Anti-bronchial asthma doxofylline injection
A technology for bronchial asthma and doxofylline, which is applied in the field of medicine, can solve problems such as discoloration, and achieve the effects of improved stability of preparations, stable and controllable quality, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
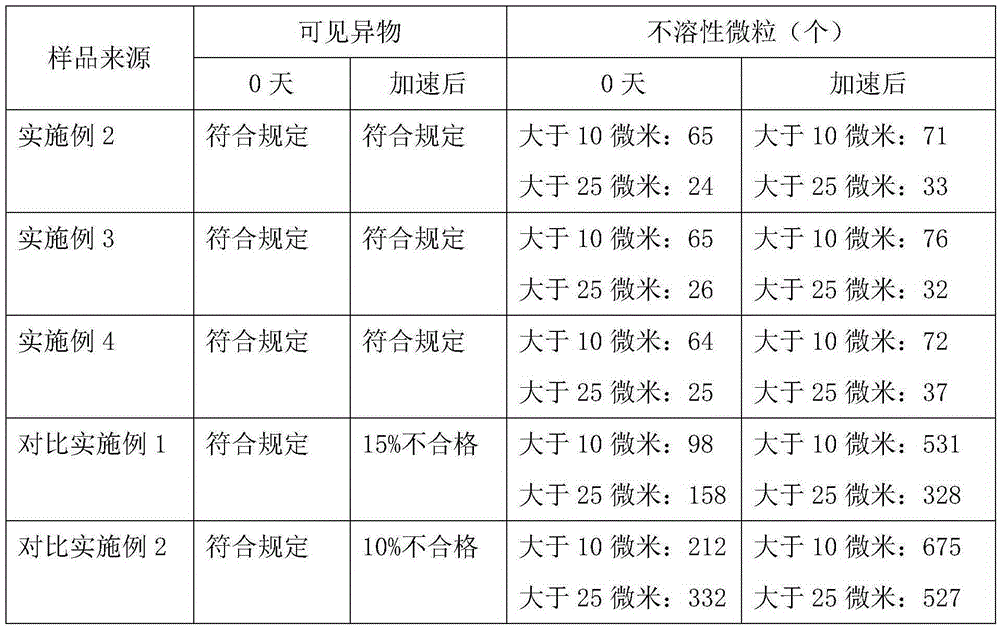
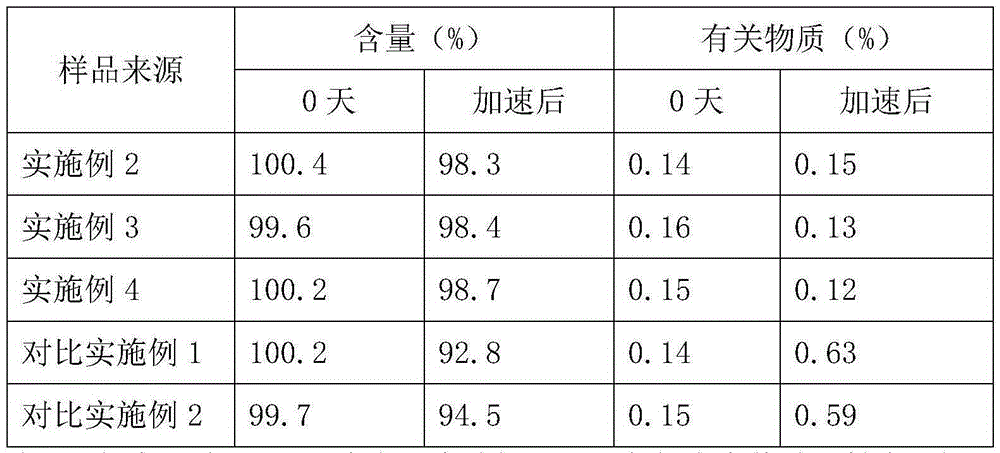
Embodiment 1
[0021] Embodiment 1: Preparation of doxofylline crystal
[0022] Dissolve doxofylline in a mixed solvent of methanol and dimethyl sulfoxide whose volume is 6 times the weight of urapidil at 35°C. The volume ratio of methanol to dimethyl sulfoxide is 5:1.5; Add the mixed solvent of butanol and ether whose volume is 9 times of the weight of doxofylline at a high speed, the volume ratio of butanol and ether is 2:3, stir while adding, control the temperature at 35°C, grow the crystal for 3 hours; then Add petroleum ether whose total volume is 7 times the weight of doxofylline at a speed of 20ml / min, and after growing the crystal for 1 hour, cool down to -5°C at a speed of 10°C / hour, and then maintain a stirring speed of 90 rpm Stir for crystallization and crystal growth for 3 hours; filter, wash, and dry under reduced pressure to obtain doxofylline crystal compound.
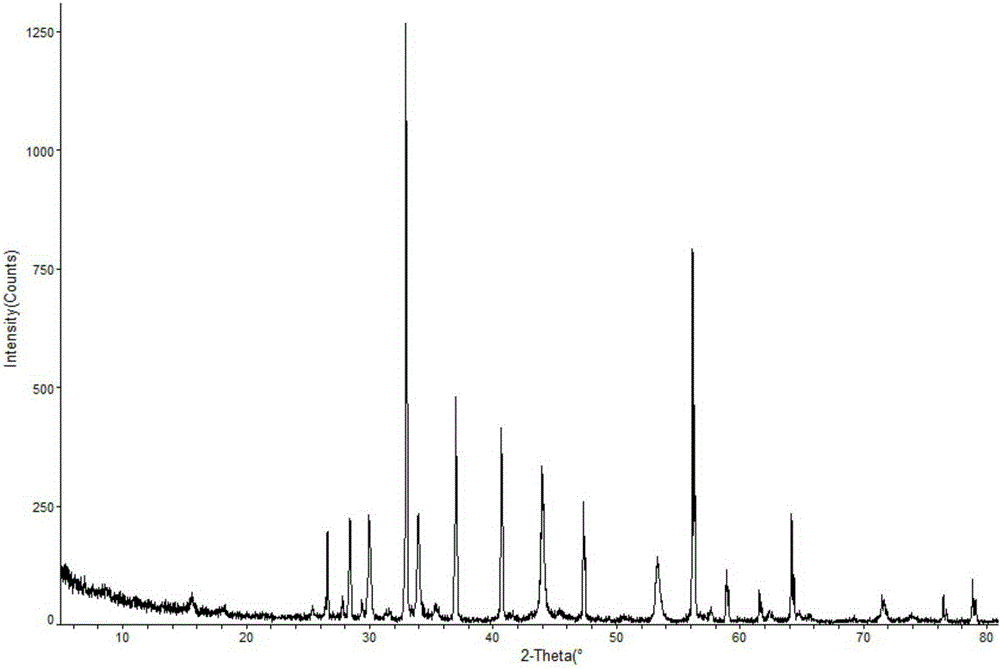
[0023] The prepared doxofylline crystal is measured by powder X-ray diffractometry, and the X-ray powder diffract...
Embodiment 2
[0024] Embodiment 2: prescription: doxofylline 2.5g, lactic acid 1.5g, 4g polyethylene glycol 400, propylene glycol 6ml, ethanol 1ml, antioxidant 0.03g, EDTA0.001g,
[0025] Preparation method: 1) mix and dissolve doxofylline with polyethylene glycol 400 and propylene glycol, and dissolve the polyethylene glycol 400 and propylene glycol solution of doxofylline; 2) mix and dissolve sodium thiosulfate with appropriate amount of water for injection, Obtain sodium thiosulfate solution; 3) Mix and dissolve lactic acid and an appropriate amount of water for injection to obtain a lactic acid solution; 4) Mix and dissolve EDTA and ethanol to obtain an EDTA ethanol solution; 5) Combine step 2), step 3) and step 4) The prepared solutions were added to the solution prepared in step 1) successively under stirring, mixed and stirred evenly; 6) 0.05% (w / v) activated carbon for needles was added to the solution obtained in step 5), and kept at 60°C Stir and adsorb for 10 minutes, filter and ...
Embodiment 3
[0027] Embodiment 3: prescription: doxofylline 4g, lactic acid 0.5g, 6g polyethylene glycol 400, propylene glycol 3ml, ethanol 1.5ml, antioxidant 0.03g, EDTA0.02g,
[0028] Preparation method: 1) mix and dissolve doxofylline with polyethylene glycol 400 and propylene glycol, and dissolve the polyethylene glycol 400 and propylene glycol solution of doxofylline; 2) mix and dissolve sodium thiosulfate with appropriate amount of water for injection, Obtain sodium thiosulfate solution; 3) Mix and dissolve lactic acid and an appropriate amount of water for injection to obtain a lactic acid solution; 4) Mix and dissolve EDTA and ethanol to obtain an EDTA ethanol solution; 5) Combine step 2), step 3) and step 4) The prepared solutions were added to the solution prepared in step 1) successively under stirring, mixed and stirred evenly; 6) 0.5% (w / v) activated carbon for needles was added to the solution obtained in step 5), and kept at 40°C Stir and adsorb for 30 minutes, filter and de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com