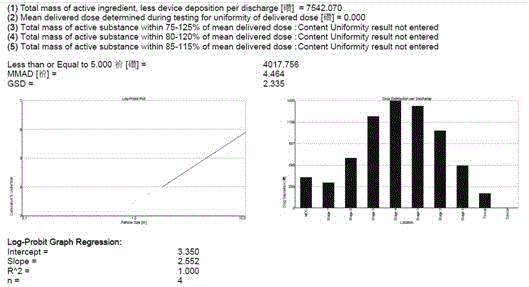
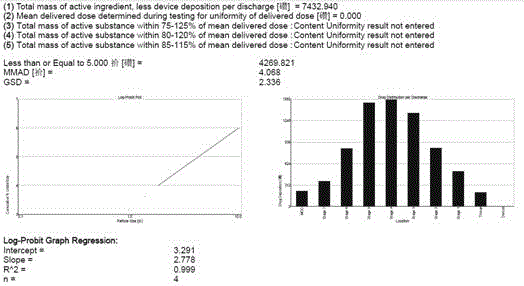
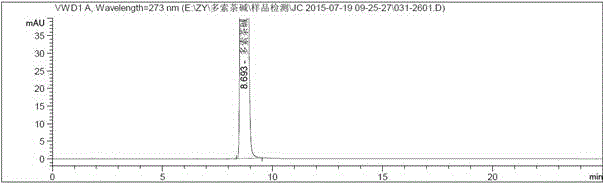
Aerosol inhalation preparation for treating bronchial asthma
A technology for bronchial asthma and atomization inhalation, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Doxofylline 1.0g tartaric acid 0.1g potassium chloride 0.7g sodium hydroxide Appropriate amount (adjust pH to 6.0) Add purified water to 100ml
[0029] Process: under heating, add all the above ingredients except sodium hydroxide and dissolve in pure water, adjust the pH of the solution to 6.0 with sodium hydroxide, filter the obtained solution through a sterile filter, and then fill the filtered medicinal solution in In 2-5ml ampoules, sterilize by high-pressure steam at 121°C for 15 minutes to obtain the doxofylline atomized inhalation preparation.
Embodiment 2
[0031] Doxofylline 1.0g Edetate disodium 0.05g Sodium chloride 0.9g sodium hydroxide Appropriate amount (adjust pH to 5.0) Add purified water to 100ml
[0032] Process: In the case of heating, add all the above ingredients except sodium hydroxide to dissolve in pure water, adjust the pH of the solution to 5.0 with sodium hydroxide, filter the obtained solution through a sterile filter, and then fill the filtered medicinal solution in In 2-5ml low borosilicate ampoule bottle, doxofylline atomized inhalation solution is obtained through aseptic treatment.
Embodiment 3
[0034] Doxofylline 1.0g Edetate disodium 0.1g Sodium chloride 0.7g sodium hydroxide Appropriate amount (adjust pH to 5.8) Add purified water to 100ml
[0035] Process: under heating, add all the above ingredients except sodium hydroxide and dissolve in pure water, adjust the pH of the solution to 5.8 with sodium hydroxide, filter the obtained solution through a sterile filter, and then fill the filtered medicinal solution in 2-5ml low-density polyethylene ampoule bottle, through aseptic treatment, the doxofylline atomized inhalation preparation is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com