Preparation method of type I clopidogrel hydrogen sulfate
A kind of technology of clopidogrel hydrogen sulfate, clopidogrel, applied in the field of chemistry, can solve problems such as low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0071] The present invention will be described in further detail below through specific implementation examples and in conjunction with the accompanying drawings.
[0072] The embodiment of the present invention provides a preparation method of type I clopidogrel bisulfate, comprising:
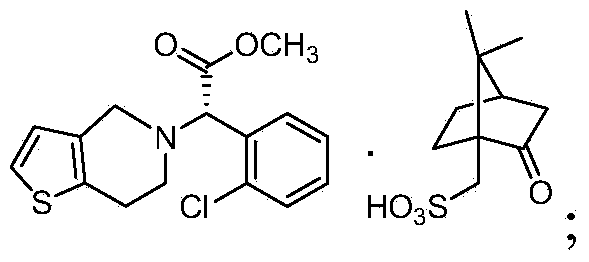
[0073] (1) (S)-α-[(2-thiophene)ethylamino]-α-(2-chlorophenyl)acetic acid methyl ester hydrochloride (S)-clopidogrel free base is generated through Mannich reaction as a raw material, and the (S)-clopidogrel free base is (S)-2-(2-chlorophenyl)-6,7-di Methyl chlorothieno[3,2-c]pyridine-5(4H)-acetate
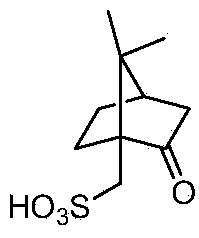
[0074] (2) Through L-camphorsulfonic acid React with the (S)-clopidogrel free base, and remove impurities to obtain (S)-α-(2-chlorophenyl)-6,7-dichlorothieno[3,2-c]pyridine- 5(4H)-Methyl acetate-(L)-camsylate
[0075] (3) The (S)-α-(2-chlorophenyl)-6,7-dichlorothieno[3,2-c]pyridine-5(4H)-acetic acid methyl ester by acetone-petroleum ether -(L)-Camphorsulfonate is purified to remove impur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com