Ambroxol hydrochloride atomized inhalation solution and preparation method thereof
A technology of ambroxol hydrochloride and atomized inhalation, which is applied in aerosol delivery, pharmaceutical formulation, spray delivery, etc., can solve the problems of lack of systematic evaluation, etc., achieve good curative effect, good reproducibility, and easy large-scale industrial production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
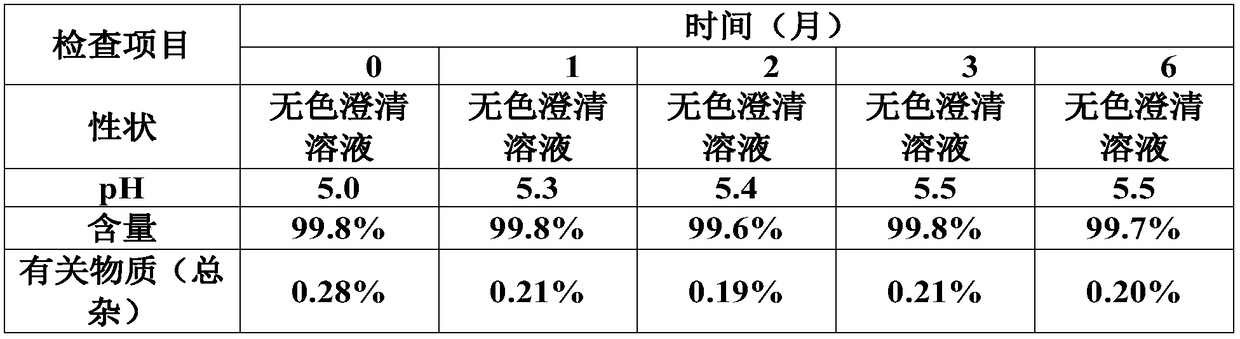
[0032] The embodiment of the present invention provides a kind of preparation method of the above-mentioned ambroxol hydrochloride atomization inhalation solution, and it comprises the following steps:
[0033] (1) Add ambroxol hydrochloride and clenbuterol hydrochloride into a solvent of 50%-80% of the recipe quantity, stir evenly, and the temperature is 50-70°C.
[0034] (2) Add the buffer pair, stir until it is completely dissolved, and the temperature is 50-70°C.
[0035] (3) Add isotonicity regulator and stir until dissolved.
[0036] (4) Add 0.01%-0.1% (w / v) of activated carbon for injection, the temperature is 50-70° C., stir for 15-30 min, and filter for decarburization.
[0037] (5) Add the solvent to the full amount, stir evenly, check the pH and content, and adjust the pH between 4.5-5.5. In this embodiment, a pH adjusting agent is used to adjust the pH value, and the pH adjusting agent includes at least one selected from the group consisting of citric acid, malic...
Embodiment 1
[0049] The present embodiment provides a kind of ambroxol hydrochloride atomization inhalation solution, which adopts the following preparation method to make:
[0050] (1) Add 1 g of ambroxol hydrochloride and 0.05 g of clenbuterol hydrochloride to 80 ml of water for injection, stir evenly, and the temperature is 60°C.
[0051] (2) Add citric acid-sodium citrate, stir until completely dissolved, and the temperature is 60°C.
[0052] (3) Add 2 g of sodium chloride and stir until dissolved.
[0053] (4) 0.05% activated carbon for injection was added, the temperature was 60° C., stirred for 20 min, and filtered for decarburization.
[0054] (5) Add water for injection to a total volume of 100ml, stir evenly, detect pH and content, and adjust pH to be between 5.
[0055] (6) Filter the above-mentioned medicinal liquid through a 1.0 μm titanium filter element, detect the pH value and content of the intermediate, and adjust and control the pH to be between 4 and 101%.
[0056] (...
Embodiment 2
[0060] The present embodiment provides a kind of ambroxol hydrochloride atomization inhalation solution, which adopts the following preparation method to make:
[0061] (1) Add 0.5 g of ambroxol hydrochloride and 0.1 g of clenbuterol hydrochloride into 50 ml of water for injection, stir evenly, and the temperature is 70°C.
[0062] (2) Add citric acid-disodium hydrogen phosphate, stir until completely dissolved, and the temperature is 70°C.
[0063] (3) Add 1 g of magnesium chloride and glucose, and stir until dissolved.
[0064] (4) 0.01% (w / v) of activated carbon for injection was added, the temperature was 70° C., stirred for 30 minutes, and filtered for decarburization.
[0065] (5) Add water for injection to a total volume of 100 ml, stir evenly, detect pH and content, and adjust pH between 5.5.
[0066] (6) Filter the above medicinal liquid through a 1.0 μm titanium filter element, detect the pH value and content of the intermediate, and adjust and control the pH to be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com