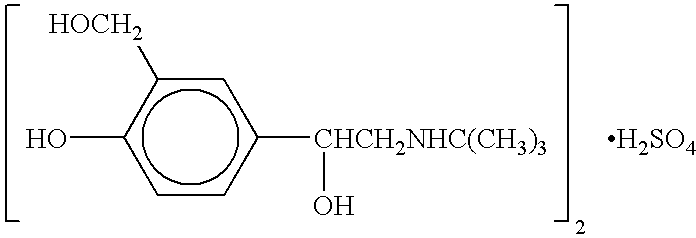
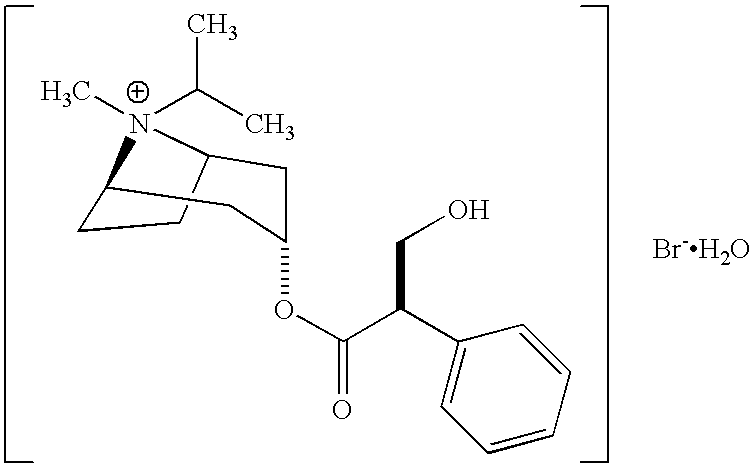
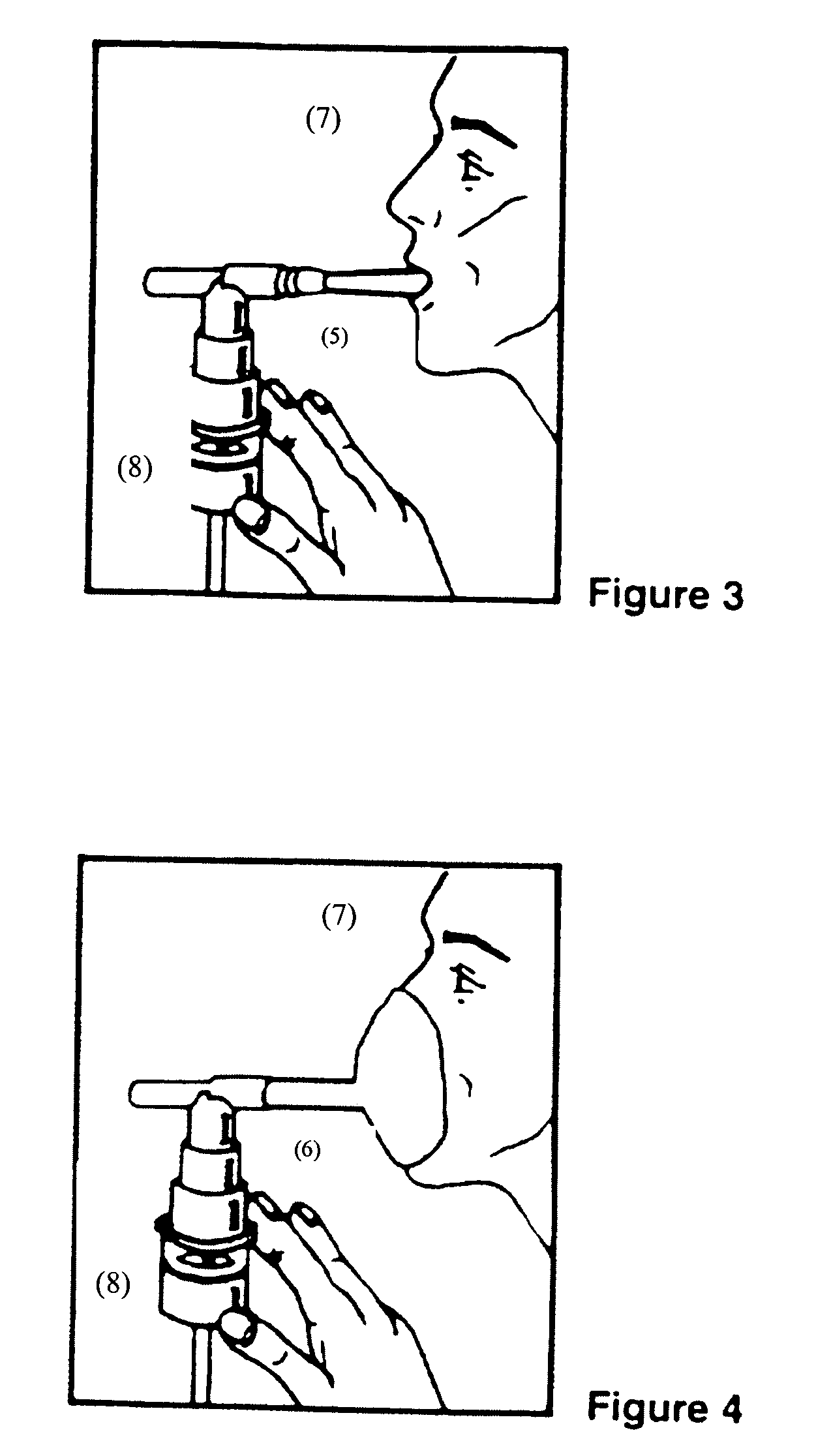
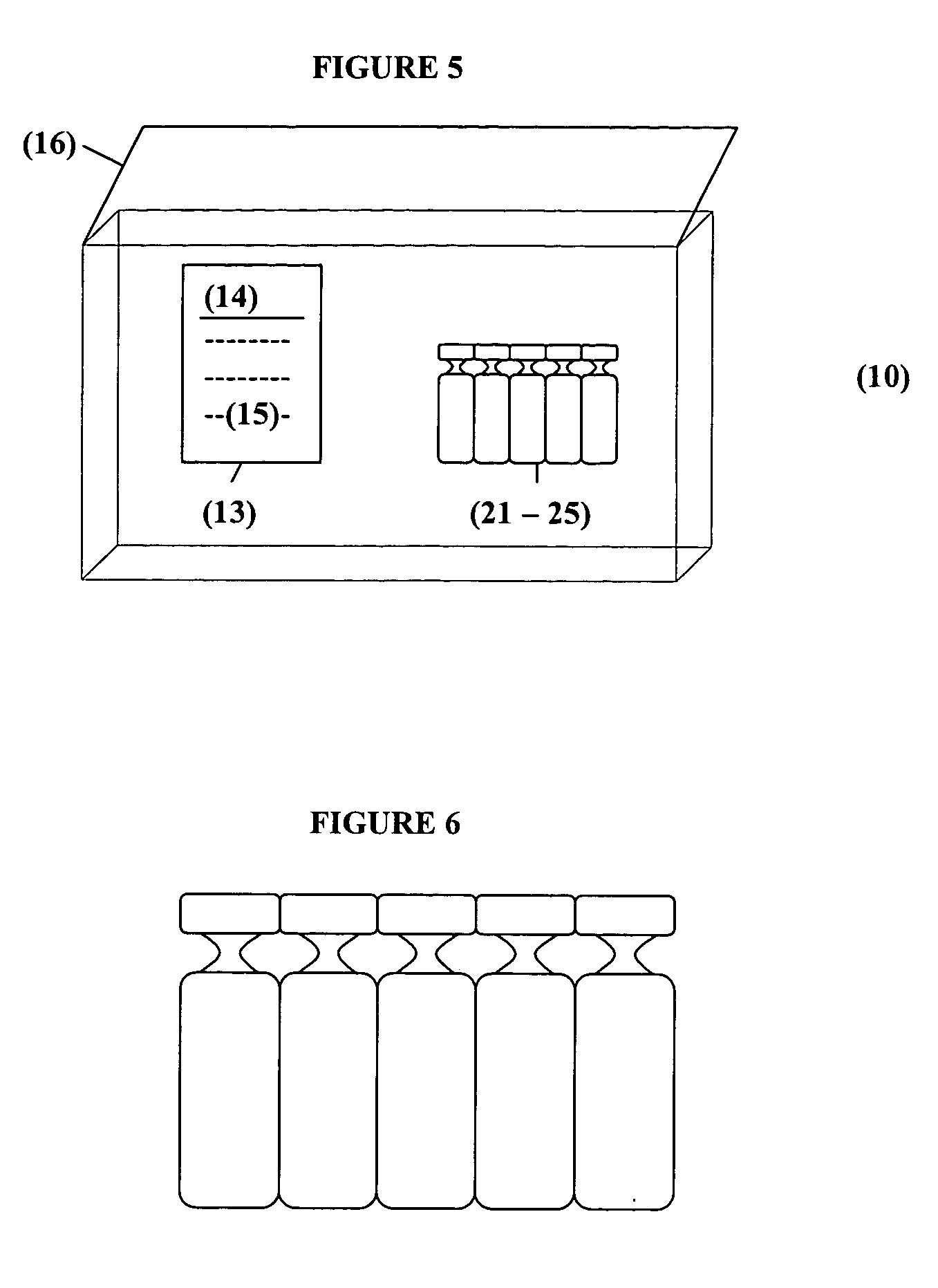
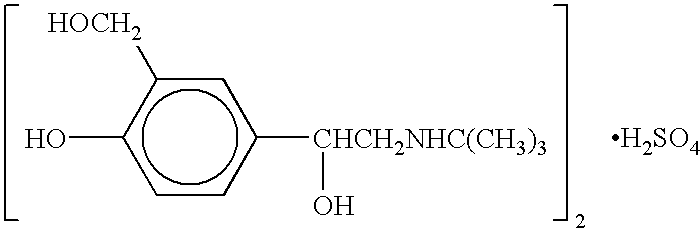
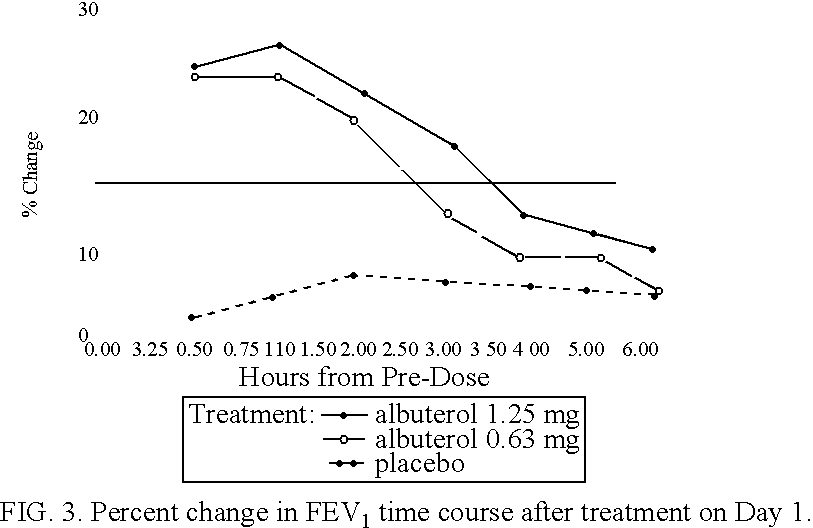
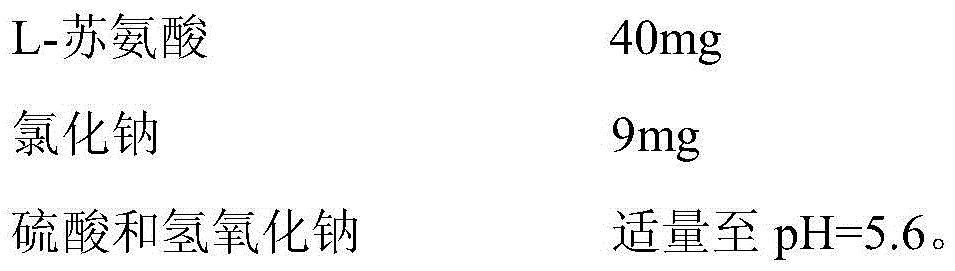Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
79 results about "Inhalation Solution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
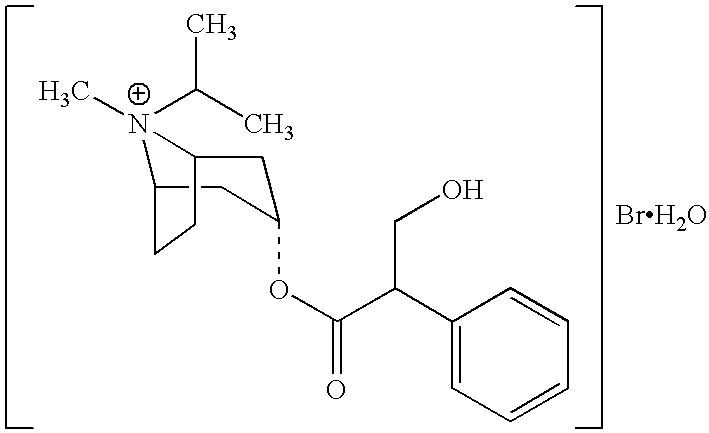
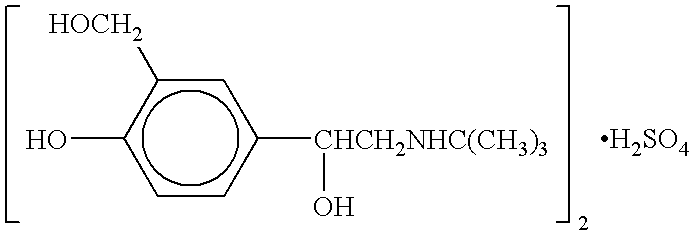
Liquid preparation containing tobramycin
The application describes a sterile aqueous inhalation solution containing the active agent tobramycin. The preparation has a high content of active agent (about 80 to 120 mg / ml of tobramycin) and contains an acidic adjuvant, but contains only a low concentration of sodium chloride (at most about 2 mg / ml). It can be injected or administered as an aerosol, for example with conventional nebuliser. It is particularly suitable for application in combination with a modern vibrating membrane nebuliser and allows the administration of a therapeutic single does in markedly less than 10 minutes.
Owner:PARI PHARMA GMBH
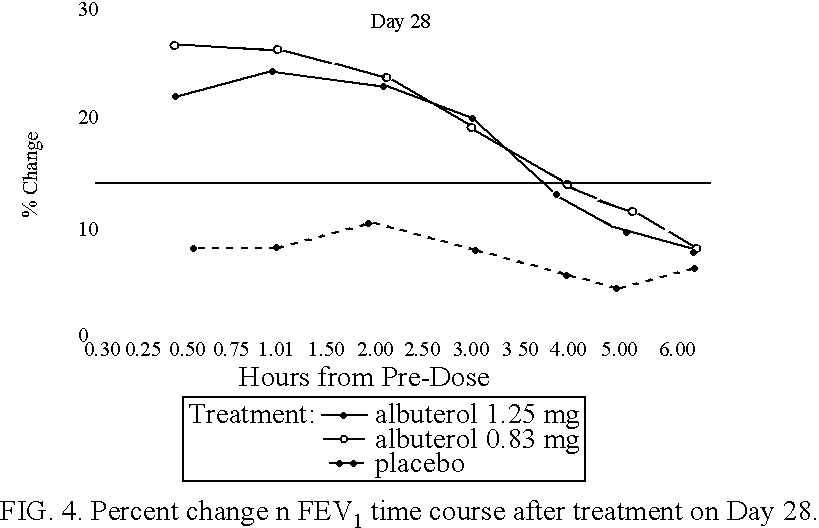
Albuterol inhalation solution, system, kit and method for relieving symptoms of pediatric asthma
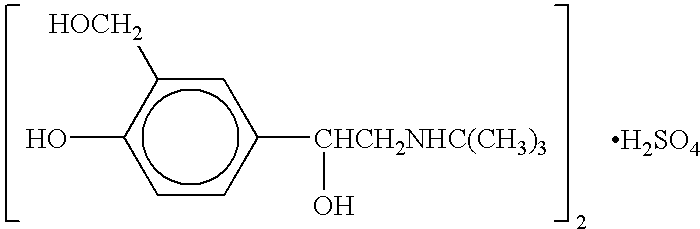
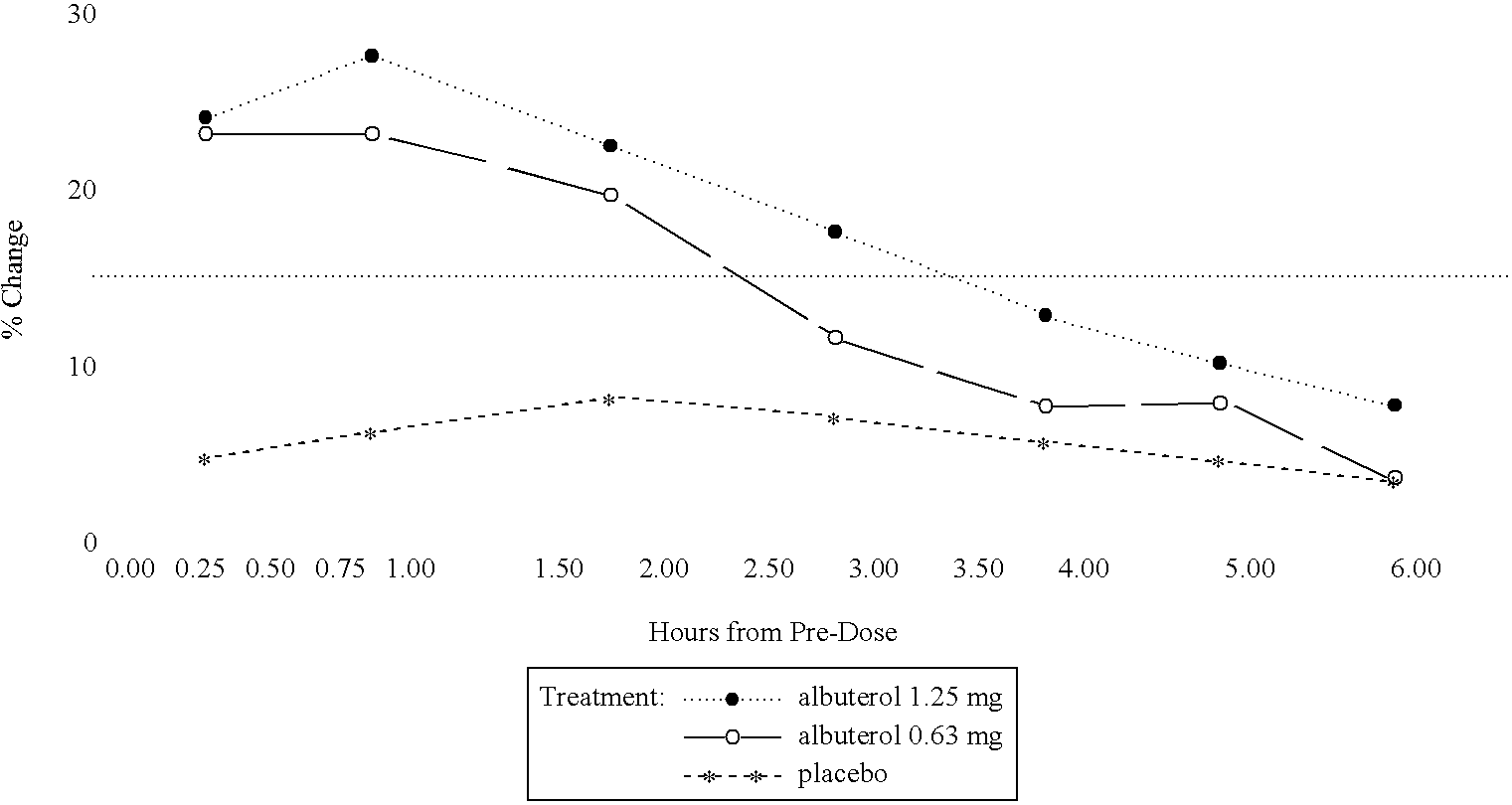
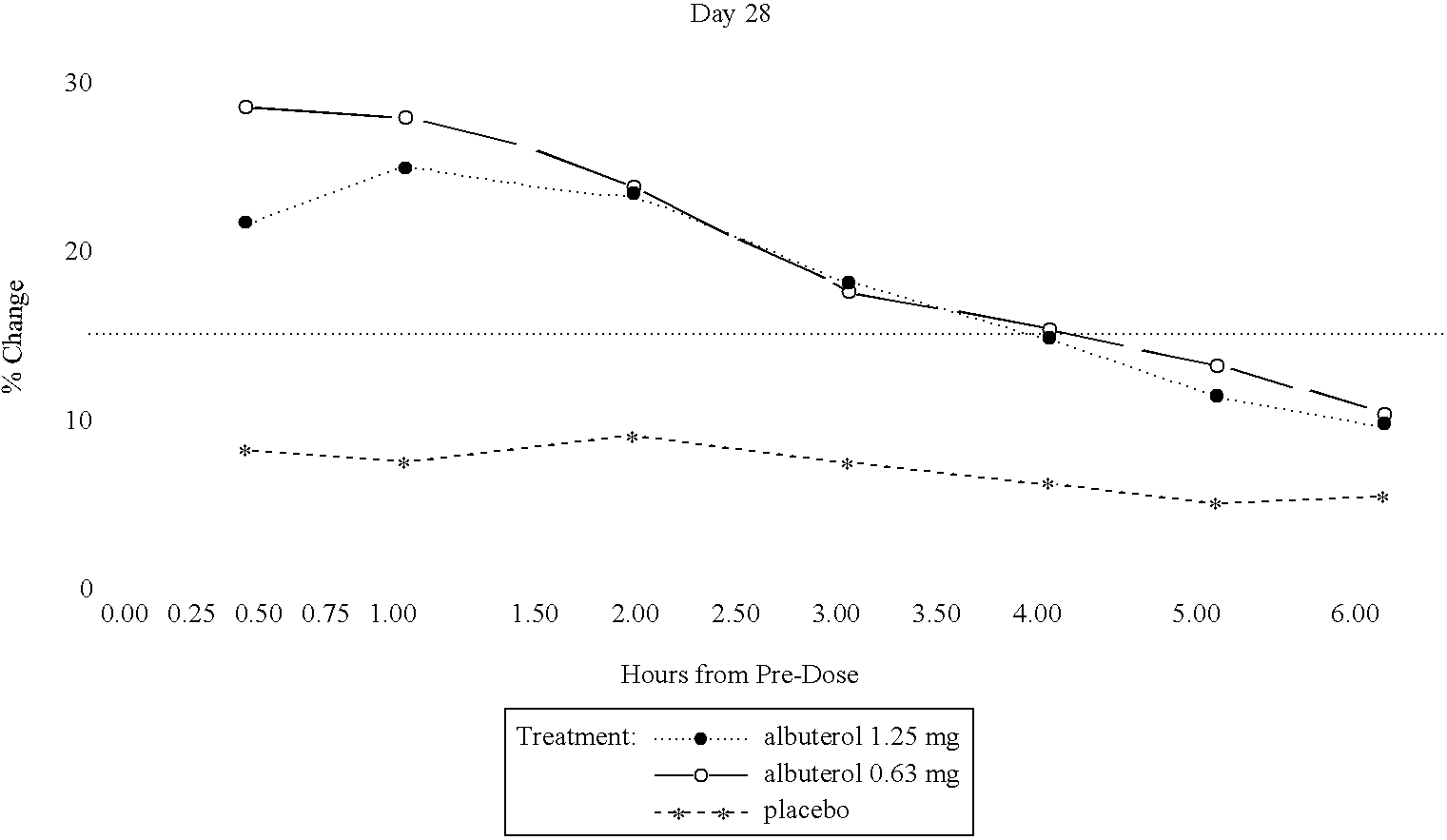
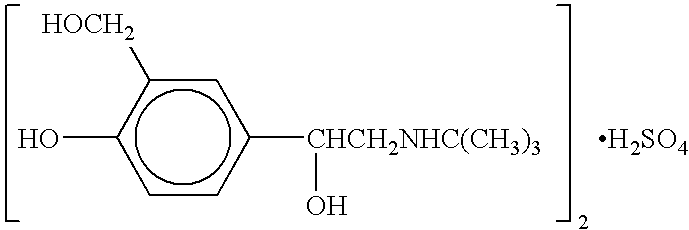
The present invention relates to an albuterol inhalation solution, system, kit and method for relieving bronchospasm in children suffering from asthma. In one alternative embodiment, the solution of the present invention is a sterile, premixed, premeasured single unit dose of albuterol for asthmatic patients 2 to 12 years of age. The present solution may be free of anti-microbial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 0.63 mg or about 1.25 mg albuterol.
Owner:MYLAN SPECIALTY
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
InactiveUS20030191151A1Relieve bronchospasmBiocideDispersion deliveryBenzalkonium chlorideSalbutamol
The present invention relates to a dual bronchodilator inhalation solution, system, kit and method for relieving bronchospasm in patients suffering from chronic obstructive pulmonary disease (COPD). In one alternative embodiment, the solution of the present invention is a prepackaged, sterile, premixed, premeasured single unit dose of albuterol and ipratropium bromide for patients suffering from COPD. The present solution may be free of antimicrobial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 2.50 mg albuterol and about 0.50 mg ipratropium bromide.
Owner:CHAUDRY IMTIAZ +1
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
InactiveUS6632842B2Relieve bronchospasmBiocidePowder deliveryBronchospasmObstructive Pulmonary Diseases
Owner:MYLAN SPECIALTY
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
InactiveUS20030149007A1Relieve bronchospasmBiocidePowder deliveryBronchospasmObstructive Pulmonary Diseases
The present invention relates to a dual bronchodilator inhalation solution, system, kit and method for relieving bronchospasm in patients suffering from chronic obstructive pulmonary disease (COPD). In one alternative embodiment, the solution of the present invention is a prepackaged, sterile, premixed, premeasured single unit dose of albuterol and ipratropium bromide for patients suffering from COPD. The present solution may be free of antimicrobial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 2.50 mg albuterol and about 0.50 mg ipratropium bromide.
Owner:MYLAN SPECIALTY
Treatment of chronic obstructive pulmonary disease with nebulized beta 2-agonist or combined nebulized beta 2-agonist and anticholinergic administration
InactiveUS20110132355A1Good curative effectExtended durationDispersion deliverySolution deliveryMuscarinic antagonistAnticholinergic agents
Inhalation solutions for administration of beta 2-agonists or combinations of muscarinic antagonists and beta 2-agonists for the treatment of breathing disorders, such as COPD, are provided. The inhalation solutions are administered by nebulization, particularly with a high efficiency nebulizer.
Owner:SUNOVION RESPIRATORY DEV
Albuterol inhalation solution, system, kit and method for relieving symptoms of pediatric asthma
The present invention relates to an albuterol inhalation solution, system, kit and method for relieving bronchospasm in children suffering from asthma. In one alternative embodiment, the solution of the present invention is a sterile, premixed, premeasured single unit dose of albuterol for asthmatic patients 2 to 12 years of age. The present solution may be free of anti-microbial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 0.63 mg or about 1.25 mg albuterol.
Owner:MYLAN SPECIALTY
Method and system for the treatment of chronic obstructive pulmonary disease with nebulized anticholinergic administrations
Inhalation solutions for administration of muscarinic antagonists for the treatment of breathing disorders, such as COPD, are provided.
Owner:SUNOVION RESPIRATORY DEV
Zanamivir inhalation solution and application thereof
InactiveCN101773491AIncrease deposition rateDoes not cause depositionOrganic active ingredientsPharmaceutical delivery mechanismPharmacyMedicine
The invention belongs to the fields of pharmacy and influenza, and discloses a zanamivir inhalation solution and the application thereof. The zanamivir inhalation solution is prepared by being added with a certain dosage of surface active agent and osmotic pressure regulator, and comprises the components by weight percent: 0.1-10wt percent of zanamivir, 0.01-0.5wt percent of surface active agent, 0.9-8wt % of osmotic pressure regulator and balance water. The inhalation solution is beneficial to forming aerosol granules with small grain diameter by ultrasonic atomization, and prevents the zanamivir liquid drops from merging and gathering after aerosol is formed, thus effectively transmitting the medicine into the lung of a patient, and ensuring the medicine to take the best effect.
Owner:JIANGSU SIMCERE PHARMACEUTICAL R & D CO LTD
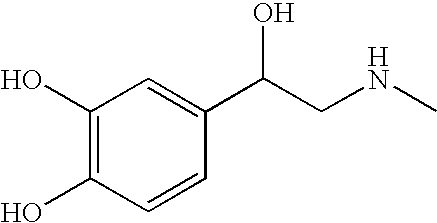
Injection-grade ambroxol hydrochloride and solution for inhalation of injection-grade ambroxol hydrochloride
InactiveCN102924302AImprove stabilityQuality improvementOrganic active ingredientsOrganic compound preparationInhalationAqueous ethanol
The invention relates to a method for refining injection-grade ambroxol hydrochloride. The method is characterized by comprising the steps of adding oral-taking-grade ambroxol hydrochloride with the proportion smaller than or equal to 20:1 into ethanol water solution with the volume ratio of 5-20%; performing heating to completely dissolve the oral-taking-grade ambroxol hydrochloride; stopping heating and performing cooling to seed out ambroxol hydrochloride; and filtering solvent to obtain crystals and drying the crystals to obtain the injection-grade ambroxol hydrochloride. The invention further relates to ambroxol hydrochloride solution for inhalation prepared by utilizing the prepared injection-grade ambroxol hydrochloride as the raw material. The atomization inhalation solution comprises the ambroxol hydrochloride, a stabilizer, a pH conditioning agent and an osmotic pressure conditioning agent. By means of the scientific preparation adopting the carrier, fewest degradation products of the atomization inhalation preparation can be produced under the high temperature of 121 DEG C for 15 minutes, the atomization inhalation preparation is stable, and the service life of the atomization inhalation preparation is prolonged to 5 years.
Owner:HC SYNTHETIC PHARMA CO LTD
Andrographis paniculata atomization inhalation solution preparation and preparation method thereof
ActiveCN107789340AAvoid damageImprove complianceDispersion deliverySolution deliveryDose ReducedSolvent
The invention relates to an andrographis paniculata atomization inhalation solution preparation, and belongs to the field of pharmaceutical pharmacy, wherein the andrographis paniculata atomization inhalation solution preparation comprises (1) an andrographis paniculata effective component, and (2) an isotonic agent and a solvent. According to the present invention, the prepared andrographis paniculata atomization inhalation solution preparation makes up for the gap in the current domestic market, and is specifically designed for atomization inhalation patients, wherein the andrographis paniculata atomization inhalation solution preparation is directly inhaled into the respiratory system in an atomized form after the andrographis paniculata atomization inhalation solution preparation is atomized by an ultrasonic atomizer or an air compression type atomizer, such that the use is convenient; and compared to the andrographis paniculata oral liquid, the andrographis paniculata atomizationinhalation solution preparation of the present invention has the different administration route, has advantages of use dose reducing, rapid effect and improved safety, and can well treat upper respiratory tract infections, sore throat, cough with lung heat and the like.
Owner:INCREASEPHARM HENGQIN INST CO LTD
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
InactiveUS20030203930A1Relieve bronchospasmBiocideDispersion deliveryBronchospasmObstructive Pulmonary Diseases
The present invention relates to a dual bronchodilator inhalation solution, system, kit and method for relieving bronchospasm in patients suffering from chronic obstructive pulmonary disease (COPD). In one alternative embodiment, the solution of the present invention is a prepackaged, sterile, premixed, premeasured single unit dose of albuterol and ipratropium bromide for patients suffering from COPD. The present solution may be free of antimicrobial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 2.50 mg albuterol and about 0.50 mg ipratropium bromide.
Owner:CHAUDRY IMTIAZ +1
Albuterol inhalation solution, system, kit and method for relieving symptoms of pediatric asthma
The present invention relates to an albuterol inhalation solution, system, kit and method for relieving bronchospasm in children suffering from asthma. In one alternative embodiment, the solution of the present invention is a sterile, premixed, premeasured single unit dose of albuterol for asthmatic patients 2 to 12 years of age. The present solution may be free of anti-microbial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 0.75 mg or about 1.5 mg albuterol sulfate.
Owner:DEY
Albuterol inhalation soultion, system, kit and method for relieving symptoms of pediatric asthma
InactiveUS20030140920A1Relieve bronchospasmRespiratorsOrganic active ingredientsBronchospasmSalbutamol
The present invention relates to an albuterol inhalation solution, system, kit and method for relieving bronchospasm in children suffering from asthma. In one alternative embodiment, the solution of the present invention is a sterile, premixed, premeasured single unit dose of albuterol for asthmatic patients 2 to 12 years of age. The present solution may be free of anti-microbial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 0.63 mg or about 1.25 mg albuterol.
Owner:DEY
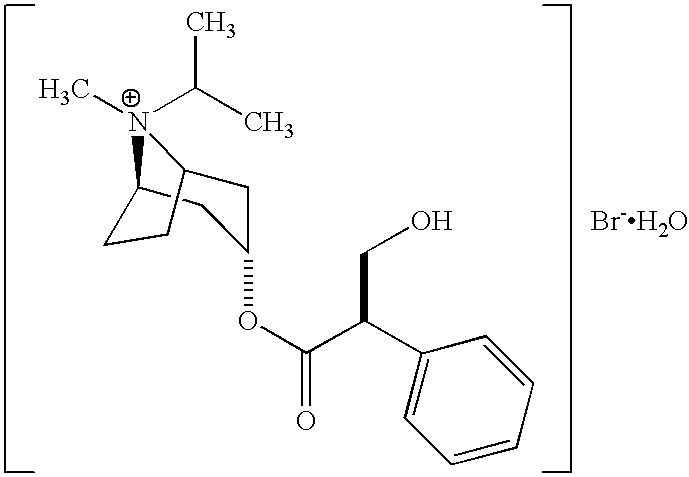
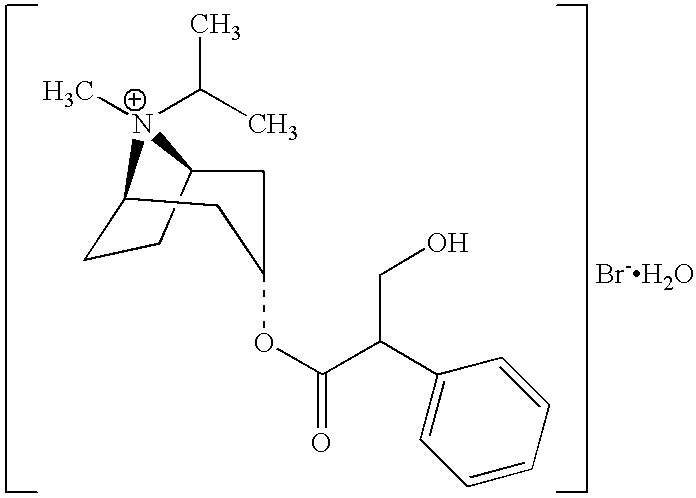
Tiotropium inhalation solution for nebulization
ActiveUS9757365B2Improve complianceQuality improvementDispersion deliveryAerosol deliveryDiseaseTiotropium bromide
Owner:GLENMARK SPECIALTY
Tiotropium inhalation solution for nebulization
ActiveUS9987260B2Improve complianceQuality improvementDispersion deliveryInorganic non-active ingredientsDiseaseTiotropium bromide
Owner:GLENMARK SPECIALTY +1
Exocarpium citri grandis produtive cough atomization inhalation solution preparation and preparation method thereof
InactiveCN107789556AThe treatment effect is easy to compareEfficacy standardDispersion deliverySolution deliveryCommon coldAir compression
The invention relates to an exocarpium citri grandis produtive cough atomization inhalation solution preparation, and belongs to the field of pharmaceutical pharmacy, wherein the exocarpium citri grandis produtive cough atomization inhalation solution preparation comprises (1) an exocarpium citri grandis produtive cough effective component, and (2) an isotonic agent and a solvent. According to thepresent invention, the prepared exocarpium citri grandis produtive cough atomization inhalation solution preparation makes up for the gap in the current domestic market, and is specifically designedfor atomization inhalation patients, wherein the exocarpium citri grandis produtive cough atomization inhalation solution preparation is directly inhaled into the respiratory system in an atomized form after the exocarpium citri grandis produtive cough atomization inhalation solution preparation is atomized by an ultrasonic atomizer or an air compression type atomizer, such that the use is convenient; and compared to the exocarpium citri grandis produtive cough liquid, the exocarpium citri grandis produtive cough atomization inhalation solution preparation of the present invention has the different administration route, has advantages of use dose reducing, rapid effect and improved safety, can well treat cough, asthma and excessive phlegm caused by phlegm stagnating lung, common cold, bronchitis and pharyngitis, and can provide other effects.
Owner:BEIJING INCREASEPHARM CORP LTD
Ambroxol hydrochloride atomized inhalation solution and preparation method thereof
InactiveCN108904476AAccurate measurementGood curative effectPowder deliveryOrganic active ingredientsPreservative freeSide effect
The invention discloses an ambroxol hydrochloride atomized inhalation solution and a preparation method thereof, and relates to the field of pharmaceutical nanotechnology. According to the ambroxol hydrochloride atomized inhalation solution, every 100ml of ambroxol hydrochloride atomized inhalation solution comprises 0.5-1g of ambroxol hydrochloride, 0.05-0.1g of clenbuterol hydrochloride, a buffer pair and isoosmotic adjusting agents and the balance of solvents. The single-dose specification of the ambroxol hydrochloride atomized inhalation solution is 2ml, measurement is accurate, and the curative effect is good; and the ambroxol hydrochloride atomized inhalation solution has no preservative, stable quality and little side effects. The preparation method of the ambroxol hydrochloride atomized inhalation solution comprises the steps that the ambroxol hydrochloride and the clenbuterol hydrochloride are added to part of the solvents and stirred well; and the buffer pair and the isoosmotic adjusting agents are added and stirred until dissolved, enough solvent is replenished after filtration, and fine filtration is performed. The process has good reproducibility and product stability,and is easy for large-scale industrial production.
Owner:JIANMIN PHARMA GRP CO LTD
Heparin aerosol inhalation solution preparation and preparation method thereof
InactiveCN109260181AImprove imbalanceImprove systemic blood coagulation abnormalitiesOrganic active ingredientsDispersion deliveryDiseaseObstructive Pulmonary Diseases
The invention discloses a novel atomized inhalation solution preparation of heparin pharmaceutically acceptable salt and derivative thereof and a preparation method thereof; a liquid preparation for atomizing inhalation comprises a pharmaceutically acceptable salt of heparin, a surfactant, an isotonic agent, an appropriate amount of a PH modulator and purify water, and can be used for treating lung diseases, in particular chronic obstructive pulmonary disease (COPD), acute lung injury and acute respiratory distress syndrome.
Owner:BEIJING INCREASE INNOVATIVE DRUG RESEARCH CO LTD
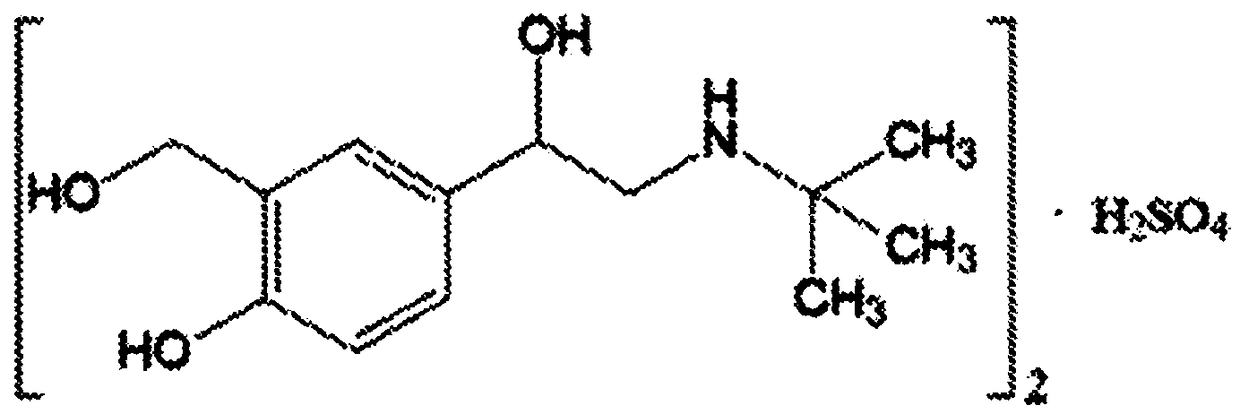
A kind of compound albuterol sulfate and ambroxol hydrochloride inhalation solution
InactiveCN102258504AImprove bioavailabilityIncrease blood concentrationOrganic active ingredientsPharmaceutical delivery mechanismSalbutamolInhalation
A compound salbutamol sulfate and ambroxol hydrochloride inhalation solution. The invention relates to an inhalation solution with salbutamol sulfate and ambroxol hydrochloride as active components and a preparation method thereof. The purpose of the present invention is to provide a kind of bioavailability that can improve medicine to vast patients and medical worker, bring into play medicine curative effect fully, reduce untoward reaction, preparation method is simple, is suitable for the new preparation of large-scale production -- salbutamol sulfate and hydrochloric acid Ambroxol inhalation solution. The present invention uses albuterol sulfate and ambroxol hydrochloride as active components, adds some specific types and proportions of auxiliary materials, and prepares the inhalation solution according to the conventional technology of pharmacy.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Tobramycin inhalation solution and preparing method
InactiveCN105534961AHigh percentage of missing expected valuesLow incidence of adverse reactionsAntibacterial agentsOrganic active ingredientsL-threonineStability study
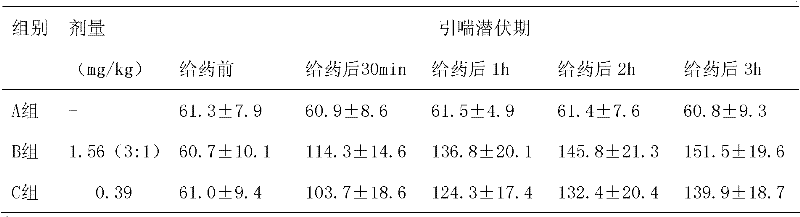
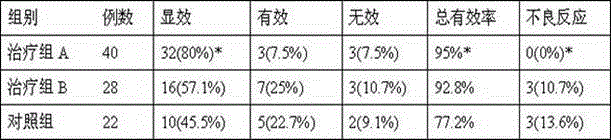
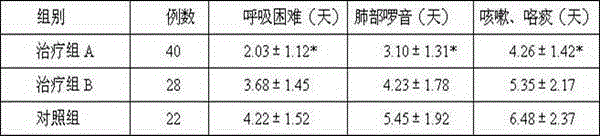
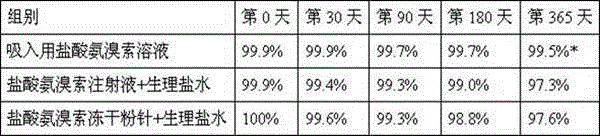
The invention discloses a tobramycin inhalation solution. The tobramycin inhalation solution comprises tobramycin and L-threonine which are dissolved in a 4.5mg / mL sodium chloride water solution, the concentration of tobramycin is 75mg / mL, the concentration of L-threonine is 20-30mg / mL, and pH of the inhalation solution is 5.6-6.0. According to the tobramycin inhalation solution, L-threonine is added, pH adjustment is conducted, and the long-term stability study shows that the inhalation solution is stable at least for 24 months at the indoor temperature in the end and is stable for 6 months on the acceleration condition. The FEV1% predicted value deletion proportion is far higher than that of public products, and the adverse reaction occurrence rate is obviously reduced.
Owner:孙红娟 +1
Tiotropium inhalation solution for nebulization
ActiveUS20160339003A1Improve the quality of lifeImproving user complianceDispersion deliveryAerosol deliveryDiseaseTiotropium bromide
The present invention relates to a sterile pharmaceutical composition comprising tiotropium or a pharmaceutically acceptable salt thereof, for inhalation via nebulization to a subject (e.g. a human). The invention also relates to a process for preparing the pharmaceutical composition and its use in the treatment of respiratory diseases such as chronic obstructive pulmonary disease (COPD) in a subject.
Owner:GLENMARK SPECIALTY
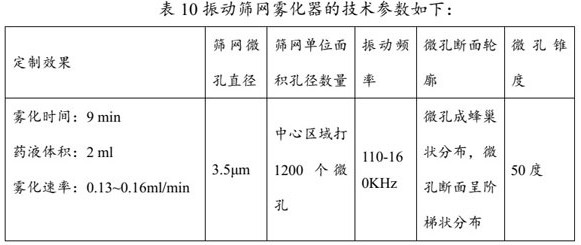
Medicine component containing tobramycin inhalation solution and application thereof
ActiveCN113952320AGood expansion effectReduce volumeOrganic active ingredientsDispersion deliveryPharmaceutical drugBronchial epithelium
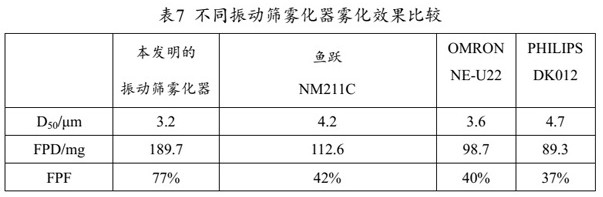
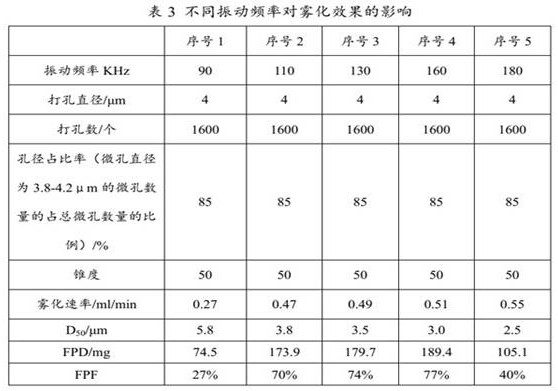
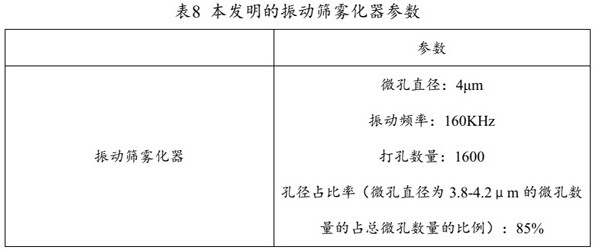
The present invention provides a medicine component containing a tobramycin inhalation solution and an application thereof. The medicine component comprises: (a) the tobramycin inhalation solution; and (b) the vibrating screen atomizer used in cooperation with the tobramycin inhalation solution. The central area of a metal mesh of the vibrating screen atomizer has 1400-1800 micropores. The fogdrop particle size of the tobramycin inhalation solution is as follows: D10 ranges from 0.5 [mu]m to 2.5 [mu]m, D50 ranges from 2.0 [mu]m to 4.2 [mu]m, and D90 ranges from 6.0 [mu]m to 9.0 [mu]m. The mass percentage of the particles with the aerodynamic particle size smaller than 5.39 [mu]m in the tobramycin inhalation solution is not smaller than 45%. The medicine component has a remarkable effect on clinically treating bronchiectasia.
Owner:JIANKANGYUAN PHARMA GROUP +1
Pharmaceutical assembly containing formoterol fumarate inhalation solution and application of pharmaceutical assembly
ActiveCN113768910AEasy to useEasy to carryOrganic active ingredientsDispersion deliveryPharmaceutical drugObstructive Pulmonary Diseases
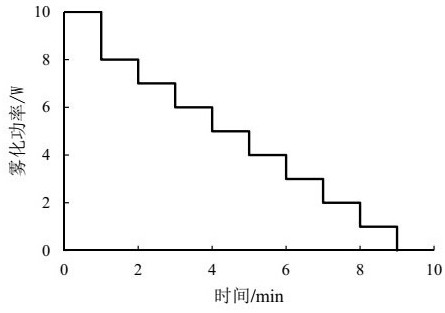
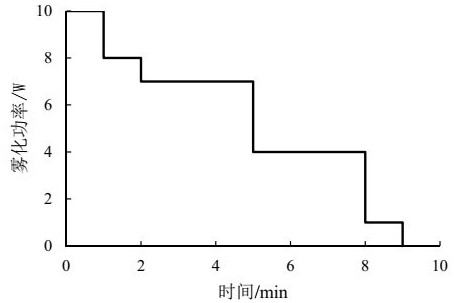
The invention relates to a pharmaceutical assembly containing a formoterol fumarate inhalation solution and an application of the pharmaceutical assembly. The pharmaceutical assembly comprises: (a) the formoterol fumarate inhalation solution; and (b) a vibrating screen atomizer used in cooperation with the formoterol fumarate inhalation solution, wherein the atomization power of the vibrating screen atomizer is decreased from 10 watts to 0 watt in a stepped decreasing mode, and the fogdrop particle sizes of the formoterol fumarate inhalation solution are as follows: D<10> is 0.5-1.5 [mu]m, D<50> is 2.5-4.5 [mu]m, and D<90> is 6.0-12.0 [mu]m. The mass percentage of the particles with the aerodynamic particle size smaller than 5.39 [mu]m in the formoterol fumarate inhalation solution is not smaller than 30%. According to the pharmaceutical assembly, the good effect of clinically treating the chronic obstructive pulmonary disease is achieved, and besides, the used vibrating screen atomizer has the advantages of being small in residual medicine liquid size, convenient to use and carry and low in noise.
Owner:JIANKANGYUAN PHARMA GROUP +1
Albuterol sulfate atomizing inhalation solution and preparation method thereof
InactiveCN108743569AImprove stabilityReduce riskOrganic active ingredientsDispersion deliveryAntioxidantAdditive ingredient
The invention belongs to the technical field of medicines, and particularly relates to an albuterol sulfate atomizing inhalation solution and a preparation method thereof. The albuterol sulfate atomizing inhalation solution is prepared from albuterol sulfate, sodium gluconate and a pH regulator. The albuterol sulfate atomizing inhalation solution provided by the invention has excellent stability,the content of the related substances is less, the ingredients are simple, excipients, such as a preservative, a stabilizer, an antioxidant, an osmotic pressure regulator and a metal ion complexing agent (edetate disodium), are not contained, consequently, the risk of clinical medication is decreased, and the safety of medication is improved; moreover, the preparation process is simple, high-temperature sterilization is not required, energy consumption is low, and the cost of mass production is reduced.
Owner:深圳大佛药业股份有限公司
Compositions for treatement of croup and methods of administering same
The invention relates to a racemic epinephrine inhalation solution, system, kit, and method for the treatment of croup. In particular, the racemic epinephrine solution is premixed, sterile, premeasured single unit dose of racemic epinephrine for the treatment of croup. More particularly, the racemic inhalation solution is free of antimicrobial preservatives and other preservatives. Additionally, the invention also relates to a comprehensive kit to administer racemic epinephrine aerosol therapy for the treatment of croup. More particularly, the invention relates to an inhalation therapy kit to provide a seamless conduit for a patient to receive in-office or hospital inhalation treatments and access to drugs and equipment for continuing home use.
Owner:PRE HLDG
A kind of ambroxol hydrochloride solution for inhalation
ActiveCN103462942BEasy dischargeImprove absorption efficiencyOrganic active ingredientsPharmaceutical delivery mechanismSolventPharmacology
The invention discloses an ambroxol hydrochloride solution for inhalation, which is characterized in that: every 100mL of solvent contains 0.15-0.3g of ambroxol hydrochloride, a surfactant whose mass is 4-6% of ambroxol hydrochloride and an appropriate amount of antioxidant and preservatives, the pH value of the solution is 4.6-5.8, wherein the solvent is selected from water for injection, physiological saline, and isotonic glucose solution. The ambroxol hydrochloride solution for inhalation of the present invention is sprayed into the oral cavity of a patient to form an aerosol, which directly enters the respiratory tract, has a high inhalation rate and a high local concentration, achieves safe and targeted treatment purposes, and effectively promotes the discharge of sputum in patients. The dosage is small, the drug effect is high, and the safety and stability are good, it is not easy to be polluted or oxidized and deteriorated, and the storage time is long.
Owner:赛隆药业集团股份有限公司
Umeclidinium bromide atomization inhalation solution and preparation method thereof
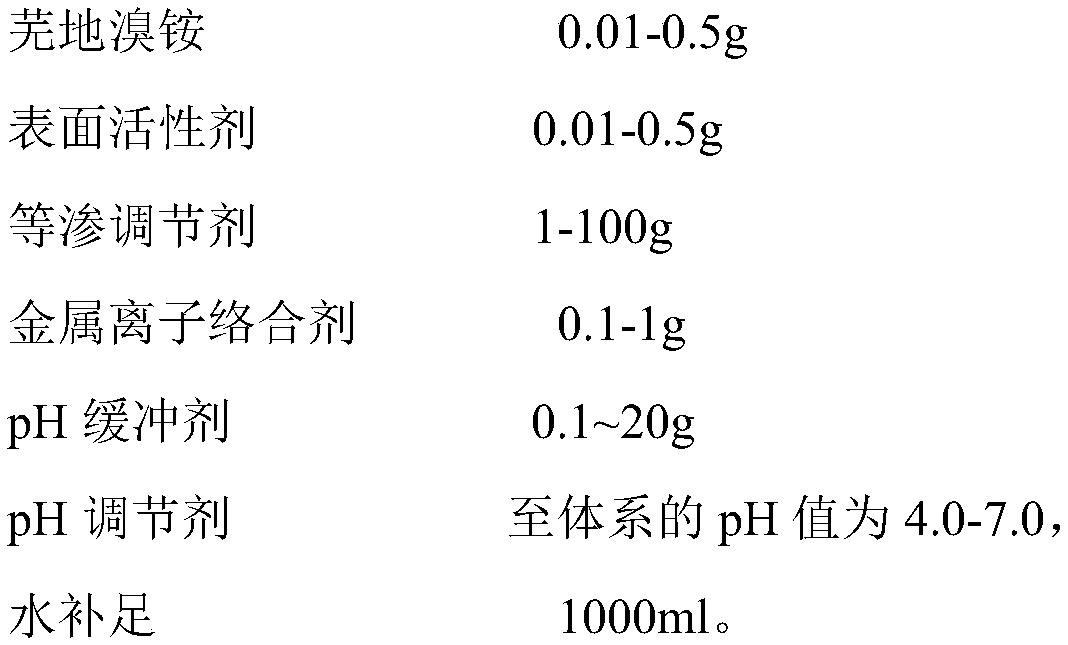
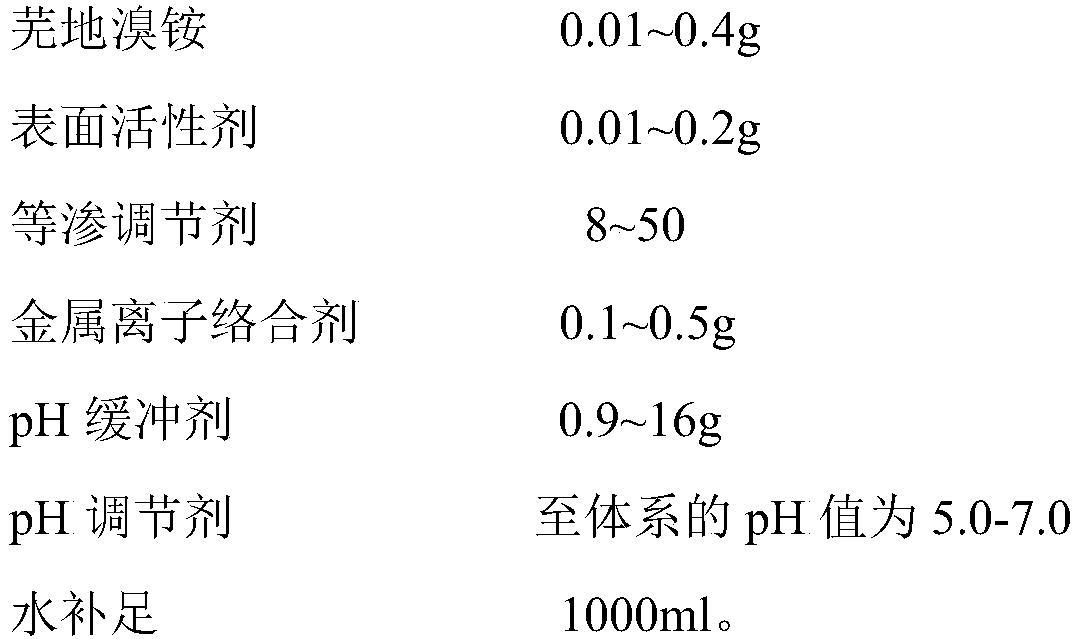
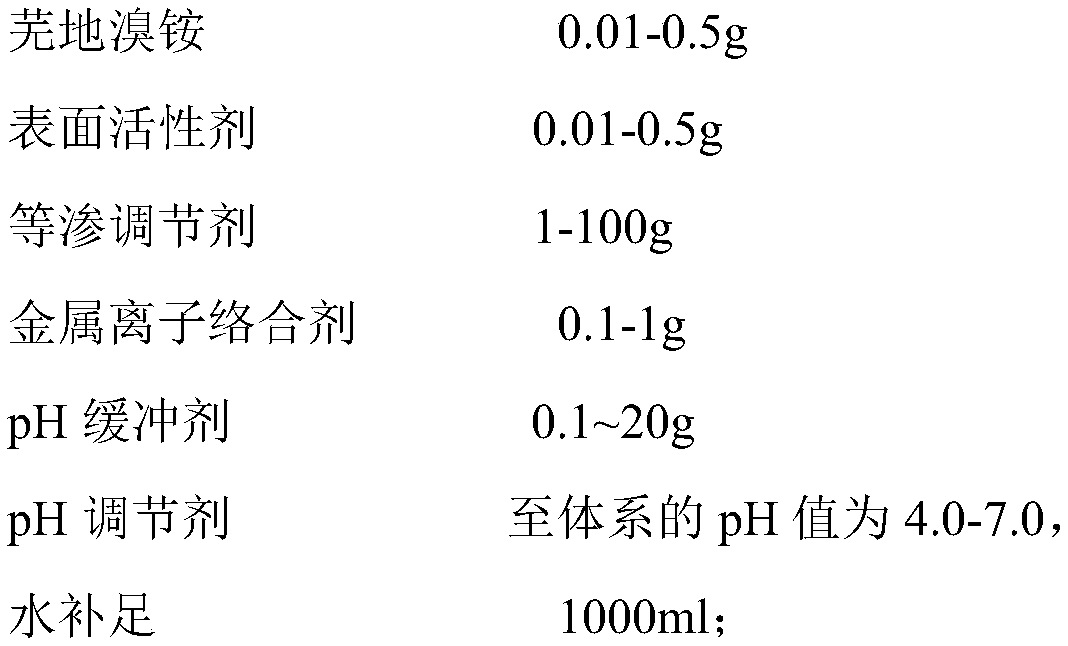
InactiveCN110292559AAvoid potential hazardsDelivery dose is accurate and controllablePowder deliveryOrganic active ingredientsSide effectBalance water
The invention discloses an umeclidinium bromide atomization inhalation solution and a preparation method thereof. The umeclidinium bromide atomization inhalation solution per 1,000 ml contains 0.01-0.5 g of umeclidinium bromide, 0.01-0.5 g of a surfactant, 1-100 g of an isoosmotic adjusting agent, 0.1-1 g of a metal ion complexing agent and 0.1-20 g of a pH buffering agent, a pH adjusting agent adjusting the pH of the system to 4.0-7.0 and the balance water, and the total amount is 100 ml. According to the umeclidinium bromide atomization inhalation solution and the preparation method thereof,not only can the potential harm of a lactose carrier in dry powder inhalation be avoided, but also the conveyed dosage is accurate and controllable, the requirement for the breathing air flow is low,and the compliance is good. The preparation method is simple to implement, the required equipment is simple, and the sterility requirement of the product can be met. According to the umeclidinium bromide atomization inhalation solution, the surfactant is added, and therefore side effects generated after the atomization inhalation solution is inhaled by a normal person in the atomization process are reduced, and the utilization rate of the medicine is also increased.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
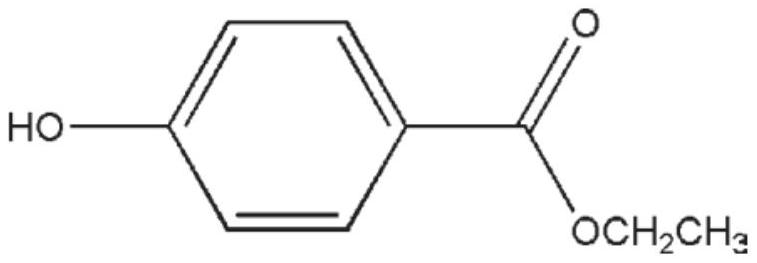
Application of ethyl p-hydroxybenzoate to resistance to coronavirus infection
PendingCN114146078AStrong noveltyGood effectOrganic active ingredientsAntiviralsEthyl hydroxybenzoateViral infectious disease
The invention belongs to the field of drug treatment, and particularly relates to application of ethyl p-hydroxybenzoate in preparation of drugs for preventing or treating virus infectious diseases. The invention discloses application of ethyl p-hydroxybenzoate and an inhalation preparation thereof in treatment of coronavirus infectious diseases. The new application of the ethyl p-hydroxybenzoate inhalation solution is verified in a mouse test infected with human coronavirus HCoV-229E, the technical scheme has novelty, a curative effect test of the m-cresol inhalation solution in treatment of pneumonia caused by HCoV-229E virus infection is carried out, and the result shows that the m-cresol inhalation solution can be used for treating pneumonia caused by HCoV-229E virus infection. The ethyl p-hydroxybenzoate inhalation solution shows a good drug effect on the lung index and the inhibition rate of a pneumonia mouse infected with the HCoV-229E virus, and the lung index of the mouse can be remarkably reduced.
Owner:INCREASEPHARM HENGQIN INST CO LTD
Oxygen-driven atomized inhalation solution for infant asthmatic suffocating pneumonia
InactiveCN102114027ARelieves spasmodic contractionsInhibition releasePharmaceutical delivery mechanismAntiviralsDiseaseSide effect
The invention belongs to the medical technology field, in particular discloses an oxygen-driven atomized inhalation solution for infant asthmatic suffocating pneumonia. The atomized inhalation solution is prepared by using budesonide, terbutaline sulfate, ambroxol hydrochloride, aminophylline and ribavirin as raw materials, respectively and proportionally compounding according to different characteristics of the medicaments, uniformly mixing, packaging into 5 ml / injection, sealing, sterilizing and labeling. The invention has the advantages of effectively relieving the symptoms of the infant asthmatic suffocating pneumonia, increasing the curative rate, shortening the course of disease, lessening adverse reactions, being convenient and safe to use, and the like. Tested by 150 clinical cases of infants with asthmatic suffocating pneumonia, the total curative rate reaches 92 percent without any special toxic or side effect.
Owner:李运智
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com