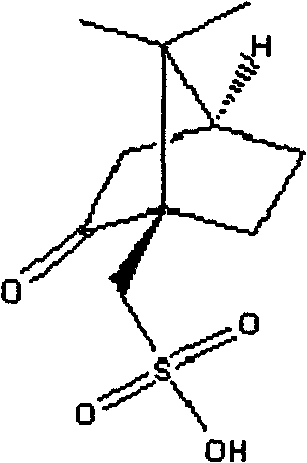
Recovering and applying method of clopidogrel resolving agent
A technology for clopidogrel and clopidogrel hydrochloride, applied in the field of organic chemical synthesis, can solve the problems of difficult separation of levocamphorsulfonic acid, poor optical purity, complicated process, etc., and achieves short recovery reaction time, low production cost, and optical purity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The mother liquor in the above reaction was adjusted to pH = 6 with sodium carbonate, crystallized at room temperature for 0.5 hours, filtered to obtain solid sodium camphorsulfonate, added 70ml (2 times) ethyl acetate, adjusted to pH = 1 with sulfuric acid, and crystallized at room temperature for 2 hours , filtered to obtain 14.5g of L-camphorsulfonic acid, mp193-195℃, [α] D =-42.9°, the recovery rate was 96.03%. It should be noted that this mother liquor was used as a raw material mother liquor for the invention described below.
Embodiment 2
[0022] The mother liquor in the above reaction was adjusted to pH = 7 with sodium bicarbonate, crystallized at room temperature for 1 hour, filtered to obtain solid sodium camphorsulfonate, added 140ml (4 times) ethyl acetate, adjusted to pH = 3 with sulfuric acid, and crystallized at room temperature 4 hour, filtered to obtain 14.9g of L-camphorsulfonic acid, mp194-195℃, [α] D =-43.5°, the recovery rate was 98.68%.
Embodiment 3
[0024] The mother liquor in the above reaction was adjusted to pH=8 with sodium carbonate, crystallized at room temperature for 2 hours, filtered to obtain solid sodium camphorsulfonate, added 70ml (2 times) ethyl acetate, adjusted to pH=5 with phosphoric acid, and crystallized at room temperature for 8 hours , filtered to obtain 13.7g of L-camphorsulfonic acid, mp193-194℃, [α] D =-41.5°, the recovery rate was 90.73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com