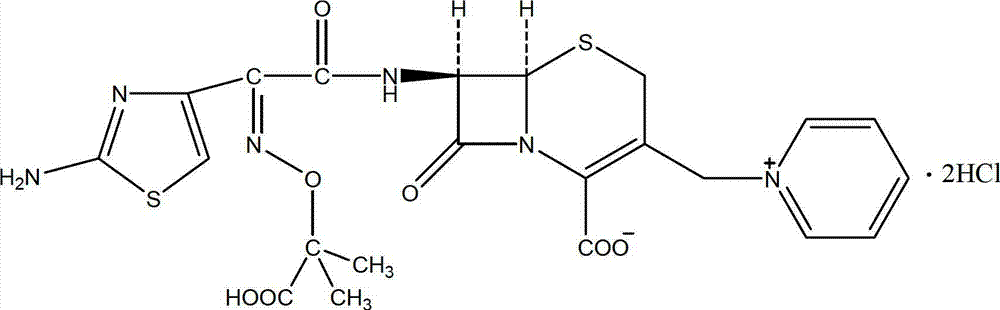
Method for preparing ceftazidime hydrochloride
A technology of ceftazidime and hydrochloride, applied in the field of chemical pharmacy, can solve the problems of low total yield, high cost, low purity and the like, and achieve the effects of improving production efficiency, reducing production cost and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The embodiment of the present invention provides a kind of preparation method of ceftazidime hydrochloride, comprises the following steps:
[0023] Step S01, acylation to ester reaction:
[0024] Add 7-APCA and ceftazidime side chain active ester to the organic solvent, adjust the system temperature to -10~-5℃, add organic base, adjust the system temperature to 0~5℃ and react for 22~24 hours, adjust the temperature to- 10~-5℃, add acid to adjust the pH to 0.5~1, add water and adsorbent to stir the reaction, for example, 30 minutes, filter and collect the filtrate, the organic solvent is selected from one or a combination of methanol, ethanol, DMF, DMSO More than one, the acid is selected from one or more of hydrochloric acid, phosphoric acid, sulfuric acid, nitric acid, formic acid or acetic acid;
[0025] Step S02, hydrolysis:
[0026] Adjust the temperature of the filtrate to 0-5°C, add concentrated hydrochloric acid, react for 10-12 hours, add a crystallization sol...
Embodiment 1
[0040] Disperse 20g of 7-APCA and 25g of ceftazidime side chain active ester in 50mL of methanol, cool down to ~10°C, slowly add 10mL of triethylamine dropwise, and control the temperature at 0~5°C for 24 hours; after the reaction, cool down to ~10°C ℃, slowly add concentrated hydrochloric acid dropwise to adjust the pH value to 1.0, add 60mL water and 5g alumina to absorb for 30 minutes, and collect the filtrate by filtration;
[0041] Add dropwise 20mL of concentrated hydrochloric acid to the filtrate, control the temperature at 0~5°C for 12 hours, slowly add 400mL of acetone dropwise, stir and grow the crystal for 1h, filter, wash, and dry to obtain 28.4g of ceftazidime hydrochloride crystals. The molar yield is 89.0%, the purity is 99.2%.
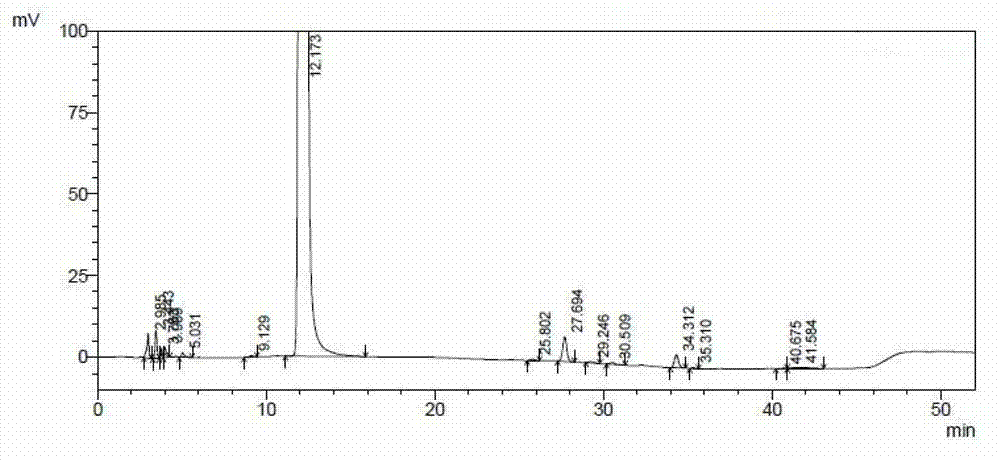
[0042] see figure 1 , figure 1Show the chromatogram of the ceftazidime hydrochloride prepared by the embodiment of the present invention 1, please combine the following table:
[0043]
[0044] From figure 1 And it can be seen fr...
Embodiment 2
[0046] Disperse 25g of 7-APCA and 36g of ceftazidime side chain active ester in 40mL of DMF, cool down to ~10°C, slowly add 15mL of pyridine dropwise, and control the temperature at 0~5°C for 24 hours; after the reaction, cool down to ~10°C, Slowly add concentrated hydrochloric acid to adjust the pH value to 0.5, add 80mL water and 6.5g alumina to absorb for 30 minutes, and collect the filtrate by filtration;
[0047] Add 25mL of concentrated hydrochloric acid dropwise to the filtrate, control the temperature at 0~5°C for 12 hours; slowly add 500mL of isopropyl acetate dropwise, stir and grow the crystal for 1h, filter, wash, and dry to obtain 36.3g of ceftazidime hydrochloride crystals, mol The yield was 89.6%, and the purity was 99.3%.
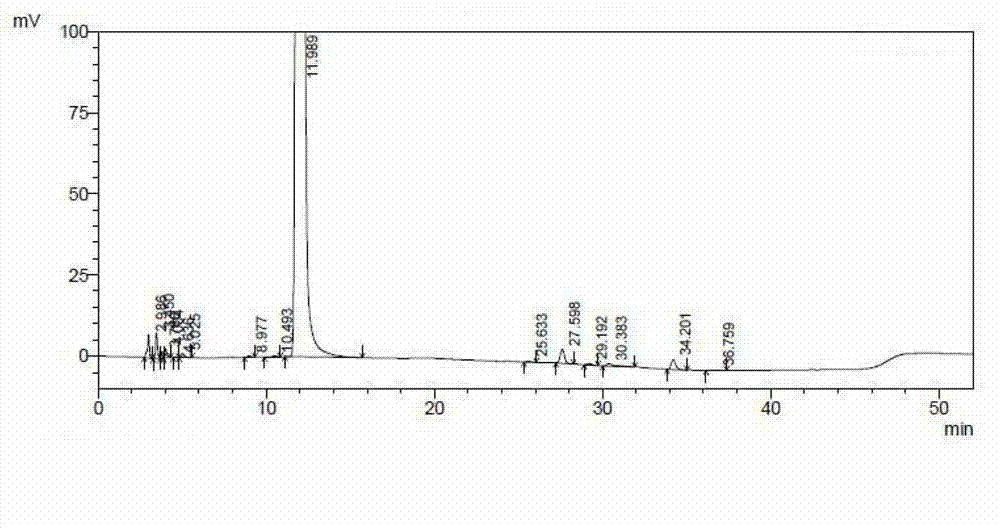
[0048] see figure 2 , figure 2 Show the chromatogram of the ceftazidime hydrochloride prepared by the embodiment of the present invention 2, please combine the following table again:
[0049]
[0050]
[0051] From figure 2 And ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com