room temperature, stir evenly, and add Sulfuric acid 2ml, heated to 70°C and refluxed for 24 hours to end the reaction, evaporated the methanol under reduced pressure, added the solid to water to form a suspension, adjusted the pH value to about 8 with saturated sodium carbonate solution, stood still for 24 hours, and filtered the solid , washed with distilled water, and dried to obtain 46.5 g of the product, with a yield of 86.7%, and mp: 147-150°C. HPLC detection content: 98.8% (detection conditions: chromatographic column: amino column (250 × 4.6mm, 5 μm); mobile phase: acetonitrile-water solution (87:13); temperature is 30 ° C; flow rate: 1.0mL / min; detection wavelength : 245nm).
Embodiment 2
[0022] Synthesis of 5-nitro-N-(2,3-dihydroxypropyl)-N'-(2-hydroxyethyl)-1,3-benzenedicarboxamide:
[0023] In a flask equipped with a stirrer and a reflux condenser, add 53.6 g (0.2 mol) of methyl 5-nitro-N-(2-hydroxyethyl)-m-formamide benzoate and 200 ml of methanol at room temperature, and stir evenly Finally, add 20g (0.22mol) of 3-amino-1,2-propanediol, heat up to 70°C and reflux for 48 hours to complete the reaction, cool the reaction solution to 4°C, precipitate a solid, filter, wash with a small amount of methanol, and dry to obtain the product 62.9g, yield 96.2%, mp: 142-144°C. 1 H-NMR (DMSO-D 6 , 400MHzδ(ppm): 3.17~3.73(m, 9H, 4×CH 2 , 1×CH), 4.55~4.84(m, 3H, 3×OH), 8.76~8.81(m, 3H, 3×Ar-H), 8.86~8.93(t, 2H, 2×ArCONH); MS: m / z(%): 328.1(M + +H, 100). HPLC detection content: 99.1% (detection conditions: chromatographic column: amino column (250 × 4.6mm, 5 μm); mobile phase: acetonitrile-water solution (87:13); temperature is 30 ° C; flow rate: 1.0mL / min; detectio...
Embodiment 3
[0026] Synthesis of 5-amino-N-(2,3-dihydroxypropyl)-N'-(2-hydroxyethyl)-2,4,6-triiodo-1,3-benzenedicarboxamide:
[0027]In a flask equipped with a stirrer and a reflux condenser, 5-nitro-N-(2,3-dihydroxypropyl)-N'-(2-hydroxyethyl)-1,3-benzene Dissolve 65.4g (0.2mol) of diformamide in 250ml of water, add 5ml of hydrochloric acid and heat up to 80°C, add 56g (1mol) of iron powder in 5 times, continue to stir for one hour after the addition, filter, wash the filter cake with distilled water, Combine the filtrate and washing liquid, adjust the pH value to 1 with concentrated hydrochloric acid, raise the temperature to 80°C, add 107g (0.66mol) of iodine monochloride dropwise, react at 90°C for 3 hours to complete the reaction, and cool the reaction solution to 4°C , precipitated a solid, filtered, washed with a small amount of ethanol, and dried to obtain 110.8 g of the product, with a yield of 82.1%, mp: 220-225°C. 1 H-NMR (DMSO-D 6 , 400MHz) δ (ppm): 3.18~3.76 (m, 9H, 4×CH 2 ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com