Mesenchymal stem cell preservation liquid, and preparation method and application thereof
A technology of mesenchymal stem cells and preservation solution, applied in the field of biomedicine, can solve the problems of legality, reagent cost, unfavorable clinical promotion and use of mesenchymal stem cells, short viability of mesenchymal stem cells, and limited application range of mesenchymal stem cells, etc. , to achieve safe and reliable clinical application, normal cell morphology, and reduce the possibility of exogenous virus contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
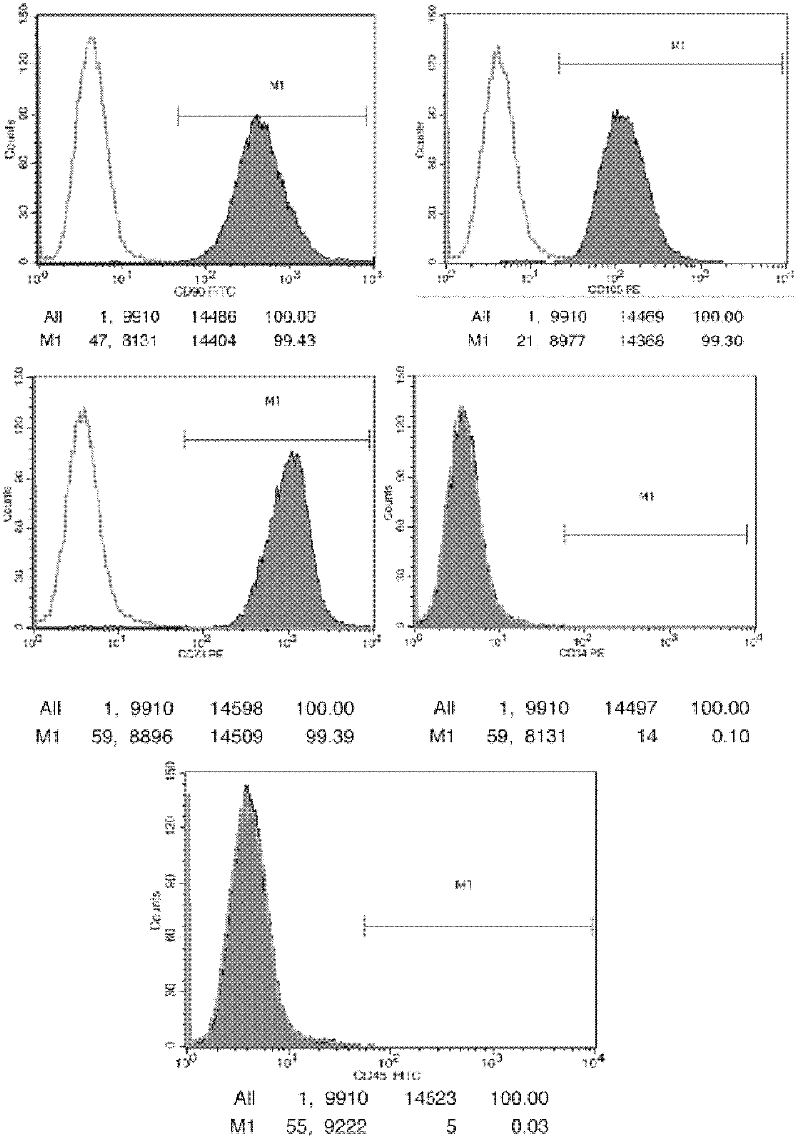
[0029] 1. Isolation, culture and phenotypic identification of umbilical cord mesenchymal stem cells
[0030] (1) Soak the umbilical cord in physiological saline for injection containing 100IU / L penicillin and 100mg / L streptomycin for 10-15 minutes.
[0031] (2) Rinse the umbilical cord fully with normal saline for injection, and then cut the umbilical cord tissue pieces into 1mm 3 of small pieces.
[0032] (3) Add LONZA medium to suspend tissue pieces, inoculate all tissue pieces into cell culture dishes, and place at 37°C, 5% CO 2 cultured in a saturated humidity incubator (Thermo, USA).
[0033] (4) After the tissue block was cultured for 1 week, the LONZA medium was completely replaced, and the tissue block and non-adherent cells were discarded.
[0034] (5) Change the LONZA medium every 3 days in half, observe the cell growth state every day, and when the cells reach 80% confluence, digest the cells with 0.25% trypsin (GIBCO, USA) by volume fraction, and adjust the rati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com