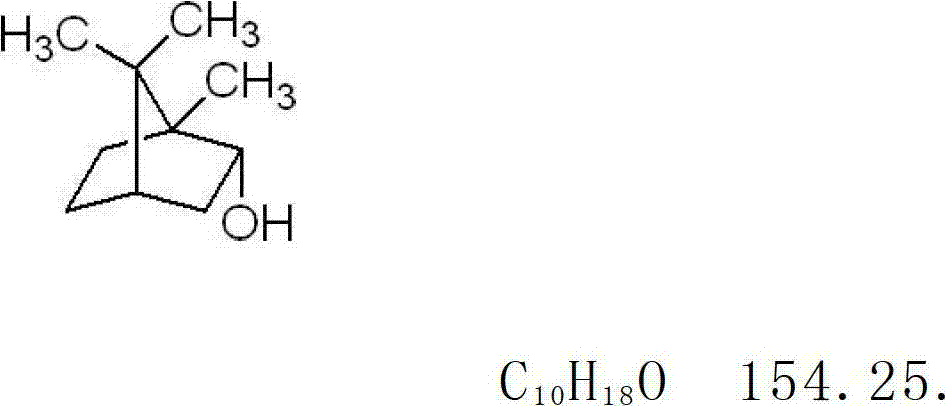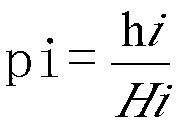Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
193results about How to "Clinical application safety" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
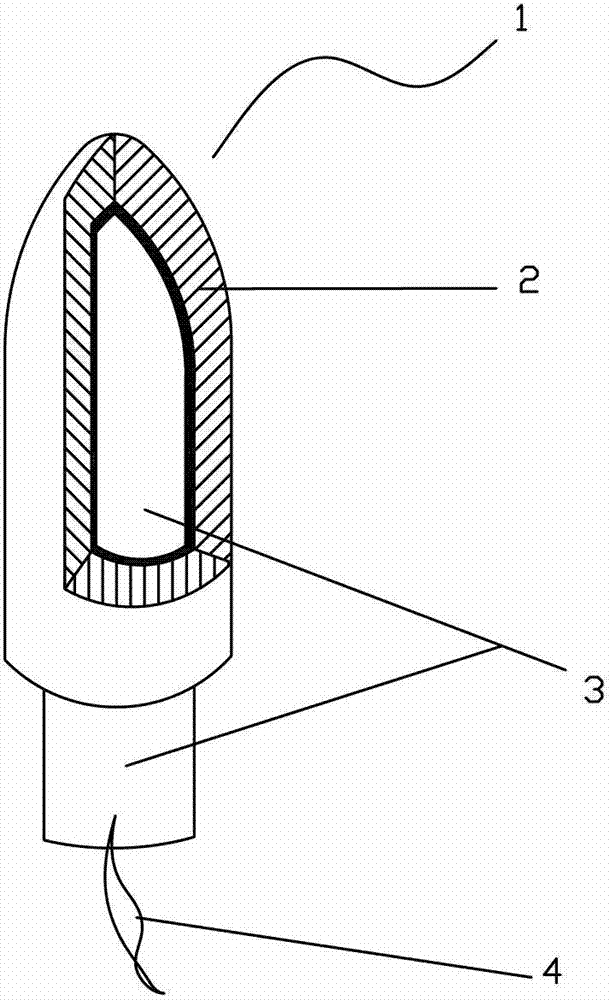
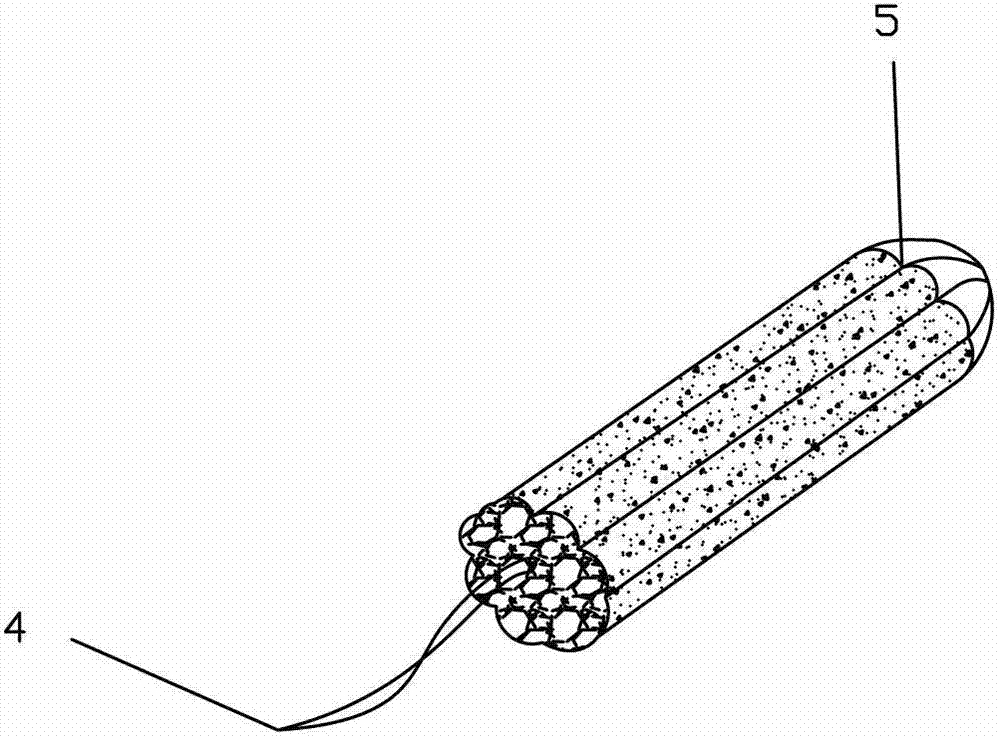
Baofukang suppository with expansion carrier
ActiveCN103041311AClinical application safetyHydroxy compound active ingredientsSuppositories deliveryVaginal SuppositoryZedoary oil
The invention relates to a Baofukang suppository with an expansion carrier, which is a vaginal suppository prepared by the following components in parts by weight: 60-100 parts of zedoary oil, 60-100 parts of borneol and matrix and 500-2,000 parts of expansion carrier; the surface of the expansion carrier is coated with a drug containing layer formed by at least a part of active ingredients and at least a part of matrix, and the expansion value of the expansion carrier is greater than 1.5. The Baofukang suppository has the benefits that the Baofukang suppository is convenient to use and long in effect time and takes effect fast; the pollution to clothes is avoided; an active drug can be uniformly distributed on a whole vaginal wall and uterine cervix, and permeated into a deep site of mucosa ruffles; surprisingly, the technological effects can still be realized under the conditions that even polyethylene glycol is not used and water is almost not used; the effective rate to mycotic vaginitis is 96.2%; and the effective rate to senile vaginitis is 96.6%.
Owner:哈尔滨田美药业股份有限公司
Chinese medicinal ointment with antibacterial and antipruritic effects and preparation method thereof
ActiveCN101721594AHigh content of active ingredientsStrong pharmacological effectHydroxy compound active ingredientsAerosol deliveryBiologyAloin
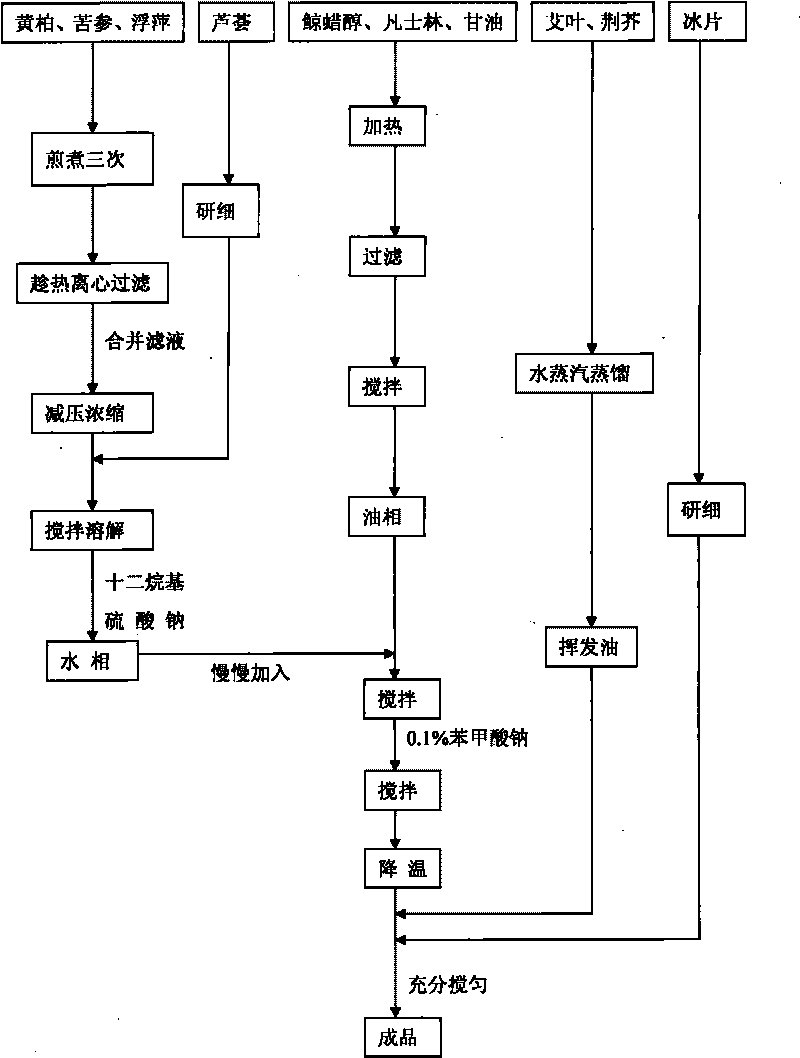
The invention discloses a Chinese medicinal ointment with antibacterial and antipruritic effects and a preparation method thereof. The Chinese medicinal ointment comprises the following raw materials: amur corktree bark, lightyellow sophora root, common ducksmeat herb, aloe, argy wormwood leaf, fineleaf schizonepeta herb, borneol and the like. The invention also discloses a preparation method forthe Chinese medicinal ointment, which comprises the following steps: preparing a water phase from the amur corktree bark, the lightyellow sophora root, the common ducksmeat herb and the aloe; collecting volatile oil from the argy wormwood leaf and the fineleaf schizonepeta herb; grinding the borneol into fine powder; preparing an oil phase from cetyl alcohol, vaseline and glycerin; and preparing the ointment by normal working procedures. When the Chinese medicinal ointment is prepared, a water decoction heat filter method and a steam distillation method are respectively adopted to make effective medicaments give full play. When used, the Chinese medicinal ointment has the characteristics that the medicaments are directly acted on disease positions; and the response is quick. The ointment of the invention has good antibacterial, anti-inflammatory and antipruritic effects and is in a dose-response relationship. The long-term toxicity research proves that the Chinese medicinal ointment is safe.
Owner:CHENGDU YONGKANG PHARM CO LTD
Flurbiprofen acetaminophen resin lipid microsphere injection, freeze-drying lipid microsphere injection and preparation methods
ActiveCN103054800AImprove solubilityImprove physical stabilityPowder deliveryOrganic active ingredientsSolubilityTreatment effect
The invention relates to a flurbiprofen acetaminophen resin lipid microsphere injection, a freeze-drying lipid microsphere injection and a preparation method. Through a reasonable composition proportion and a preparation process with controllable parameters, good stability and strong operability, the flurbiprofen acetaminophen resin which is an indissolvable drug is wrapped in an oil phase and an interfacial film of a lipid microsphere, so that not only is the solubility of the drug remarkably strengthened, but also precipitation and oxidation do not occur easily, the physical and chemical stabilities of the drug are greatly improved, the devitrification phenomenon is avoided in the use and storage processes, a slow release effect is exerted and the action time of the drug in the blood plasma is prolonged, meanwhile, as the lipid microsphere targets to an inflammation part, the toxicity and the vessel stimulation during injection are remarkably reduced, the clinical application of the injections is safer, the treatment effect is more remarkable, and the injections and the methods are suitable for industrialized production and clinical application.
Owner:WUXI ERYUN TECH CO LTD
Medicine for treating schizophrenia
ActiveCN102078566ALittle side effectsLow recurrence rateHeavy metal active ingredientsNervous disorderPsychosis drugDisease
The invention relates to a medicine for treating schizophrenia, relating to a medicine composition for treating schizophrenia, in particular to a medicine for treating schizophrenia by using fossil, ore, Chinese animal medicine and Chinese herbal medicines as raw materials. The formula comprises the following components: raw keel, ochre, rhubarb, wrinkled gianthyssop, tabasheer, lapis chloriti, arisaema with bile, albizia bark, datura flower, japanese sweetflag, radix curcumae, radix bupleuri, eupatorium, burreed tuber, curcuma zedoary, poria with hostwood and magnet. The medicine has therapeutic effects on schizophrenia, is safe and effective, has quick effect-taking, high total effective rate, few side effects and low recurernce rate when matched with a low dose of antipsychotic for treating schizophrenia, and is low in price, convenient to carry and take, and especially suitable for long-term maintenance treatment in a recovery phase and a stable phase.
Owner:苏建峰
Mesenchymal stem cell preservation liquid, and preparation method and application thereof
ActiveCN102365933AProlong the duration of activityEasy to prepareDead animal preservationCord blood stem cellMedicine
The invention belongs to the field of biologic medicines and discloses mesenchymal stem cell preservation liquid, and a preparation method and application thereof. The mesenchymal stem cell preservation liquid contains AB cord blood plasma which is 5-15% of the volume of the preservation liquid and low molecular weight heparin calcium which is 70-80 AXaIU / ml. The AB cord blood plasma and mesenchymal stem cells come from the same provider. By means of the mesenchymal stem cell preservation liquid provided by the invention, the activity maintaining time of the mesenchymal stem cells can be prolonged (above 90% of activity is kept at 4-15 DEG C for 72 h); after the mesenchymal stem cells are preserved in the preservation liquid for 72h, the cells are normal in form, the proliferation and amplification capability of the cells cannot be influenced, and the phenotypic characteristics of the mesenchymal stem cells cannot be influenced; the mesenchymal stem cell preservation liquid disclosed by the invention is safe and reliable for clinical application; and, because the AB cord blood plasma and the cord mesenchymal stem cells come from the same placenta and belong to auto-plasma, the possibility of exogenous virus pollution is reduced.
Owner:广州市天河诺亚生物工程有限公司
Medicine for treating gastric ulcer and preparation method thereof
InactiveCN101822813ADefinite curative effectNo side effectsDigestive systemPharmaceutical delivery mechanismSide effectGLYCYRRHIZA EXTRACT
The invention discloses a medicine for treating gastric ulcer, which comprises raw materials of straight ladybell root, codonopsis pilosula, angelica, dendrobium, rhizome atractylodis, bighead atractylodes rhizome, golden thread, bitter orange, red thorowax root, charred triplet, medicinal cyathula root, cardamom, white paeony root,uttlebone, astragalus root, dried ginger and liquorice. The medicine can be prepared into various clinical drugs and the preferred drug form is oral liquid. The invention is applied to soothing sliver and harmonizing stomach, nourishing the stomach Yin, tonifying middle-Jiao, Qi and spleen, promoting blood circulation, relieving pain, relieving hyperacidity and stopping bleeding, has good efficacy for gastric ulcer and other diseases caused by the weakness of spleen and stomach, inadequate stomach Yin and the like and has the advantages of high efficiency, good safety and reliability of clinical use and no obvious toxic and side effect.
Owner:赵晚乐
Medicine for preventing and curing cerebrovascular dementia
InactiveCN1454608AIncrease excitabilityImprove hypoxia toleranceNervous disorderLeech/worm material medical ingredientsSalvia miltiorrhizaMeninges
The invention discloses a kind of medicine for preventing and curing cerebrovascular dementia. The materials are: ginseng 3-9g, pseudo-ginseng 3-9g, the fruit of Chinese magnoliavine 3-9g, cornus 6-15g, boiled glutinous rehmannia 10-30g, polygonum ultiforun 10-20g, aweto 5-10g fried gutta-percha 10-15g, pilose deer horn 1-3g, leech 1.5-3g, stone calamus 5-10g, polygala root 3-10g, salvia miltiorrhiza 3-15g, tortoise plastron 10-30g. The invention uses the speciality of the Chinese traditional medicine to enhance the oxygen absorbing ability under ordinary pressure and improve the micro-circulation of meninges, enhance the imminity and memory. It not only can cure the cerebrovascular dementia effectively, but also can prevent the disease.
Owner:马合奎
Method for preparing compound famotidine chewing tablet
ActiveCN1768744AImprove stabilityClinical application safetyOrganic active ingredientsDigestive systemTherapeutic effectFamotidine
The invention relates to a process for preparing compound Famotidine chewable tablets, which comprises separating Famotidin from calcium carbonate and magnesium hydroxide, thus no reactions with be produced between the effective compositions, thus the product stability can be improved.
Owner:四川泰华堂制药有限公司
Composition for alleviating eye fatigue
InactiveCN102441070AEasy to acceptWide range of medicinesSenses disorderPlant ingredientsTraditional medicineEye Fatigue
The invention relates to a composition for alleviating eye fatigue and specifically to a Chinese herbal compound composition capable of treating myopia and alleviating eye fatigue and a preparation method thereof. The Chinese herbal compound composition in the invention is prepared from the following Chinese herbal bulk drugs: 100 to 500 parts of semen celosiae, 100 to 500 parts of mulberry fruit, 100 to 500 parts of wolfberry, 100 to 500 parts of cassia seed and 100 to 500 parts of chrysanthemum.
Owner:北京同御康泰科技有限公司
Medicine for treating cor pulmonale and its preparation method
InactiveCN1442162AIncrease generation levelPromote generationUnknown materialsCardiovascular disorderAlcoholTraditional medicine
A Chinese medicine in the form of capsule, stable, particle, or oral liquid for treating pulmonary heart disease is prepared from 3 Chinese medicinal materials including astragalus root, red peony root and psoralea fruit through decocting twice (each time for 1 hr.), filtering, concentrating up to relative density of 1.05-1.15 at 80 deg.C, depositing in alcohol standing still for 24 hr, recovering alcohol, continuously concentrating up to relative density of 1.35-1.40 at 80 deg.C concentrating, mixing with red peony root powder, stirring, drying, pulverizing and granulating.
Owner:李湛明 +1
Bone joint disease capsule and its manufacturing metod
InactiveCN1559540AClinical application safetyThe effect appearsSkeletal disorderUnknown materialsDiseaseBones joints
A Chinese medicine in the form of capsule for treating hyperosteogeny, lumbar intervertebral disk protrusion, osteoarthropathy and osteoporosis is prepared from 10 Chinese-medicinal materials including eucommia bark, pubescent angelica root, Chinese angelica root, antler glue, etc. Its preparing process is also disclosed.
Owner:李莛岐
Standardized processing technology of Chinese herbal piece stewed aconite
InactiveCN103463220AQuick effectIncrease the strength of actionBlood disorderCardiovascular disorderMedicineRoom temperature
The invention discloses a standardized processing technology of a Chinese herbal piece stewed aconite. The standardized processing technology is characterized by comprising the following steps of: soaking salted aconite, taking out and draining water when soaking water is 25 DEG C and electrical conductivity is lowered to below 6000mu s / cm; uniformly and flatly spreading salted aconite with expanded part up in clean and burnt fine bran ash with thickness of 10cm-40cm, spreading a plurality of layers of clean ginger slices over the bran ash, and flatly covering 1-5 pieces of coarse paper on the ginger slices, spreading a layer of fine clean bran ash with thickness of 3cm-10cm on the coarse paper, and flatly spreading dry bran hulls with thickness of 5cm-30cm on the ash; igniting the dry bran hulls at four corners, controlling the upper-end temperature of the aconite to 110-180 DEG C, controlling the lower-end temperature of the aconite to 70-130 DEG C, continuously baking for 36-72 hours, taking out the aconite when the bran ash is cooled, placing in a heating container, continuously steaming for 6-12 hours, pouring out, spreading and airing to the room temperature, slicing, and drying at 60 DEG C-80 DEG C until the moisture content is 5%-13%, thus obtaining the Chinese herbal piece stewed aconite. The standardized processing technology has obvious advantages of reducing toxicity, enhancing efficacy and reducing active ingredient loss in comparison with a normal steaming method and a cooking method, so that the stewed aconite pieces are high-quality.
Owner:江西中医药高等专科学校
Compound metronidazole expansion suppository, preparation technology and detection method thereof
InactiveCN103285055AGuaranteed effective concentrationPrevent outflowOrganic active ingredientsSuppositories deliveryBULK ACTIVE INGREDIENTHuman engineering
The invention relates to a compound metronidazole expansion suppository. The compound metronidazole expansion suppository is vaginal suppository prepared by metronidazole, total saponins of ginseng stems and leaves, and vitamin E which are taken as active ingredients, and proper matrix; an expandable expansion carrier is arranged in the suppository; a medicated layer formed by the active ingredients and the matrix is coated on the surface of the expansion carrier; after saturated water absorption, the expansion carrier has the radial expansion value more than 1.1. The invention also provides a detection method of the compound metronidazole expansion suppository. The method comprises the contents such as expansion value measurement, weight difference measurement, etc. The expansion suppository applies six original technologies including 'one-time suppository filling integrated forming' technology, 'inner core expansion factor control' technology, 'human engineering suppository type' technology, 'treatment-cleaning integration' dosage form, 'vaginal inner-attached administration' technology and 'side leakage prevention suppository body design'; the medicated layer of the compound metronidazole expansion suppository is fully contacted with the inner wall of the vagina, so that liquid medicine is prevented from flowing outside; the compound metronidazole expansion suppository also has the effect of cleaning the vagina so as to prevent superinfection.
Owner:HARBIN OT PHARMA +1
Spleen fortifying qi boosting capsule and its manufacturing method
InactiveCN1559541AClinical application safetyGood effectDigestive systemUnknown materialsTangerine PeelGelatin
A Chinese medicine in the form of capsule for invigorating the function of spleen and replenishing vital energy is prepared from 10 Chinese-medicinal materials including astragalus root, tangerine peel, haw, donkey-hide gelatin, etc. Its preparing process is also disclosed.
Owner:李莛岐
Cefathiamidine compound
The invention relates to the field of medicines, and particularly relates to a cefathiamidine compound. The cefathiamidine is a compound which is obtained by virtue of Cu-K alpha ray measurement and shown as an X-ray powder diffraction pattern 3. The main particle size of the cefathiamidine compound is 650-950mu m, and the distribution width of the cefathiamidine compound is 450-1150mu m. The prepared cefathiamidine crystal compound is excellent in stability, the color of the cefathiamidine compound solution is thinner than a No.3 yellow green colorimetric solution after six-month accelerated test, the hygroscopicity is lower than that of the prior art, the bioavailability is improved, and the cefathiamidine compound is more suitable for clinical use.
Owner:YOUCARE PHARMA GROUP +1
Medicament for treating pharyngitis and preparation method thereof
InactiveCN102228624ADefinite curative effectNo side effectsRespiratory disorderPlant ingredientsCurative effectRhizome
The invention discloses a medicament for treating pharyngitis, prepared from dangshen, angelica sinensis, tuckahoe, Chinese yam, dendrobe, ophiopogon root, balloonflower, magnolia obavata, arisaema root, licorice and bighead atractylodes rhizome as raw materials. The medicament can be prepared into various medicaments for clinical use, and the preferred dosage form is capsule. The medicament disclosed by the invention has a good curative effect on various kinds of pharyngitis, and is safe and reliable in clinical application.
Owner:长治市郊区黄碾镇中心卫生院
Anethol trithione soft capsule and preparation method thereof
ActiveCN101204393AClinical application safetyOrganic active ingredientsDigestive systemVegetable oilAluminum monostearate
The invention provides a soft capsule containing an anethol trithione. The soft capsule contains the anethol trithione, a substrate, a suspending agent and a surface active agent, wherein, the weight proportion of the three matters is as follows: the anethol trithione: the substrate: the suspending agent: the surface agent is equal to 1: 2 through 75: 0.1 through 10: 0.3 through 15. The substrate can be a sesame oil, a groundnut oil, a corn oil, an olive oil, a sunflower seed oil, a soybean oil, a salad oil, a cotton oil, or a colza oil or a mixture made from a plurality of these oils. The suspending agent can be a beeswax, a yellow wax, a white wax, a mixed wax, a palm oil, a hydrogenated vegetable oil, a sterin, an aluminum monostearate, a glycerin monostearate, a fatty glyceride, a fatty glyceride mixed, an aerosil, or a silicon dioxide or a mixture made from a plurality of these matters. The surface active agent is tween. The soft capsule can strengthen the beneficial effect of the anethol trithione in human body, and meanwhile shows a relativity in and out of the human body.
Owner:江苏万高药业股份有限公司
Cell culturing method for improving curative effect and lasting effect performance of chimeric antigen receptor T cells
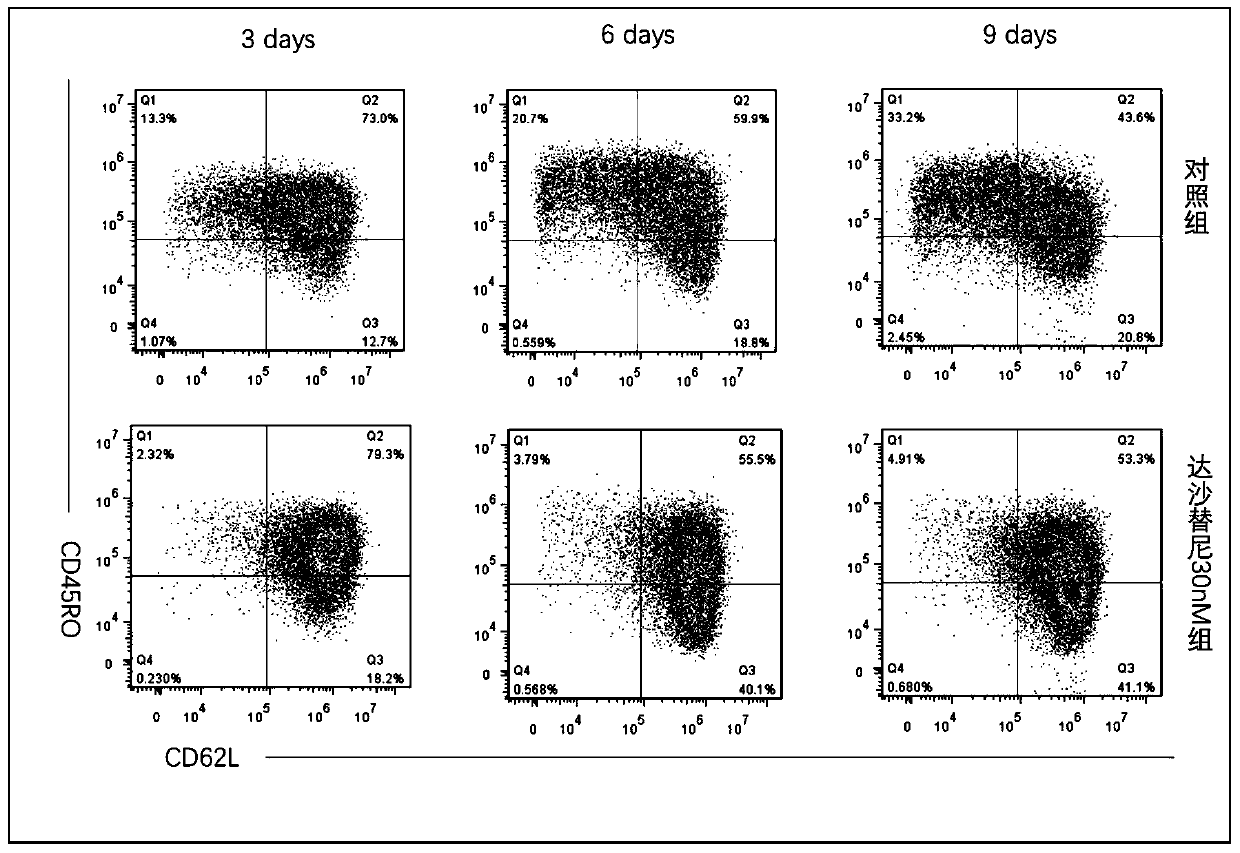
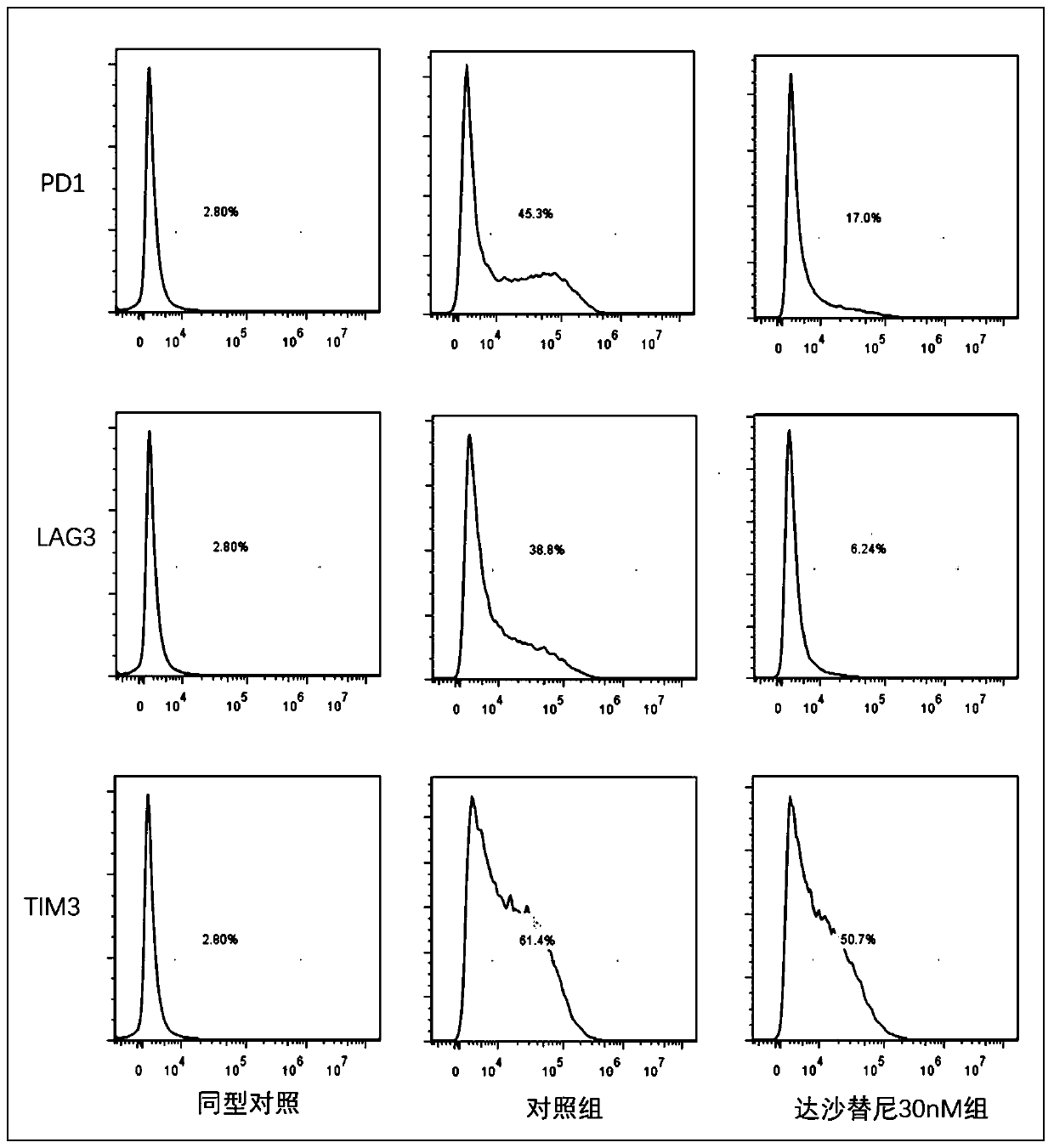
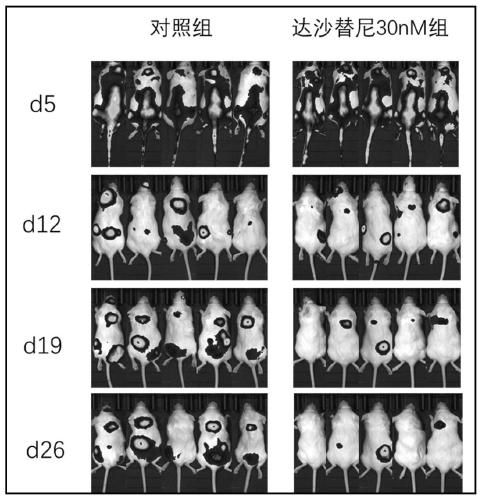
InactiveCN110157680AInhibition of activation signalingReduced activation signalingGenetically modified cellsNucleic acid vectorDasatinibTyrosine-kinase inhibitor
The invention provides a cell culturing method for improving the curative effect and lasting effect performance of chimeric antigen receptor T cells. By adding a tyrosine kinase inhibitor dasatinib (dasatinib), activation signal transmission of the CAR-T cells is reduced, the terminal differentiation of the CAR-T cells is inhibited, the proportion of naive T cells and central memory T cells in a CAR-T cell product is increased, and the depletion trend of the CAR-T cells is inhibited. The method solves the problems of the terminal differentiation and depletion trend of the CAR-T cells in the in-vitro culture process. The method is simple in culture process, easy to implement, low in cost and safe and reliable in clinic application; the proportion of the naive T cells and the central memoryT cells of the cultured CAR-T cells is high, the depletion trend of the CAR-T cells is avoided, and the good repeatability is achieved; the cultured CAR-T cells show the better curative effect and thebetter in-vivo lasting effect performance and have high application and popularization value.
Owner:ZHEJIANG UNIV
Medicament for treating gout and preparation method thereof
InactiveCN102091141ADefinite curative effectLow priceAnthropod material medical ingredientsSkeletal disorderDiseaseCentipede
The invention discloses a medicament for treating gout, which is prepared from astragalus root, ginseng, prepared aconite root, notopterygium root, white peony root, herba epimedii, angelica sinensis, jujube kernel, radix sileris, asarum, liquorice, hemlock parsley and centipede as raw materials, and the medicament can be prepared into tablets, capsules, pills, particles, powder, injection or ointment for clinical use. The medicament provided by the invention has the efficacies of benefiting qi, tonifying the blood, restoring menstrual flow and dispersing pathogenic wind, has good curative effects on gout and other diseases, and is safe and reliable in clinical application without obvious toxic side effects.
Owner:赵晚乐
Amaranth berberine tablet and preparation method thereof
ActiveCN101537037AReduce dosageGood curative effectOrganic active ingredientsDigestive systemBerberineMedicine
The invention discloses an amaranth berberine tablet and a preparation method thereof. The amaranth berberine tablet is a Chinese medicinal combined preparation comprising the following main components: copperleaf, berberine hydrochloride, liquorice, and appropriate amount of accessories. The preparation method comprises the following steps: setting the main components by the following weight percentage: 65 to 90 percent of the copperleaf, 0.5 to 10 percent of the berberine hydrochloride, and 5 to 15 percent of the liquorice; crushing the liquorice into fine powder; decocting the copperleaf in water twice, putting the decoction liquid together, filtering the mixed decoction liquid, taking the supernatant and concentrating the supernatant into thick paste, adding the liquorice fine powder, mixing the thick paste and the liquorice fine powder evenly, drying the mixture at a low temperature, crushing the mixture into fine powder, adding the berberine hydrochloride and the appropriate amount of the accessories into the fine powder, mixing the fine powder evenly with the berberine hydrochloride and the appropriate amount of the accessories, carrying out granulation and drying, adding appropriate amount of lubricant, pressing the mixture into tablets, and obtaining the finished product without coating or with a film coat. The amaranth berberine tablet consisting of the medicaments and a bulking agent, has good humidity resistance, easy carrying, quick disintegration, and small dosage.
Owner:FUZHOU JINXIANG CHINESE MEDICINE PHARMA
Preparation method of controlled-release drug
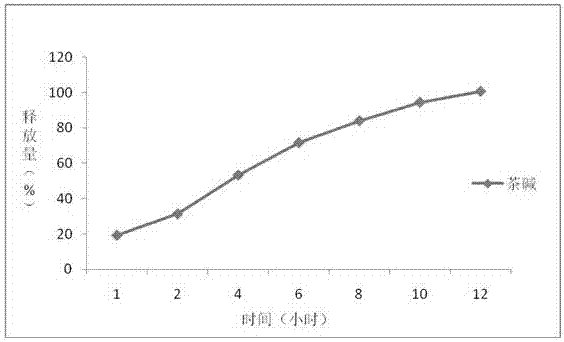
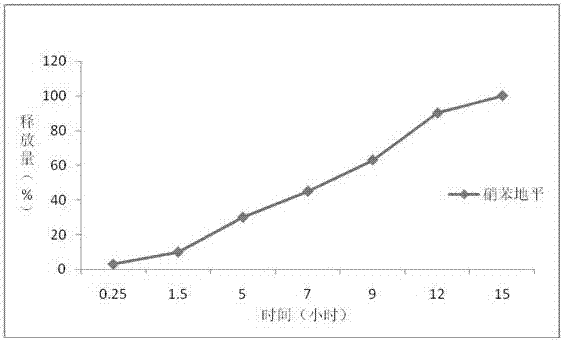
InactiveCN107441055AEvenly dispersedTightly dispersedPill deliveryMicrocapsulesNifedipineOrganic solvent
The invention belongs to the field of pharmaceutical preparations, specially relates to a preparation method of a controlled-release drug, and particularly relates to theophylline and nifedipine controlled-release tablets prepared by using the method. The controlled-release drug is prepared by adopting combination of two techniques of hot melt extrusion and membrane controlling. Compared with the prior art, the method disclosed by the invention has the advantages that the drug is subjected to melting and solidification based on that the drug is preferably controlled to release at a zero level, the quality is uniform, and the phenomenon that burst release of other drugs in controlled-release dosage forms possibly occurs is avoided; for drugs with narrow therapeutic windows, clinical application is safer; organic solvents are not used, so that the security of mass production is good.
Owner:山东则正医药技术有限公司
Anhydrous sodium sulfate powder and preparation method thereof
InactiveCN103919730AClinically convenientClinical application safetyPowder deliveryDigestive systemCitrate sodiumChemistry
The invention provides anhydrous sodium sulfate powder, which comprises the following raw materials by weight ratio: 100 parts of a main drug of anhydrous sodium sulfate, a flavouring which is composed of one or several raw materials of 0.02-0.5 parts of sodium saccharin, 0.1-0.6 parts of aspartame, 1.5-15 parts of sorbitol, 1.5-15 parts of xylitol, and 0.02-0.5 parts of asccharin; and an electrolyte of 0.5-1 part of potassium chloride and 2-3 part of sodium chloride; and a pH conditioning agent of 0.5-1 part of sodium citrate. The invention also provides a preparation method of the powder. The powder is suitable for cleaning intestinal tract before clinic operation or enteroscopy inspection, the cleaning effect of intestinal tract is good, incidence rate of adverse reaction is low, and the problems that mouthfeel of an anhydrous sodium sulfate solution is salty and bitter, the electrolyte in a process for cleaning intestinal tract is lost, so that balance of the electrolyte is disordered. The powder is suitable for people of all ages.
Owner:KECHUANG HLDG GRP
Medication for treating thyroiditis and preparation method thereof
InactiveCN101757370AElimination of systemic symptomsSignificant drug effectDrug compositionsPlant ingredientsSide effectZhebeinine
The invention relates to a medication for treating thyroiditis, which is prepared by taking Chinese herbs as raw materials. The medication is prepared from southernwood, wizened scutellariae, cortex moutan, weeping forsythia, isatis root, ptunella vulgaris, picria fel-terrae, balloonflower root and zhebeinine and can be prepared into various common clinical medicine preparations. Proved by clinical test results, the medication has the advantages of definite effect, no toxic and side effect and safe and reliable clinical application.
Owner:山西省屯留县科技情报服务中心
Tea for treating eczema
InactiveCN102302699ADefinite curative effectNo side effectsPre-extraction tea treatmentDermatological disorderSide effectTrial drug
The invention discloses tea for treating eczema. The tea is prepared from such raw materials as hairy asiabell root, Chinese angelica, shaggy-fruited dittany root-bark, Sinkiang arnebia root, liquorice, rehmannia root, double leaves, kudzu vine root, lucid ganoderma, corn, soybean and millet. As the components of the tea are reasonably compatible and are complementary and synergistic, the tea has a good curative effect on eczema. Clinical trial results prove that the health-care tea has an obvious effect and no toxic or side effects and is safe and reliable to use clinically.
Owner:申生林
Medicine composition for treating haemorrhoids and preparation method thereof
InactiveCN104147536AImprove stabilityLittle side effectsGranular deliveryCapsule deliverySide effectSargentodoxa cuneata
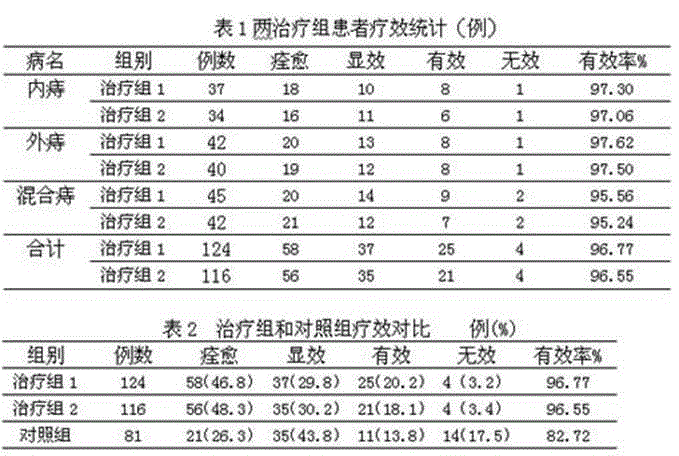
The invention relates to a medicine composition for treating haemorrhoids and a preparation method thereof. The medicine composition is characterized by consisting of following medicines, by weight: 10-30 parts of fried sophora flower buds, 10-40 parts of platycladus orientalis leaves, 5-20 parts of fructus aurantii, 5-20 parts of herba schizonepetae, 5-25 parts of bombyx batryticatus, 5-20 parts of cicada slough, 10-30 parts of radix curcuma, 5-20 parts of rhubarb, 5-20 parts of rhizome of Largeleaf Japanese ginseng and 15-40 parts of sargentodoxa cuneata. The medicine composition, after being researched for many years, is significant in clinical treatment effects, is more than 46% in curative rate and more than 96% in total effective rate and is good in curative effect, high in curative rate and free of side effects. An enteric preparation of the medicine composition can allows effective ingredients to be avoided from being damaged by internal or external environments such as strong acid in stomach, light, heat and the like. Stability of the medicine composition is improved. Active ingredients are ensured to be completely released in intestinal tracts so that the curative effect of the medicine composition is fully achieved.
Owner:LIAOCHENG PEOPLES HOSPITAL
Traditional Chinese medicinal composition for treating infectious pleuropneumonia of goats and preparation method of traditional Chinese medicinal composition
InactiveCN107349402AReduce appetiteClear boundariesAntibacterial agentsRespiratory disorderNitraria sibiricaSide effect
The invention discloses a traditional Chinese medicine composition for treating goat infectious pleuropneumonia and a preparation method thereof. 6-18 parts by weight of saxifrage, 9-27 parts by weight of Gingeria chinensis, and 10-30 parts by weight of Nitraria small fruit. The traditional Chinese medicine composition of the invention has the advantages of treating both symptoms and root causes, high overall curative effect, short course of treatment, not easy to produce drug resistance, low residue, small toxic and side effects, etc., and has very important economic and social benefits for promoting the healthy development of sheep raising industry.
Owner:JIANGSU AGRI ANIMAL HUSBANDRY VOCATIONAL COLLEGE
Medication for chronic renal failure
InactiveCN1481891AImprove fatigueImprove stool drynessUnknown materialsUrinary disorderChronic kidney failureRhizome
The present invention provides one kind of Chinese medicine for treating chronic kidney function failure is prepared with Chinese medicinal materials, including ginseng, astragalus root, white atractylodes rhizome, Chuanxiong rhizome, leech, etc. The medicine has high curative effect, can improve clinical symptoms, reduce urea nitrogen in blood, lower creatinine level, reduce exhausted urine protein, protect kidney function, promote the synthesis of plasma protein, raise plasma protein density, improve body's nutrient state and produce many other positive effects. It is safe clinically and low in production cost.
Owner:贾在金
Medicament for treating rheumatoid arthritis and preparation method thereof
InactiveCN101829312ASignificant drug effectDrug effectAntipyreticAnalgesicsSide effectCurative effect
The invention discloses a medicament for treating rheumatoid arthritis, which is prepared by using bixie sevenlobed yam rhizome, Fukuai, ginger, white atractylodes rhizome, Chinese ephedra, raw liquorice, rhizoma anemarrhenae, white paeony root, cassia twig, rhizoma alismatis and divaricate saposhnikovia root as raw materials. The medicament can be prepared into multiple preparations used clinically, and has better treatment effect on rheumatoid arthritis, no toxic and side effects and safe and reliable clinical application.
Owner:山西省屯留县科技情报服务中心
Medicine for treating coronary disease angina and preparation method thereof
InactiveCN101596302ALow priceShort course of treatmentUnknown materialsCardiovascular disorderSalvia miltiorrhizaDisease
The invention relates to a medicine for treating coronary disease angina and preparation method thereof. Chinese angelica, ligusticum wallichil, peach kernel, safflower, salvia miltiorrhiza, red paeony root, fructus aurantii, radix curcumae, eagle wood, trichosanthes kirilowii maxim, pinellia ternata, trogopterus dung, inula flower, moterwort and medicated leaven are mixed to be uniform, added with 2-4 fold weight of clean water to be soaked for 30 minutes, decocted for 15 minutes, filtered, and then added with 2-4 fold weight of clean water to be decocted for 20 minutes; the process is repeated for twice, each decoction lasts five minutes more, the four decoctions are merged, disinfected, sterilized and packaged to be oral liquid product. The medicine has obvious curative effect and rapid action time can cure the coronary disease angina in a shorter time.
Owner:王江雪
Insensitive impediment capsule and its manufacturing method
A Chinese medicine in the form of capsule for treating rhumatoid arthritis, tonic rachitis and gout is prepared from 11 Chinese-medicinal materials including eucommia bark, leech, earthworm, centipede, etc through washing, baking, proportional mixing, breaking, wetting by rice wine, steaming, baking, pulverizing and filling in capsule.
Owner:李莛岐
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com