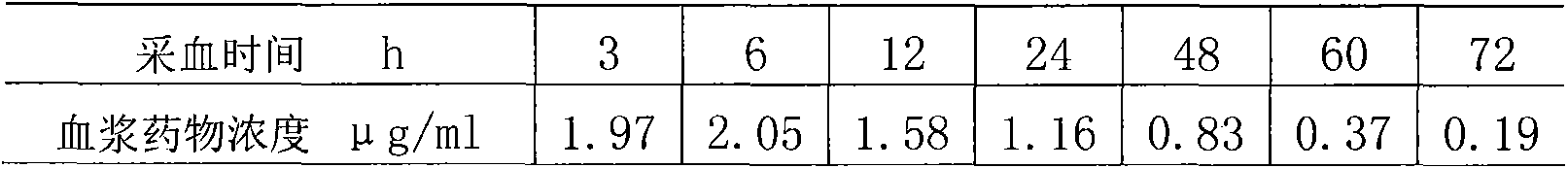Oil injection containing antibacterial agents/polyethylene glycol drug-loading particles
A technology of drug-loaded particles and antibacterial drugs, which is used in antibacterial drugs, drug delivery, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, preparation 8% ceftiofur hydrochloride injection
[0027] Preparation composition: every liter contains 90% ceftiofur hydrochloride 90g, PEG-10000 50g, H-HPC 25g, soybean oil for injection added to the final volume.
[0028] Preparation method: (1) Melt PEG at 60-70°C, add H-HPC and mix well, then add appropriate amount of methanol, after H-HPC dissolves and cool down to room temperature, add ceftiofur hydrochloride, mix well, remove under reduced pressure Clean methanol, cool, solidify, pulverize, and pass through a 40-mesh sieve to obtain drug-loaded particles containing ceftiofur hydrochloride. (2) Disperse the drug-loaded particles in some soybean oil, grind them through a colloid mill until the particle size is less than 50 μm, and further grind them with a sand mill until the particle size is less than 10 μm, add the remaining medium, and use a high-shear homogenizer to Under the condition of about 5000rpm, after several times of homogenization, cef...
Embodiment 2
[0029] Embodiment 2, preparation 8% cefquinol sulfate injection
[0030] Preparation composition: 90% cefquinol sulfate 90g, PEG-6000 90g, corn oil for injection is added to 1 liter.
[0031] Preparation method: (1) Melt PEG at 60-70°C, add cefquinol sulfate superfine powder, mix well, cool and solidify, pulverize, pass through a 40-mesh sieve to obtain drug-loaded particles containing cefquinol sulfate. (2) Disperse the drug-loaded particles in part of corn oil, grind them through a colloid mill until the particle size is less than 100 μm, and further grind them with a sand mill until the particle size is less than 20 μm, add the remaining medium, and use a high-shear homogenizer to Under the condition of about 5000rpm, after several times of homogenization, the ceftiofur hydrochloride injection with a particle size of less than 20 μm was prepared.
Embodiment 3
[0032] Embodiment 3, preparation 15% oxytetracycline hydrochloride injection
[0033] Preparation composition: every 100ml injection contains oxytetracycline hydrochloride 15g, PEG-10000 / HPMC (2:1) solid solution 6g, BHA 0.01g, BHT 0.01g, PG 0.005g, EDTA-2 sodium 0.15g, IPM added to the final volume.
[0034] Preparation method: disperse oxytetracycline hydrochloride and solid solution in part of IPM, grind with colloid mill and sand mill until the particle size is less than 10 μm, add antioxidant and remaining medium, and homogenize with high-shear homogenizer This preparation.
[0035]The preparation is intramuscularly injected into pigs, and the dosage is 15mg / kg b.w., and the blood drug concentration is still maintained in the effective concentration range (1-2 micrograms / m1) 48 hours after administration.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com