Preparation method of small-volume doxofylline freeze-dried powder injection as well as product and device thereof
A technology of doxofylline and freeze-dried powder injection, which is applied in freeze-dried delivery, powder delivery, and devices for making medicines into special physical or taking forms, etc., can solve the problem of increasing energy consumption, increasing packaging and transportation costs, etc. problems, to achieve the effect of reducing packaging volume, reducing energy consumption, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
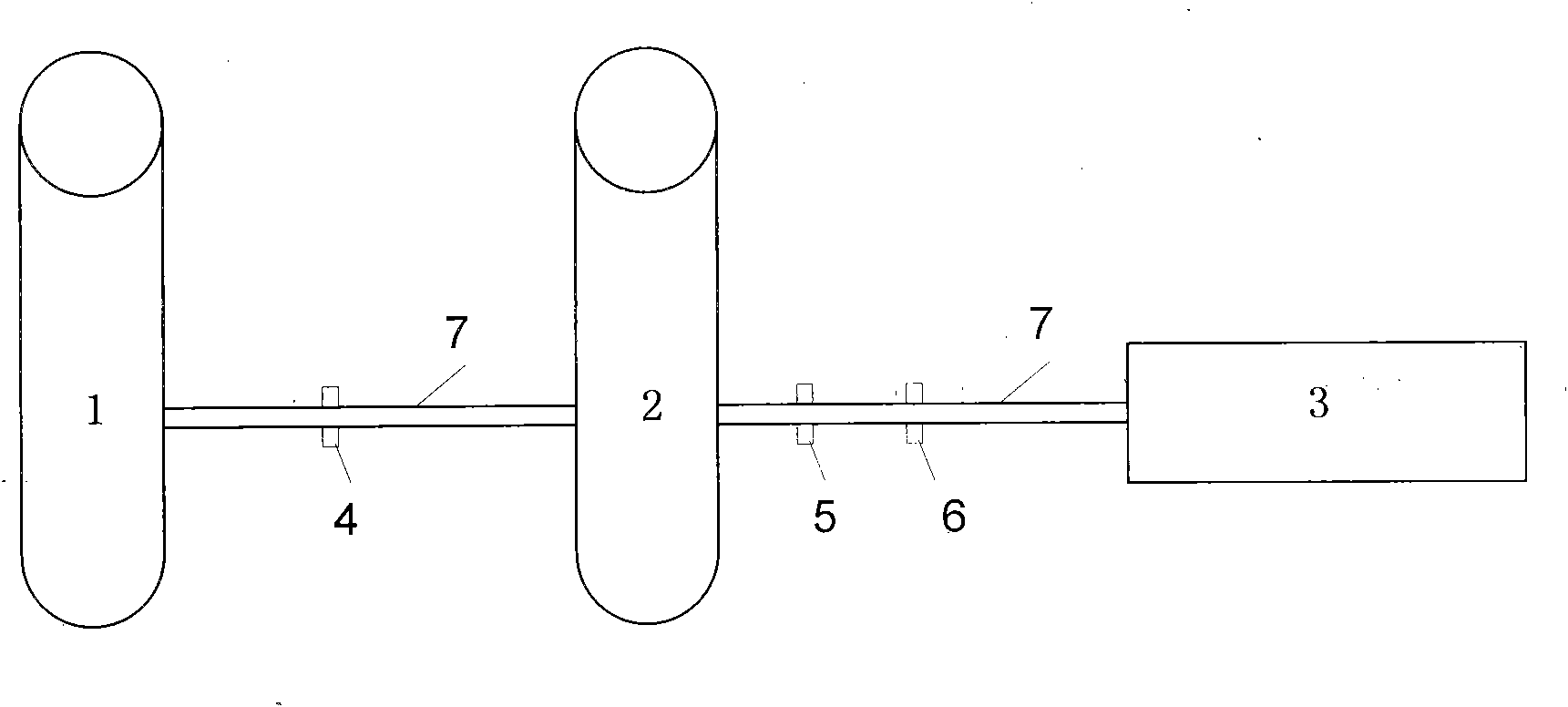
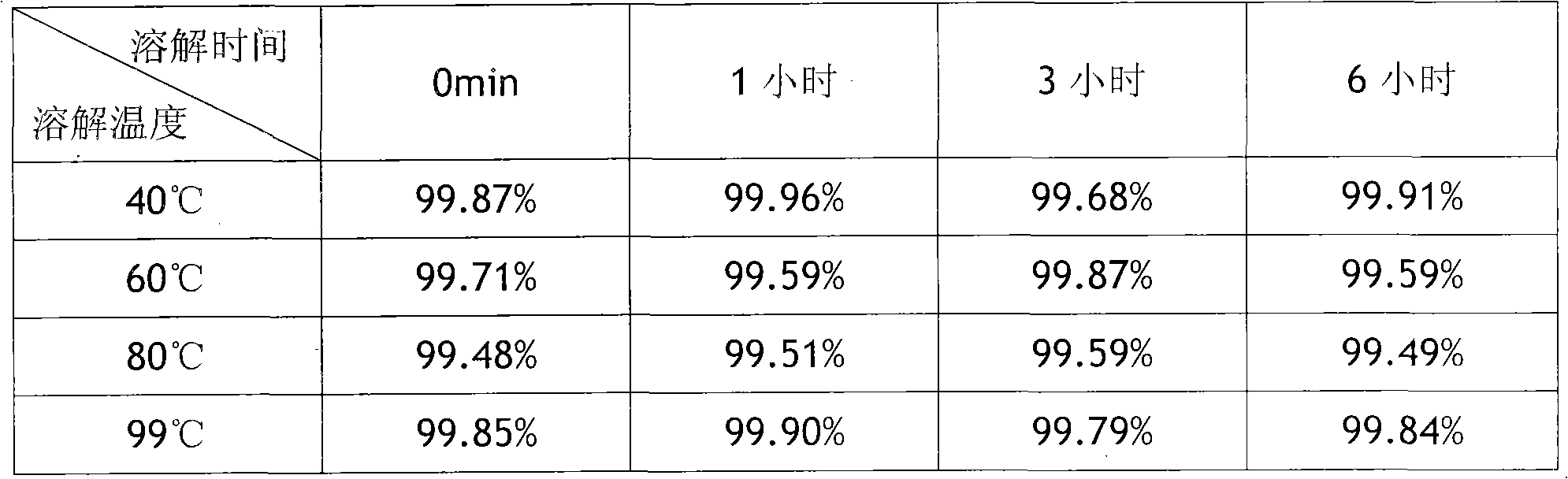
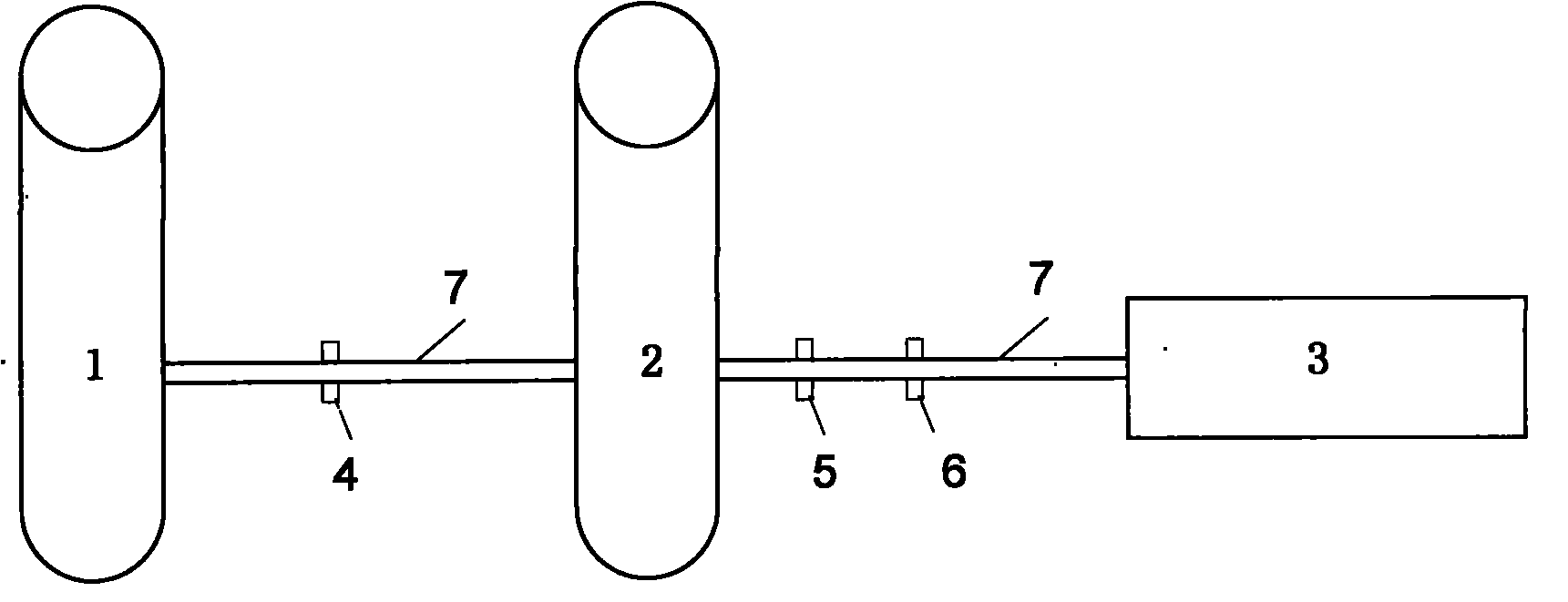
[0016] The preparation method of small-volume doxofylline freeze-dried powder injection, take 2kg of doxofylline and 2kg of mannitol, add 80°C water for injection to make a concentrated solution of 0.045g / ml, stir well to dissolve, add 0.3% Fully stir the needle with activated carbon, heat and boil for 30 minutes, filter with a 0.8um titanium rod (P≥0.6MPa), adjust the pH value to 5.5-6.5, take samples to determine the content and bacterial endotoxin, and add the qualified filtrate to the remaining preparation amount Dilute 80°C water for injection to make a dilute solution of 0.038g / ml, keep the temperature at not lower than 80°C, stir thoroughly for 20 minutes, filter through a 0.45μm microporous membrane (P≥0.23MPa), and then use 0.22 um microporous membrane for terminal filtration (P≥0.39MPa), and finally sent to the insulation storage tank through the pipeline, the temperature is kept at not lower than 80 ℃, 7.895ml is filled into a 20ml ampoule, freeze-dried, pressed Jus...
example 2
[0019] The preparation method of small-volume doxofylline freeze-dried powder injection is to take 2kg of doxofylline and 6kg of lactose, add 99°C water for injection to make a concentrated solution of 0.050g / ml, fully stir to dissolve, and add 0.3% of Stir well with activated carbon for needles, heat and boil for 30 minutes, filter with a 0.8um titanium rod (P≥0.6MPa), adjust the pH value to 5.5-6.5, take samples to determine the content and bacterial endotoxin, and add the qualified filtrate to the remaining preparation amount 99 ℃ dilute with water for injection to make a dilute solution of 0.042g / ml, keep the temperature at not lower than 80℃, stir thoroughly for 20 minutes, filter through a 0.45μm microporous membrane (P≥0.23MPa), and then use 0.22um The microporous filter membrane is used for terminal filtration (P≥0.39MPa), and finally sent to an insulated storage tank through a pipeline, and the temperature is kept at not lower than 80°C. Fill 7.14ml into a 20ml ampoule...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com