Application of different configurations of glp-1 similar peptide modified dimers and their preparation methods in the treatment of type ii diabetes
A GLP-1, homodimer technology, applied in chemical instruments and methods, medical preparations containing active ingredients, peptides, etc., can solve the problems of natural GLP1 effective, obvious side effects, etc., to protect pancreatic function, increase The time of hypoglycemic effect and the effect of promoting technology upgrading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The preparation of embodiment 1 monomer peptide and dimer
[0056] 1. Monomer peptide solid-phase synthesis process: Manual solid-phase peptide synthesis operation steps.
[0057] 1. Resin swelling: Put dichloro resin (dichlorobenzyl resin for C-terminal carboxyl group) or amino resin (amino resin for C-terminal amidation sequence) (purchased from Tianjin Nankai Synthetic Technology Co., Ltd.) into In the reaction pot, add dichloromethane (DCM, Dikma Technologies Inc.) 15ml / g resin, shake for 30min. SYMPHONY 12-channel polypeptide synthesizer (SYMPHONY model, software Version.201, Protein Technologies Inc.).
[0058] 2. Connect the first amino acid: Remove the solvent through sand core suction filtration, add 3 times the mole of the first Fmoc-AA amino acid at the C-terminal (all Fmoc-amino acids are provided by Suzhou Tianma Pharmaceutical Group Fine Chemicals Co., Ltd.), and then Add 10-fold molar amounts of 4-dimethylaminopyridine (DMAP) and N,N'-dicyclohexylcarbod...
Embodiment 2
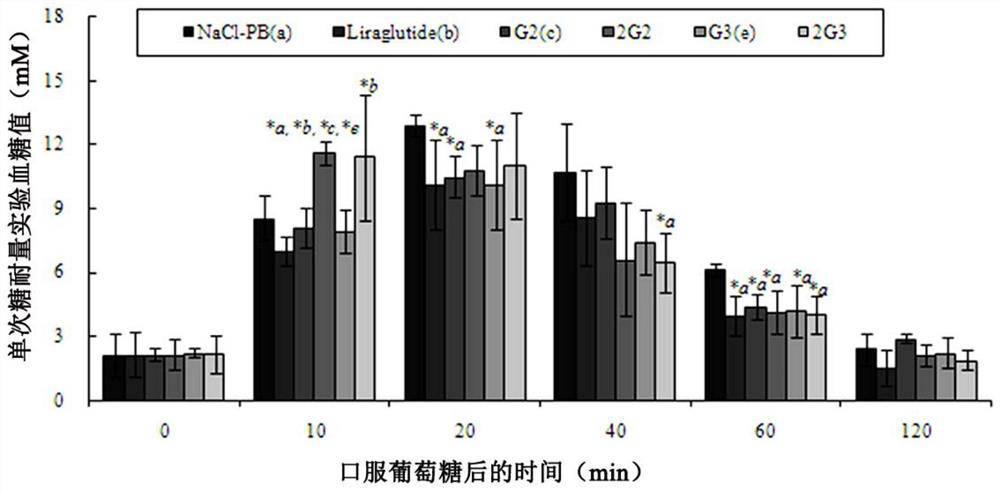
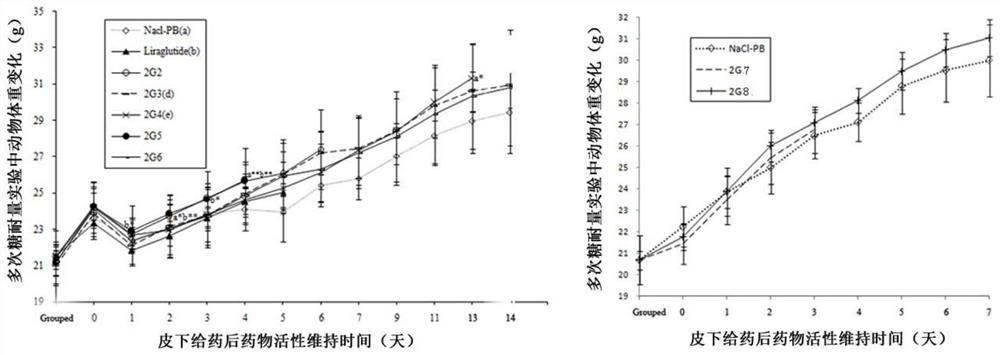
[0083] Example 2 Persistence of the hypoglycemic effect of the GLP-1 monomers and homodimers (G2-9 and 2G2-9 series) of the present invention:
[0084] 1. Experimental method: Normal KM mice were purchased from the Animal Center of Guangdong Province for glucose tolerance test (OGTT) to screen the hypoglycemic activity and persistence of drugs. According to the undifferentiated fasting blood glucose, male Kunming mice (5 weeks old) were divided into multiple groups (NaCl-PB group, Liraglutide group, monomer G2-G9 series and dimer 2G2~2G9 series group) (n=6) . After two rounds of 14-hour feeding-10-hour fasting adaptation period, KM mice were tested for glucose tolerance immediately after each 10-hour fasting. Thirty minutes after subcutaneous injection of the same dose of monomeric or dimer peptides on the back, the mice were orally gavaged with 5% glucose solution, and the blood glucose value of the rat tail was accurately measured at 35 minutes. Blood glucose meters and bl...
Embodiment 3 2
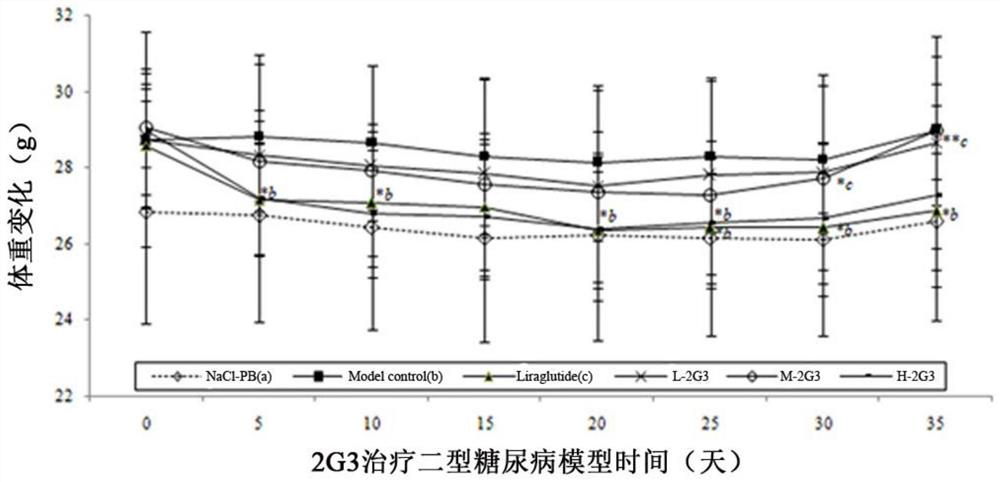
[0096] Embodiment 3 dimer is to the therapeutic effect of type 2 diabetes model
[0097] 1. Construction of type II diabetes (T2D) mouse model
[0098] C57Bl6 / J mice were placed in an SPF-level environment with standard diet and had free access to water. All experiments were performed in accordance with the institutional guidelines for the ethics and use of experimental animals. After feeding with a standard diet for 10 days, 5-week-old C57B16 / J male mice were divided into 6 groups: NaCl-PB, T2D model control group, Liraglutiade, low-middle-high dimer peptide 2G3 or 2G1 group. NaCl-PB group is blank control and T2D model control group is T2D model control, they are injected with NaCl-PB solution. The T2D model group was fed a 60kcal% high-fat diet (D12492, Changzhou Shuyishuerbiotechnology Co., Ltd.) until the end of the experiment, and the blank control group maintained a standard diet until the end of the experiment. The establishment method of the diabetes model: After f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com