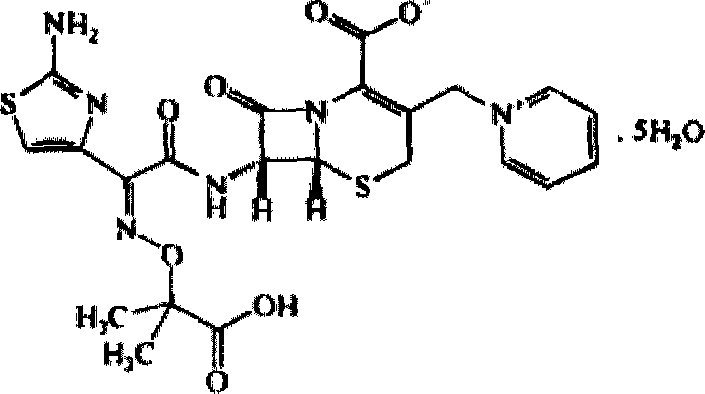
Ceftazidime crystal compound, preparation method of compound and pharmaceutical composition of compound in sterile mixed powder form
A crystalline compound, ceftazidime technology, applied in the field of medicine, can solve the problems of increased polymer content, increased risk of allergic reactions, etc., and achieves the effects of reducing the incidence, improving the safety and effectiveness of medication, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] [Example 1] Preparation of Ceftazidime Crystalline Compound
[0044] 1) Prepare the crude product solution: add 200 g of the crude product of ceftazidime into 1500 ml of a mixed solvent prepared by dimethyl sulfoxide and tetrahydrofuran in a volume ratio of 5:1, stir to dissolve, add activated carbon for decolorization, and filter to obtain the crude product solution. spare;
[0045] 2) Preparation of a crystallization solvent: prepare a crystallization solvent with acetone and ethyl acetate at a ratio of 2:10 by volume, and the volume of the crystallization solvent is 12 times the weight of the crude ceftazidime;
[0046] 3) Crystallization: under stirring, add the crystallization solvent obtained in step 2) to the crude product solution obtained in step 1), and solids are precipitated; after the addition is completed, continue to add ethanol dropwise under stirring until crystals are precipitated; stand for 5h, Filter, wash with dimethyl sulfoxide, and dry to obtain ...
Embodiment 2~9
[0048] [Example 2~9] Preparation of Ceftazidime Crystal Compound
[0049] The following are Examples 2-9 of the present invention, the steps are the same as Example 1, and the specific process parameters are shown in Table 1:
Embodiment 2-9
[0051]
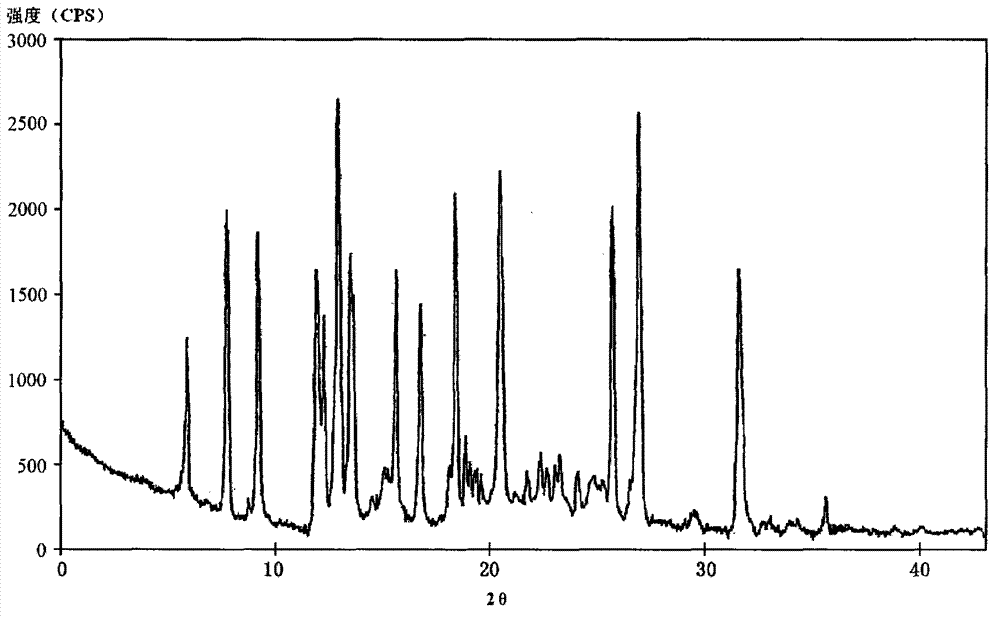
[0052] The ceftazidime crystalline compound obtained in Examples 2-9 was measured by powder X-ray diffraction measurement method, and the X-ray powder diffraction pattern represented by 2θ±0.2° diffraction angle was the same as that in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com