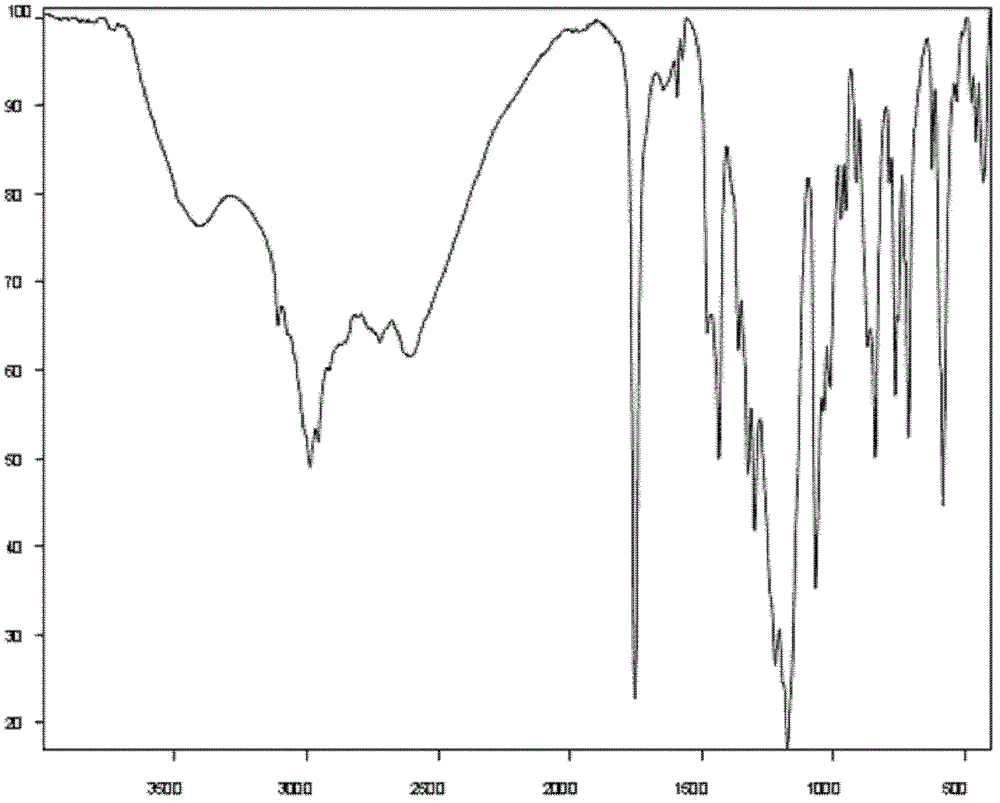
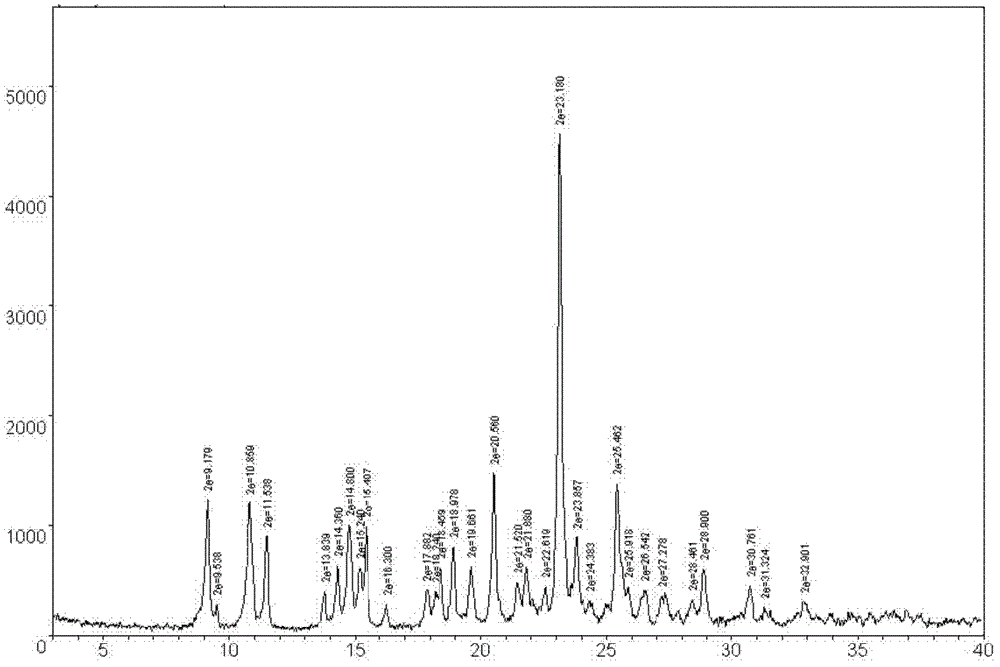
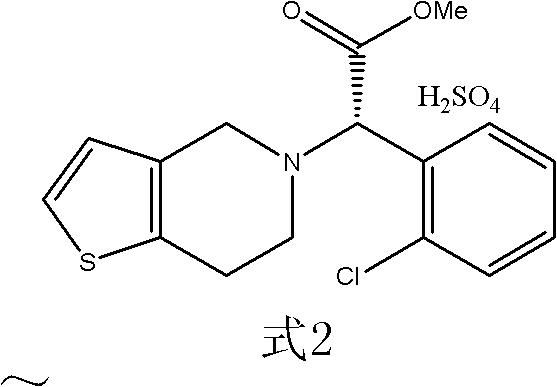
Method for preparing (+)-(S-)-clopidogrel hydrogen sulfate 1 crystal form
A technology of clopidogrel hydrogen sulfate and clopidogrel camphor sulfonate, applied in the direction of organic chemistry and the like, can solve the problems of low crystal form purity, difficult control of technological process, difficult to meet the requirements of industrialized scale-up production, etc. High purity, good crystal quality stability, not easy to agglomerate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add (+)-(S-)-clopidogrel camphorsulfonate 118g and dichloromethane 200ml in the reaction flask, stir and drop the temperature to below 5°C and control the temperature below 5°C and dropwise add sodium bicarbonate aqueous solution (20.2g Sodium bicarbonate and 300ml of water), after the dropwise addition, stir for another 10 minutes, let it stand for 5 minutes, separate the lower organic phase, extract the aqueous phase with 200ml of dichloromethane once, combine the organic phases, and use 100ml of purified water for the combined phases ×2 was washed twice, concentrated under reduced pressure to obtain an equivalent amount of (+)-(S-)-clopidogrel (free base) (64.2 g).
Embodiment 2
[0031] Inhale 100Kg of dichloromethane into the reaction kettle, add 26.2Kg of (+)-(S-)-clopidogrel camphorsulfonate, cool down to below 5°C in an ice-salt bath under stirring, and add dropwise under temperature control below 5°C Aqueous solution of sodium bicarbonate (4.8Kg sodium bicarbonate and 70Kg water), after dropwise addition, stir for 15 minutes, let stand for 10 minutes, separate the lower organic phase, extract the aqueous phase once with 100Kg of dichloromethane, and combine the organic phases , the combined phase was washed twice with purified water 35Kg×2, and concentrated under reduced pressure to obtain an equivalent amount of (+)-(S-)-clopidogrel (free base) (15.2Kg).
Embodiment 3
[0033] Dissolve the (+)-(S-)-clopidogrel prepared in Example 2 with 137Kg of ethyl acetate and add it to a stirred reactor, control the temperature at 20°C, add 1.0Kg of (+)-(S- -)-Clopidogrel bisulfate 1 crystal seed, slowly dropwise add 15.2Kg of 98% sulfuric acid ethyl acetate solution prepared in advance (the amount of 98% concentrated sulfuric acid is 5.1Kg, 0.051Kmol), drop for 1 hour After the addition is completed, stir at 25°C for 1.5h, filter, wash twice with 21Kg ethyl acetate, centrifuge, vacuum greater than 0.09Mpa, and dry at 40-50°C to obtain free (+)-(S-)-clopidogrel hydrogen sulfate Salt (1 crystal form) 19.9Kg, yield 95.1%, content of 1 crystal form 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com