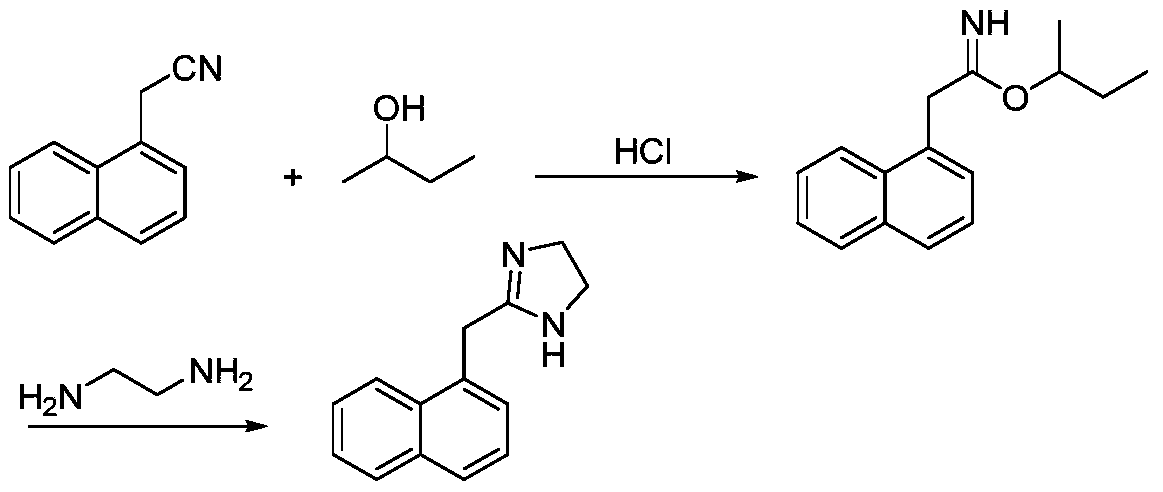
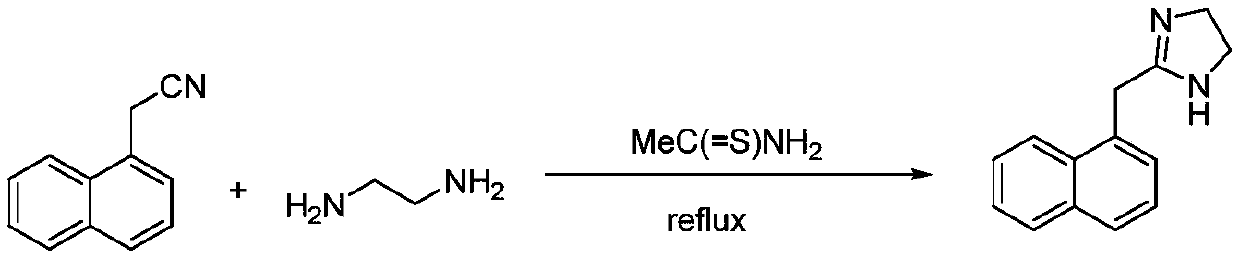
Preparation method of naphazoline hydrochloride
A technology of naphazoline hydrochloride and naphazoline, applied in directions such as organic chemistry, can solve problems such as unsatisfactory product purity and yield, and achieve the effects of good powder shape, high industrialization, and high yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Adopt the following steps to prepare naphazoline hydrochloride:
[0044] (A) Add 1000g (6.0mol) of naphthaleneacetonitrile to the reaction kettle, add 5000ml of ethylenediamine, add CS 2 45.7g (0.6mol), heat up to 110°C, stir for 2h; cool down to room temperature, add 10L of water dropwise at a rate of 1L / min, precipitate a solid, and filter to obtain naphazoline free base.
[0045] (B) Naphazoline free base was dissolved by adding 8L of acetonitrile, and 25% hydrochloric acid was added dropwise at room temperature to adjust the pH to 2, and a solid precipitated, which was filtered to obtain 1399.3 g of naphazoline hydrochloride crude product.
[0046] (C) Add 5L of methanol to the crude product in the reaction kettle, heat to 70°C, add activated carbon after dissolving, heat up to 70°C for decolorization, filter, add 1L of acetone dropwise to the filtrate, cool the filtrate, keep warm and crystallize overnight at 25°C , centrifuged, rinsed with acetone, dried under va...
Embodiment 2
[0048] Adopt the following steps to prepare naphazoline hydrochloride:
[0049] (A) Add 1000g (6.0mol) of naphthaleneacetonitrile to the reaction kettle, add 3000ml of ethylenediamine, add Na 2 S 23.4g (0.3mol), MgCl 2 .6H 2 O 10.1g (0.05mol), heated to 90°C, stirred for 10h. Cool down to 1.5°C, add 8L of water dropwise at a rate of 0.8L / 10min, a solid precipitates, and is filtered to obtain naphazoline free base.
[0050] (B) Naphazoline free base was dissolved by adding 3L of acetone, and 5% hydrochloric acid was added dropwise at room temperature to adjust the pH to 3, and a solid precipitated, which was filtered to obtain 1450.3 g of naphazoline hydrochloride crude product.
[0051] (C) Add 4L of ethanol to the crude product in the reaction kettle, heat to 78°C, add activated carbon to decolorize after dissolving, filter, add 2L of ethanol dropwise to the filtrate at the same temperature, cool down after dropping, keep warm and crystallize overnight at 20°C, Centrifuge...
Embodiment 3
[0053] Adopt the following steps to prepare naphazoline hydrochloride:
[0054] (A) Add 1000g (6.0mol) of naphthaleneacetonitrile to the reaction kettle, add 6000ml of ethylenediamine, add 54.7g (0.72mol) of thiourea, raise the temperature to 120°C, and stir for 3h. Cool down to room temperature, add 12L of water dropwise at a rate of 1.5L / min, a solid precipitates, and is filtered to obtain naphazoline free base.
[0055] (B) Naphazoline free base was dissolved in 10 L of tetrahydrofuran, and 30% hydrochloric acid was added dropwise at room temperature to adjust the pH to 1. A solid precipitated and was filtered to obtain 1433.3 g of crude naphazoline hydrochloride.
[0056] (C) Add 12L tetrahydrofuran to the reaction kettle for the crude product, heat to 60°C, add activated carbon to decolorize after dissolving, filter, add 0.5L isopropanol dropwise to the filtrate at the same temperature, cool down the filtrate, and keep warm at 30°C for crystallization Overnight, centrifu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bulk density | aaaaa | aaaaa |
| Angle of repose | aaaaa | aaaaa |
| Angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com