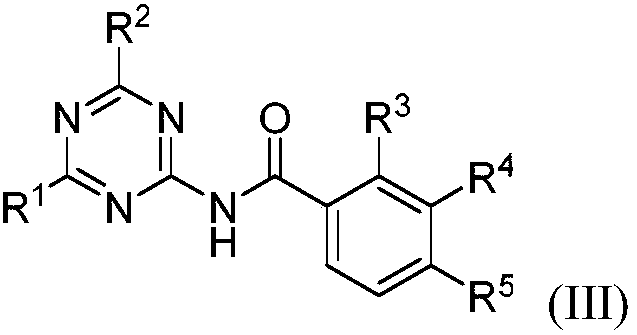
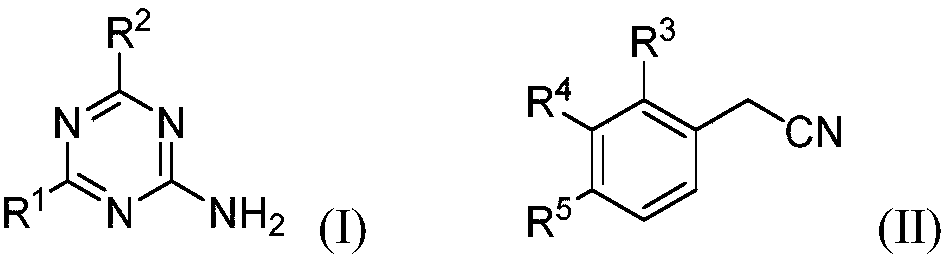
Aromatic amido replaced s-triazine compound and preparation and application
The technology of an arylamide group and compound is applied in the field of s-triazine compounds substituted with arylamide group and the preparation field thereof, which can solve the problems of difficult control of reaction conditions, poor yield, complicated steps and the like, and achieves wide industrial application prospect, The effect of easy operation and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
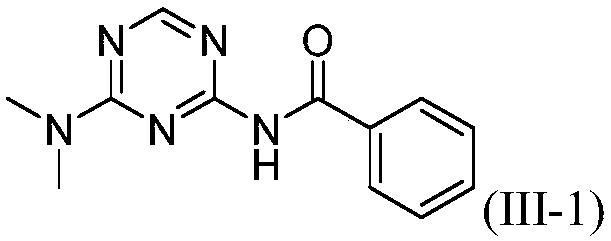
[0021] The preparation of embodiment 1 compound (III-1):
[0022] 2-Amino-4-dimethylamino-1,3,5-triazine (121.3mg, 0.87mmol), phenylacetonitrile 5a (200.3mg, 1.71mmol), CuBr (24.8mg, 0.17mmol) in o-di Mix in chlorobenzene (2.0mL), react at 125°C for 15h, after the reaction, add 50mL of water, extract with dichloromethane (20mL×3), combine the organic layers, dry with anhydrous sodium sulfate, filter, Concentrate, column chromatography (eluent is CH 2 Cl 2 : MeOH=30:1, V:V), collect R f The eluate with a value of 0.3-0.35 (monitored by TLC, the developing solvent is the same as the eluent), the solvent was distilled off under reduced pressure, and dried to obtain 169.2 mg of the target compound (III-1), with a yield of 80%. 1 H NMR (500MHz, CDCl 3 ):δ8.65(s,1H),8.38(s,1H),7.90-7.88(m,2H),7.60-7.56(m,1H),7.51-7.48(m,2H),3.21(s,3H ),3.19(s,3H)
[0023]
Embodiment 2
[0025] Change cuprous chloride to cuprous bromide (10.8mg, 0.07mmol), change the temperature to 135°C, and change the time to 30h. Other operations are the same as in Example 1. The yield is 137.5mg, and the yield is 65%.
Embodiment 3
[0027] Dichlorobenzene was changed to chlorobenzene (9 mL), the temperature was changed to 80° C., other operations were the same as in Example 1, and the yield was 52.9 mg, with a yield of 25%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com