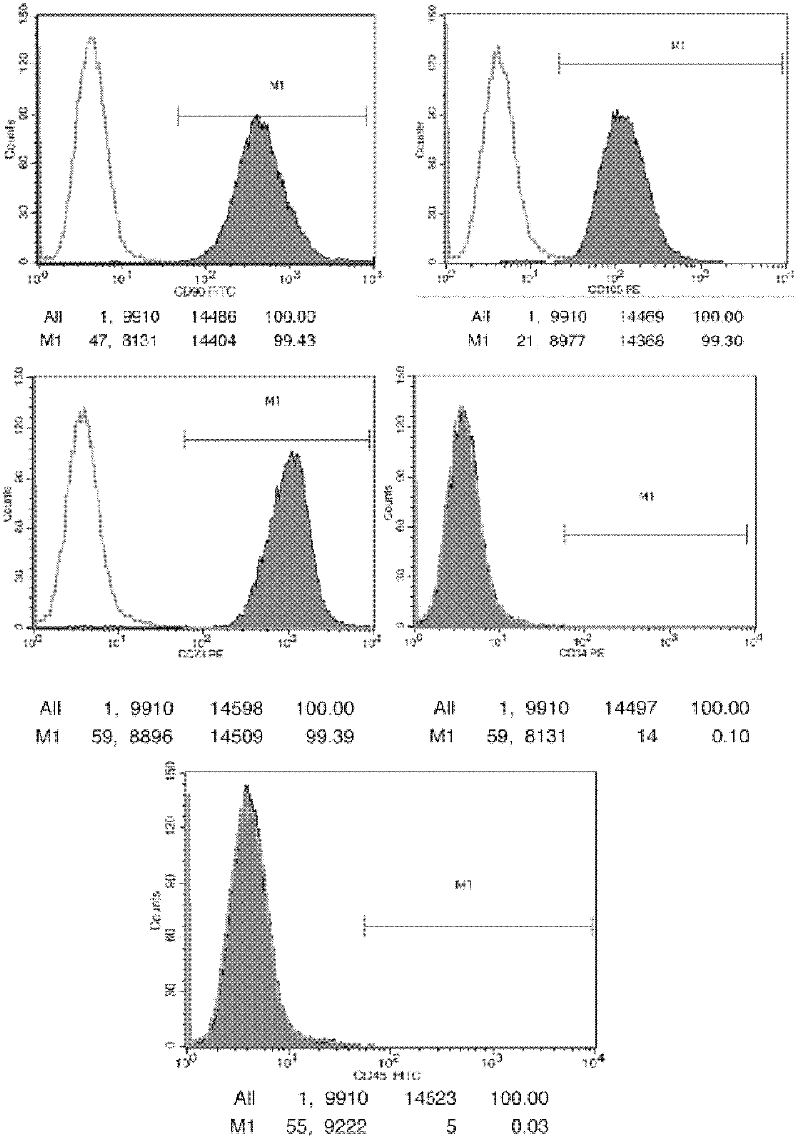
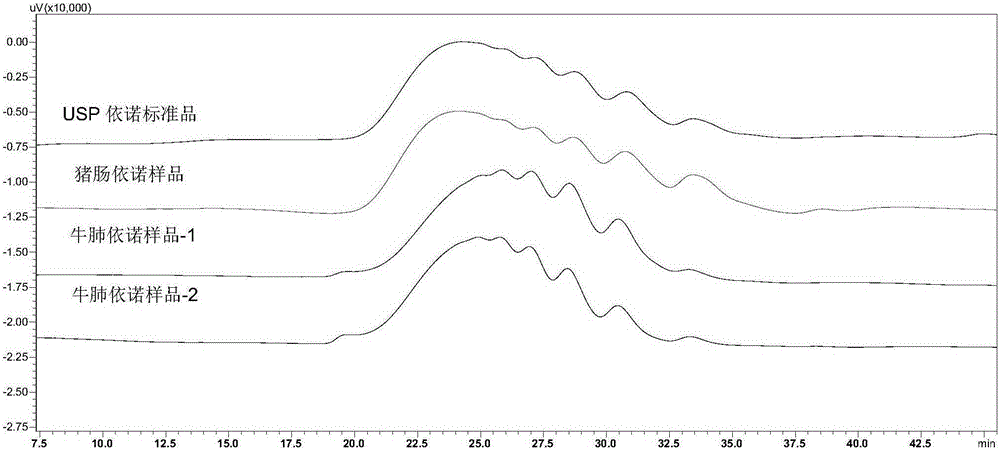
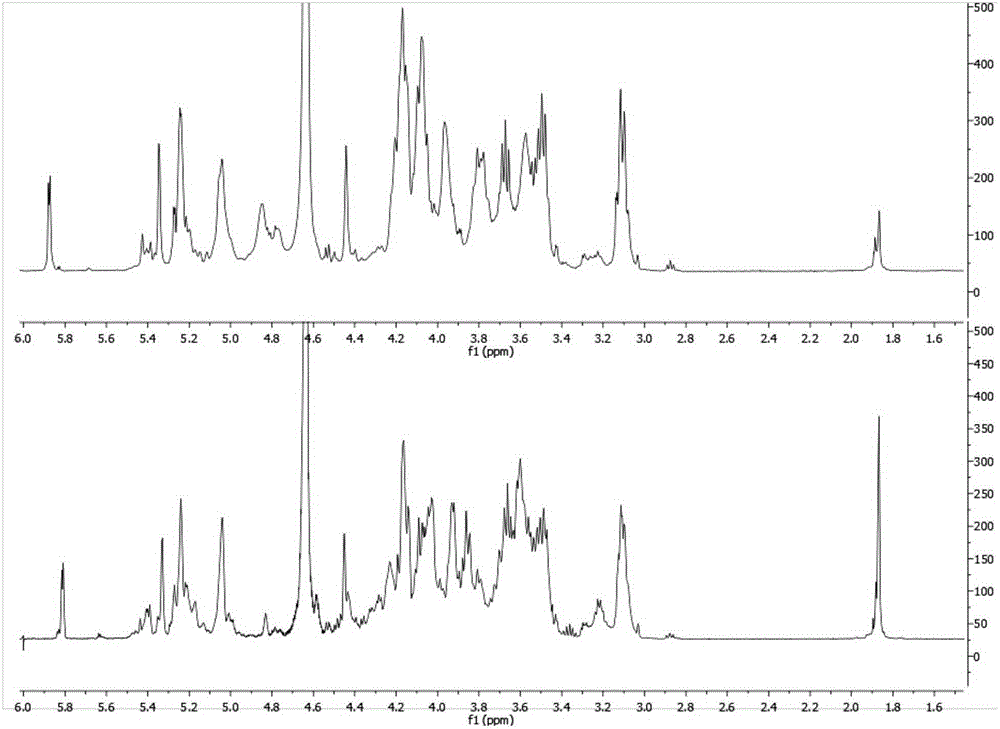
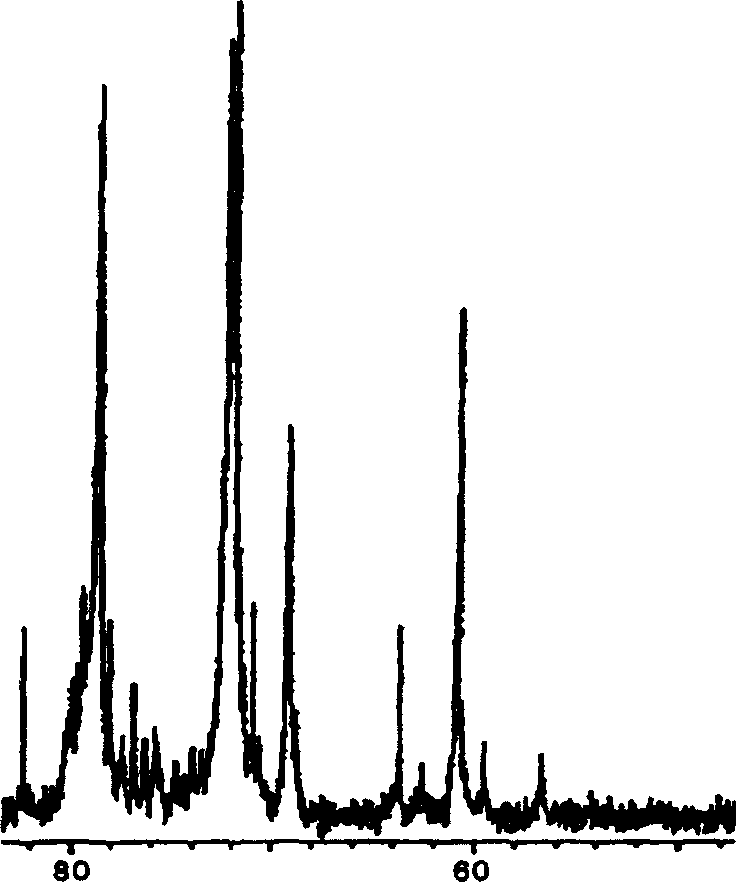
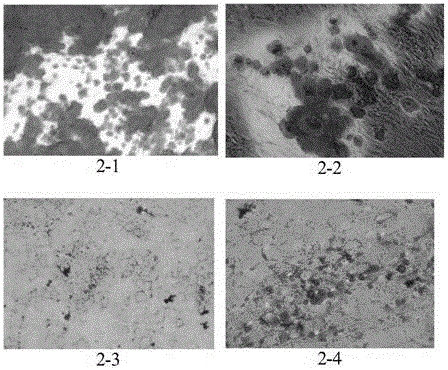
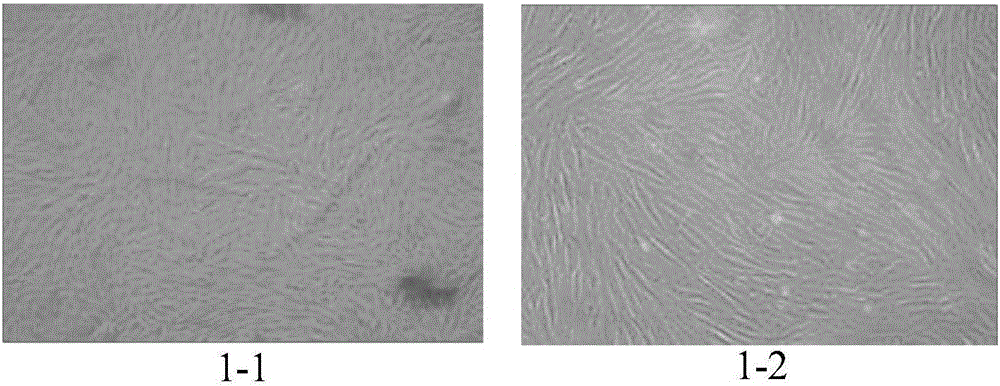
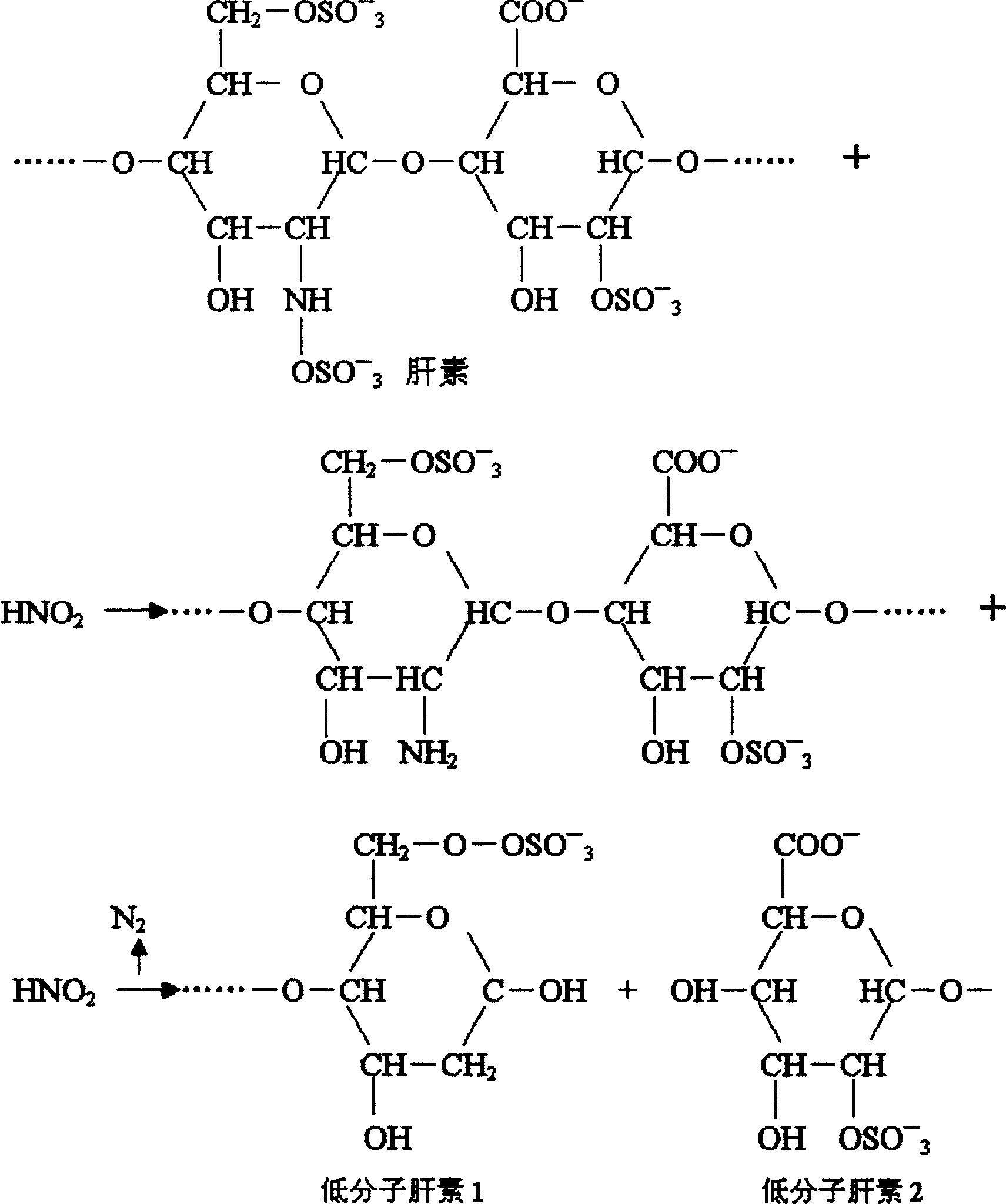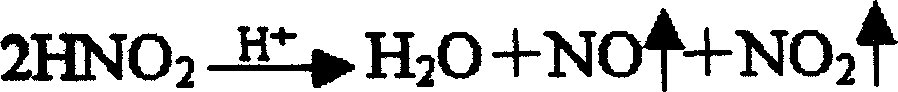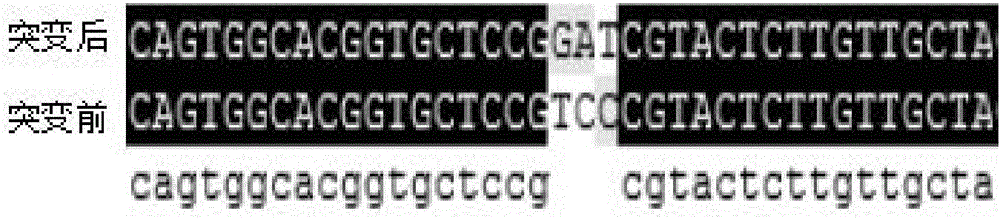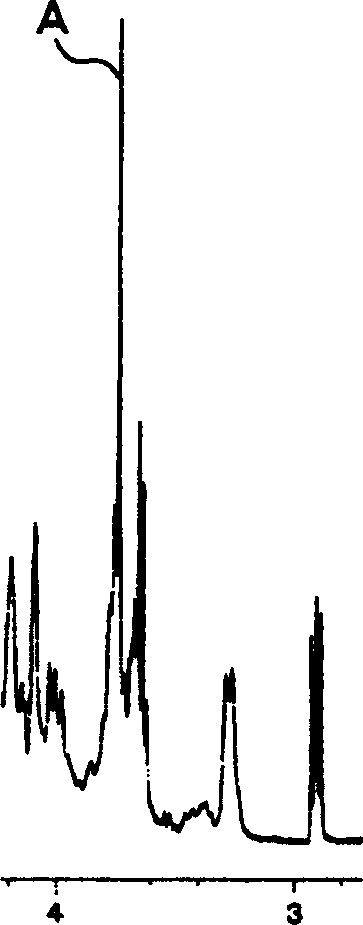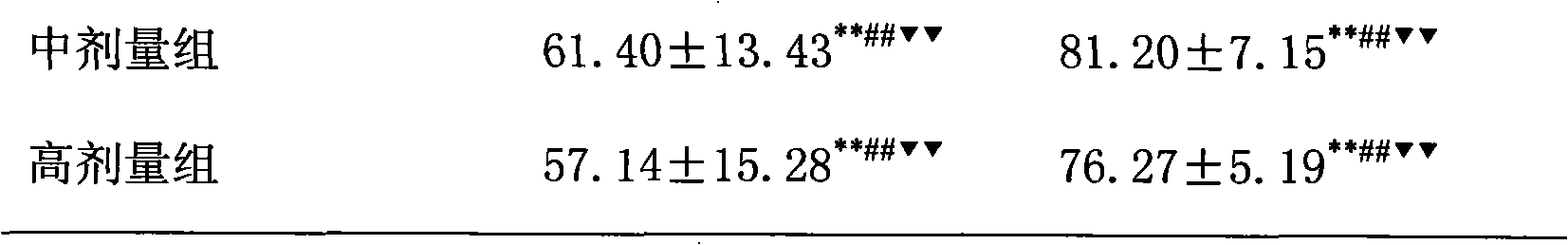Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
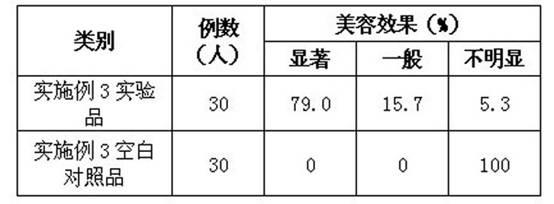
175 results about "Low molecular mass heparin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Technique for producing ultra-low molecular heparin sodium (calcium)
InactiveCN101519459AImprove securityGood and long-lasting antithrombotic effectPulmonary artery embolismDisease
Aiming at the conditions that heparin has severe bleeding side effects in clinical practice and clinical application thereof is restricted, the invention discloses a technique for producing ultra-low molecular heparin sodium (calcium). The technique comprises the following steps of: taking heparin sodium solution, adding sodium nitrite solution for cracking; adjusting the lysis buffer by using alkaline; absorbing impurities by using an anion-exchange column; washing for obtaining ultra-low molecular heparin calcium; carrying out filtration by using an ultrafiltration membrane and obtaining a precipitate by using alcohol; and after desalting, dehydration, re-precipitation, cooling and drying, obtaining a finished product of ultra-low molecular heparin calcium. The product has better and safer antithrombotic effect under low level of anticoagulation, and can be widely used for preventing and treating diseases such as deep vein thrombosis, pulmonary embolism, disseminated intravascular coagulation, and the like.
Owner:SUZHOU FAST BIOLOGICAL PHARMACY TECH
Whitening cosmetic product, preparation method and application thereof
ActiveCN101816620AGood whitening effectFor long-term storageCosmetic preparationsToilet preparationsVitamin CSide effect
The invention discloses a whitening cosmetic product, a preparation method and application thereof. The whitening cosmetic product comprises the components in percentage by mass: 0.00001to 0.001 percent of recombined human angiogenin, 0.00001 to 0.001 percent of metallothionein, 0.00001 to 0.001 percent of human epidermal growth factor, 0.00001to 0.001 percent of IL-1RA, 0.1 to 0.5 percent of superoxide dismutase, 0.1 to 0.5 percent of low molecular liquaemin, 1 to 5 percent of arbutin, 5 to 25 percent of stabilizer, 0.02 to 0.3 percent of hyaluronic acid, 0.5 to 5 percent of methylpropanediol, 0.5 to 3.5 percent of lactobacillus / mung bean fermentation liquid, 0.5 to 5 percent of dissolved protease, 0.5 to 8 percent of vitamin C sodium phosphate, 0.3 to 0.7 percent of 1,2-hexanediol or 1,2-capryl glycol, 1 percent to 5 percent of oat Beta-glucan, 1 to 5 percent of soy isoflavone and the balance of water. The whitening cosmetic product can be preserved for a long time, has no side effect on skin and is suitable for long term use.
Owner:广州泰润合投资有限公司
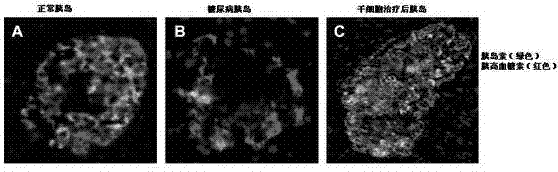
Mesenchymal stem cell injection, preparation method and application thereof in preparing medicine for treating diabetes
InactiveCN102920735AIncrease productionEasy quality controlCell dissociation methodsPeptide/protein ingredientsVitamin CSide effect
The invention discloses a mesenchymal stem cell injection, a preparation method and application of the mesenchymal stem cell injection in preparing a medicine for treating diabetes; the mesenchymal stem cells are derived from human umbilical cord and placenta; the mesenchymal stem cell injection consists of components: human mesenchymal stem cells, human albumin, low molecular heparin calcium, compound amino acid, vitamin C of 0.5% and dissolving medium; and the dissolving medium can be a compound electrolyte solution or glucose or normal saline. According to the mesenchymal stem cell injection, the preparation method and the application of the mesenchymal stem cell injection in preparing the medicine for treating the diabetes, the mesenchymal stem cell injection is used for repairing injured islet beta cells, and the blood sugar is reduced by secretion of the endogenous insulin, therefore, a purpose of foundational treating the diabetes is achieved. A disease course of the diabetes can be reversed, patients are helped in escaping out of inconvenience and toxic and side effects of taking the endogenous drug and injecting the insulin as well as serious complications caused by poor control of the blood sugar; and the 1-type and 2-type diabetes are treated thoroughly.
Owner:青岛奥克生物开发有限公司
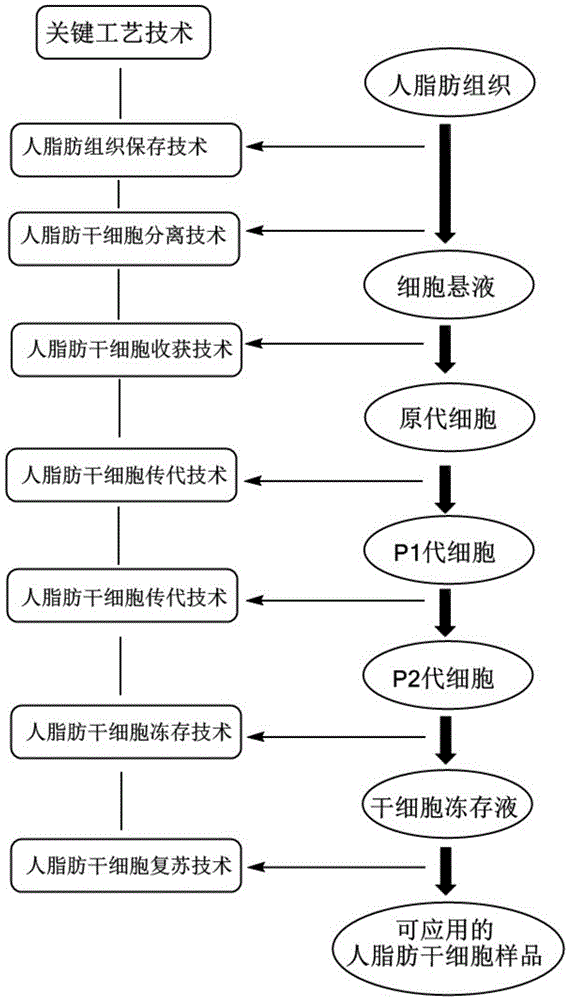
Method for separating human adipose-derived stem cells from human adipose tissues
ActiveCN104357387AHigh purityReduced differentiation inductionSkeletal/connective tissue cellsParenchymaBrown adipose tissue
The invention relates to the field of cell engineering and particularly relates to a method for separating human adipose-derived stem cells from human adipose tissues. According to the method, by adding a small amount of low-molecular-weight heparin calcium into normal saline, a mixed solution is obtained and used as a cleaning fluid to clean the human adipose tissues, residual impurities such as red blood cells in the human adipose tissues can be well removed and thus human adipose-derived stem cells with relatively higher purity can be separated, the content of the parenchyma cell is significantly reduced, the differentiation induction of the human adipose-derived stem cells is facilitated, the consumption amounts of collagenase I, trypsase and a serum-free culture medium can be reduced and the cost of the process is also decreased. In addition, by centrifuging and digesting the human adipose tissues for 1 hour at a speed of 150r / min, the human adipose-derived stem cells with significantly better activity can be obtained.
Owner:深圳市赛欧细胞技术有限公司
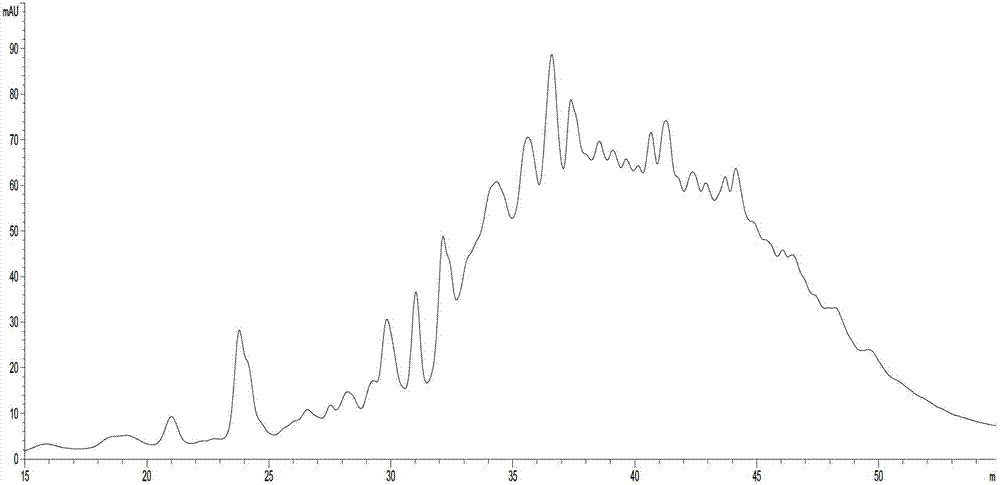
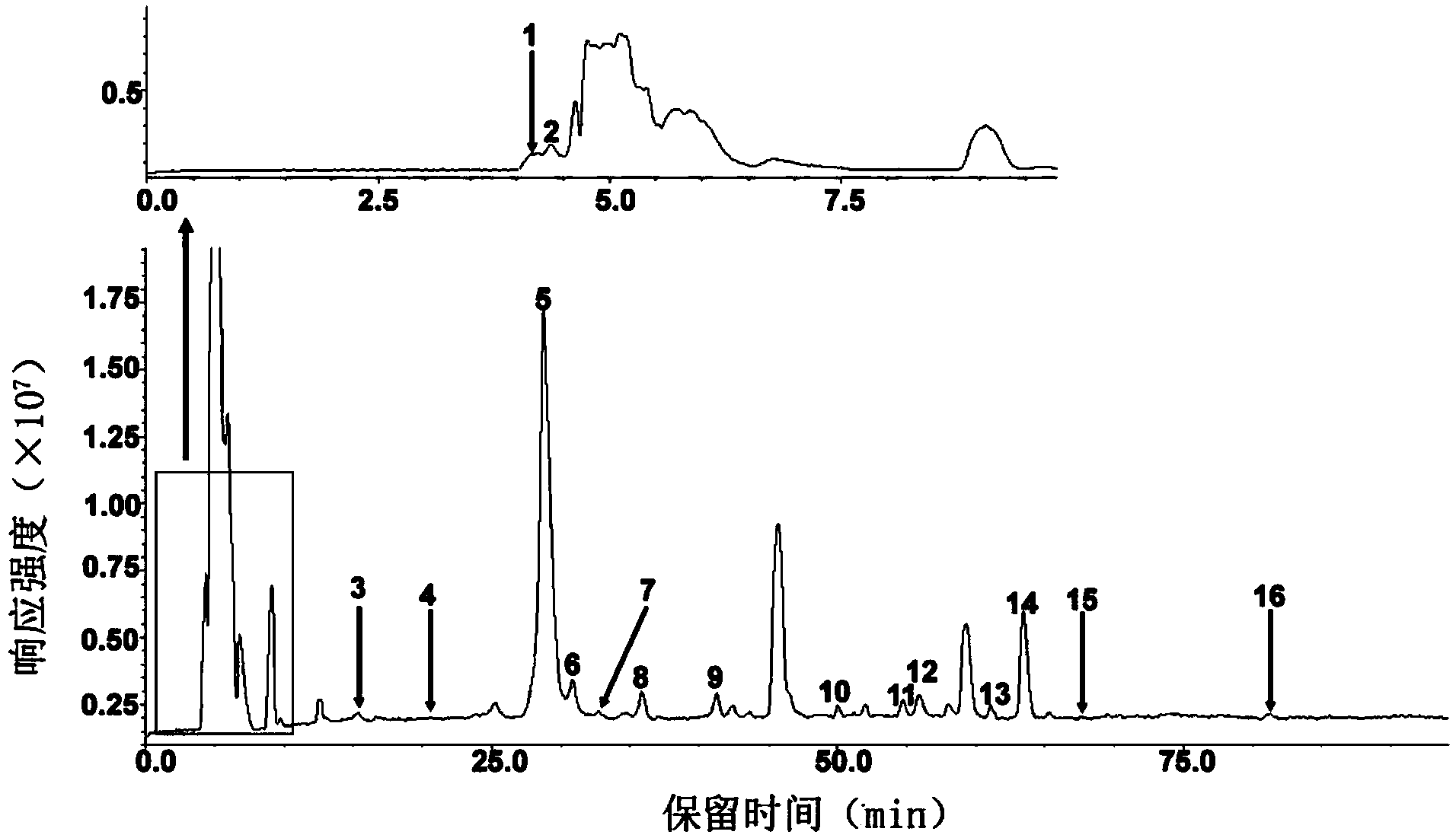
Method for detecting low-molecular-weight heparin by combining ion pair reversed phase chronmatogaphy and mass spectrum
ActiveCN102759596AImprove detection levelEnsure safetyComponent separationChromatographic separationMass spectrometry
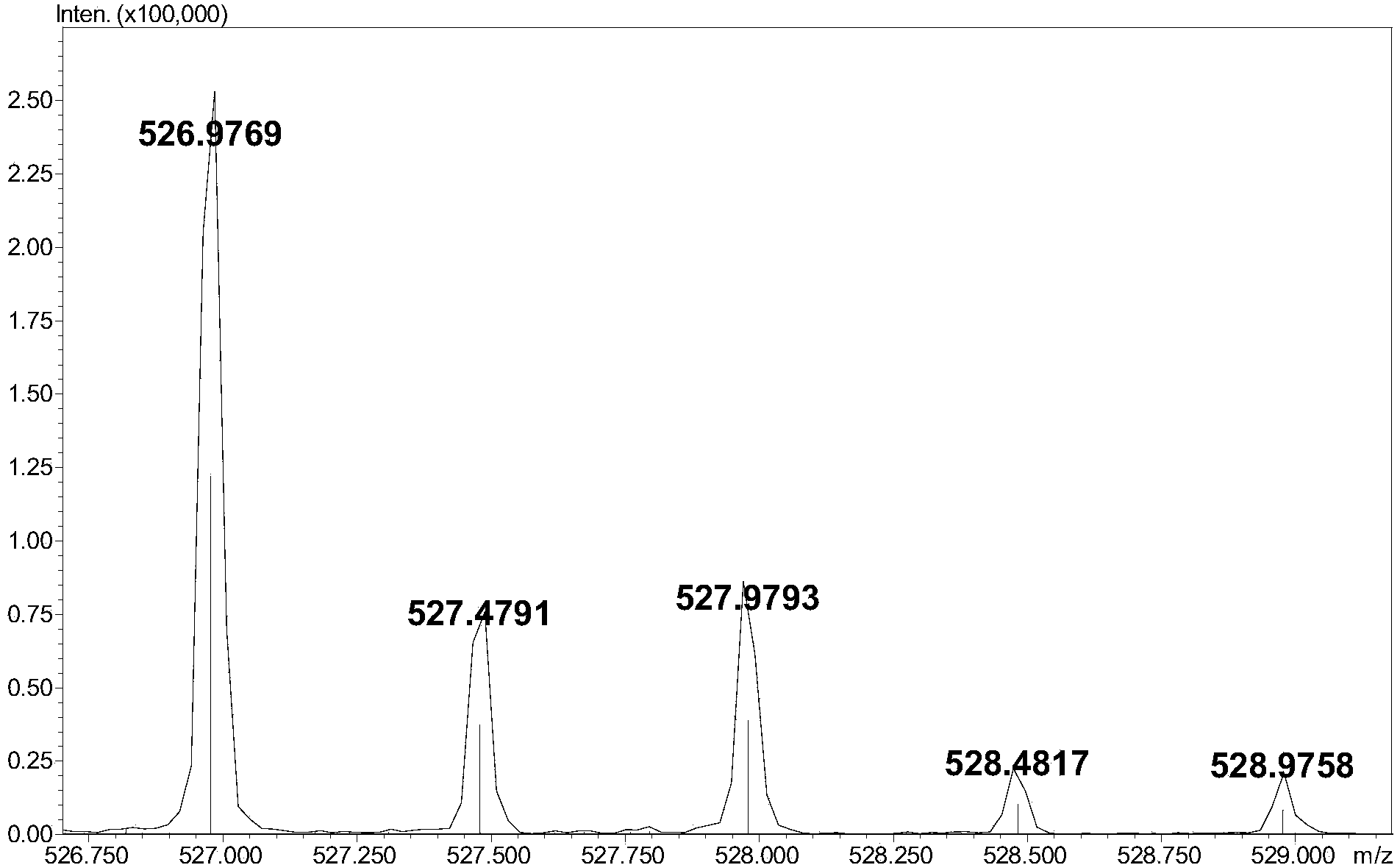
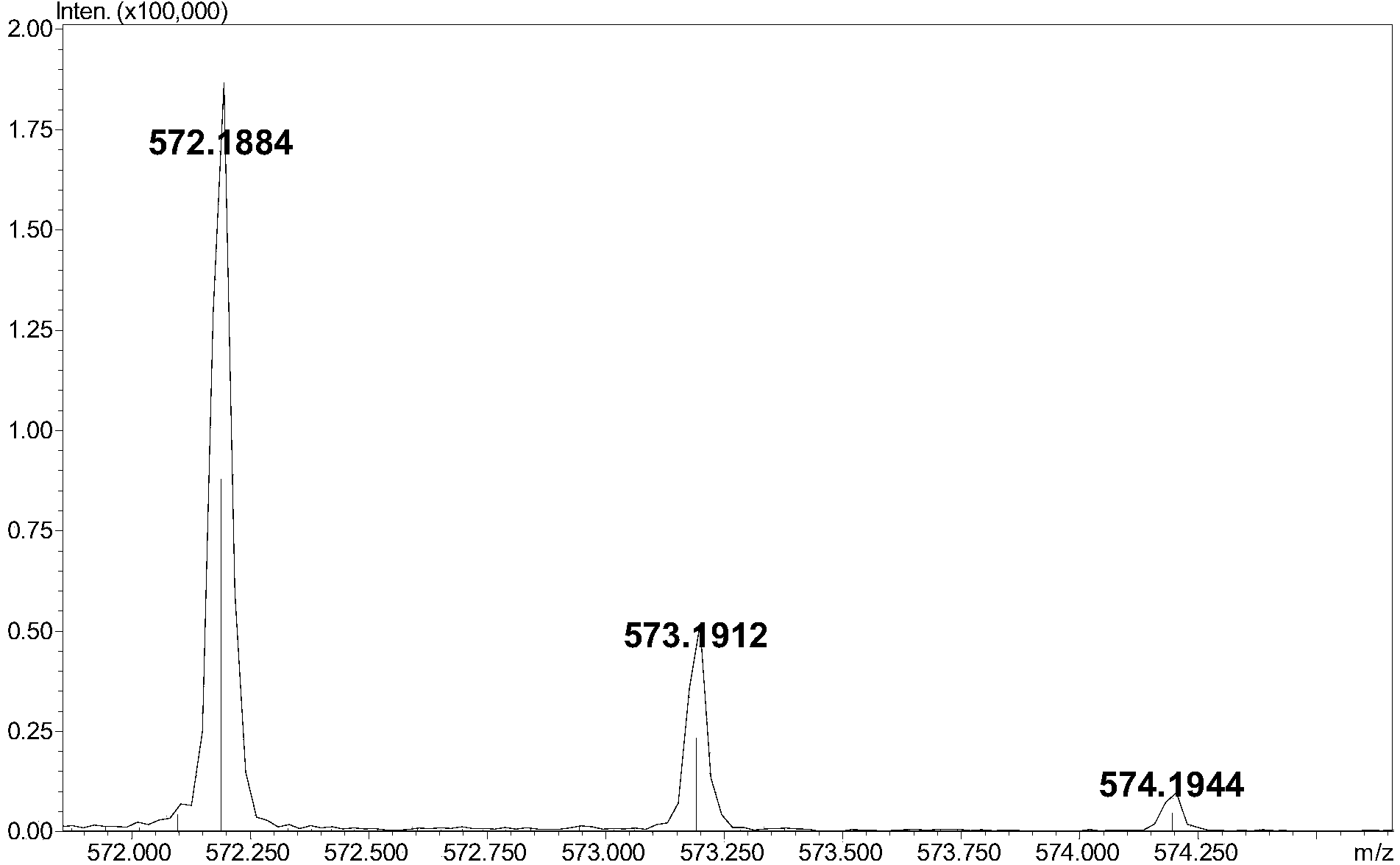
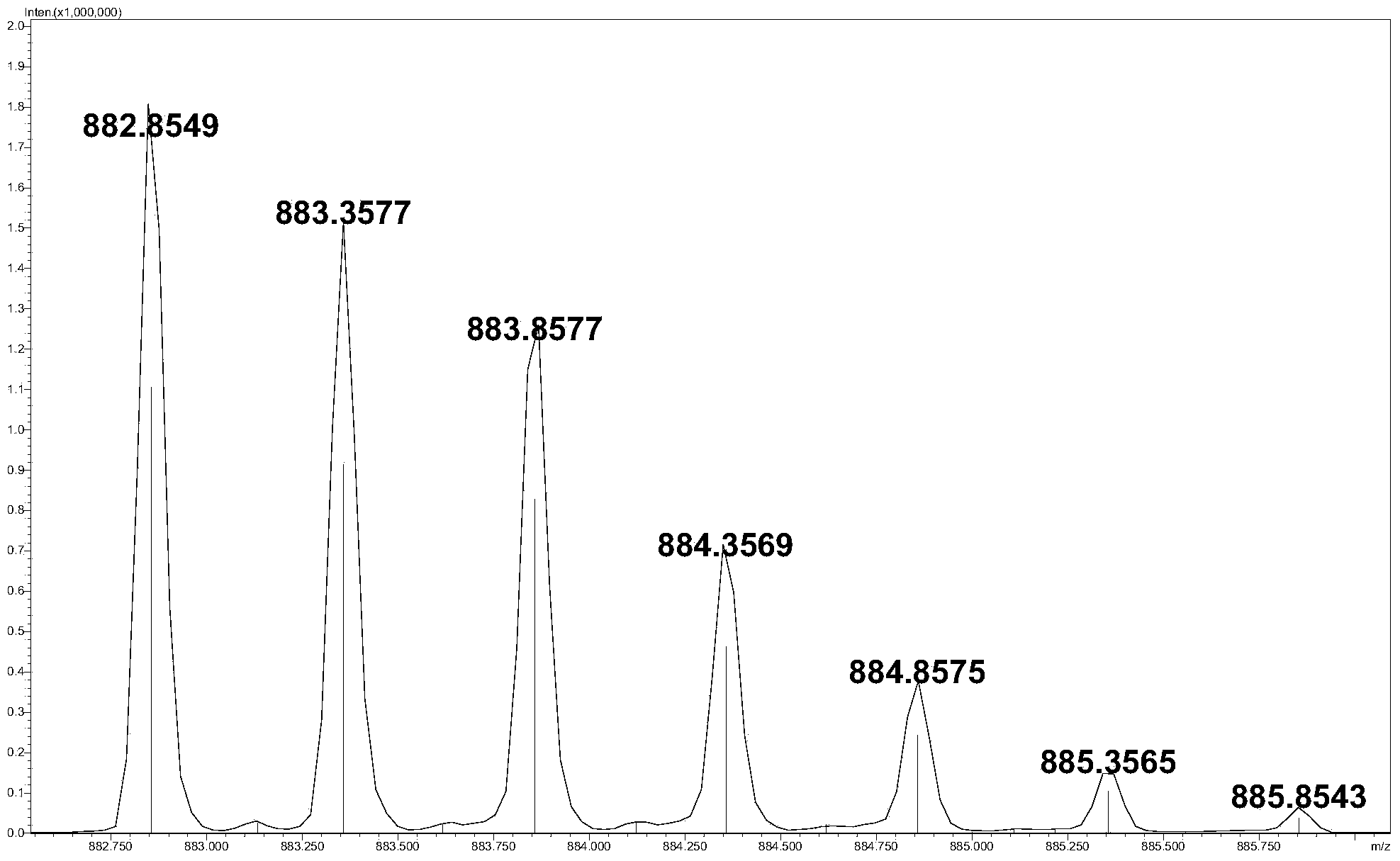
The invention relates to a method for detecting low-molecular-weight heparin by combining ion pair reversed phase chronmatogaphy and mass spectrum. The method comprises the steps of: detecting all compositions of low-molecular-weight heparin by combining ion pair reversed phase chronmatogaphy and high-resolution mass spectrum, separating main compositions from a sample through the ion pair reversed phase chronmatogaphy, obtaining precise molecular weight by the high-resolution mass spectrum, and calculating information of a carbohydrate chain sequence, including structures of two ends, length of a carbohydrate chain and substitution number of ethanoyl and sulfate, thus carrying out fine surface features on the low-molecular-weight heparin. The method has great practical values on improving the heparin detection level and ensuring drug safety.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
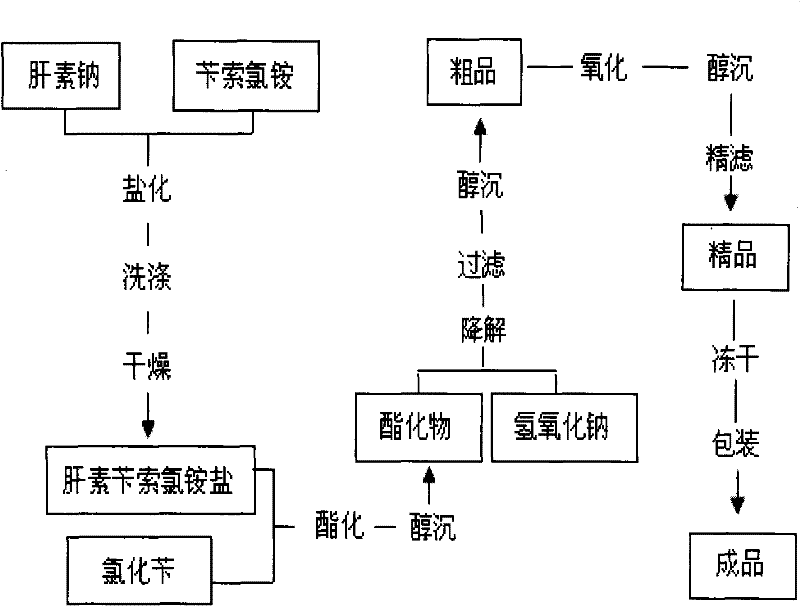
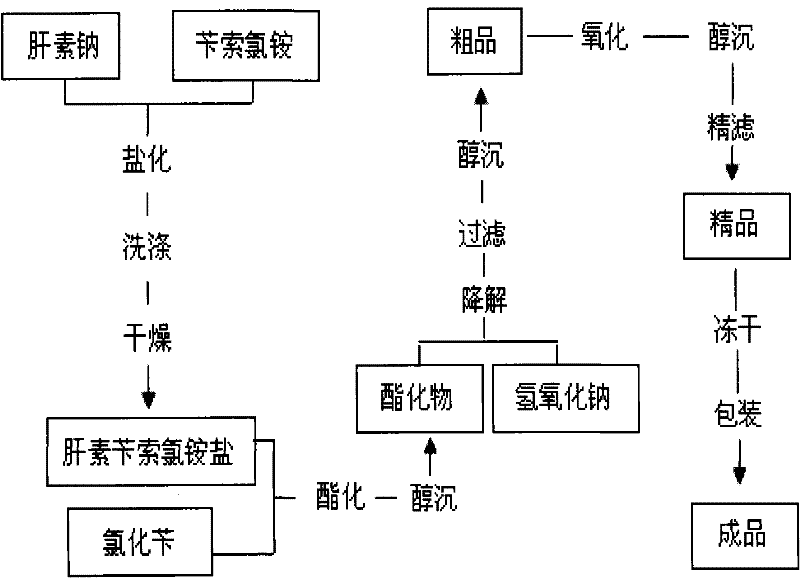
Method for preparing enoxaparin sodium
The invention discloses a method for preparing enoxaparin sodium, comprising the steps of salinizing, drying, esterfying, alcohol precipitating, oxidizing, alcohol precipitating, fine filtering and freeze drying. In the method provided by the invention, a hydrophilic liquid phase reaction, a hydrophobic liquid phase reaction and a solid phase reaction are adopted, so that macromolecule sodium heparin is degraded into micromolecule sodium heparin with a specific structure, and the molecular weights of products and molecular weight distribution ranges are controlled, thus anti-FIIa activity resulting in bleeding risk is greatly reduced, the anti-FXa activity is relatively improved, and the product effectiveness and safety advantages are obvious. The enoxaparin sodium can be used for effectively preventing venous thromboembolism and pulmonary embolism, can be used for thrombosis before and after operations of orthopedic surgery and neurosurgery, and can be used for greatly reducing apoplexy risk, more effectively reducing death, cardiac failure and recurrent angina of patients suffering from unstable coronary artery syndromes, reducing hypertriglyceridemia and effectively eliminating the side effects of haemorrhage, osteoporosis and induced thrombocytopenia after long-term use of common unfractionated heparin sodium and derivates of common unfractionated heparin sodium.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Process for preparing high-purity low-molecular heparin sodium
The invention discloses a process for preparing high-purity low-molecular heparin sodium and belongs to the field of bioengineering. The process mainly comprises the following steps of: salting-out and impurity removal, supplementary material warming and oxidization, gel elution, ion exchange, ethanol precipitation, vacuum drying and the like. The problems that the existing heparin sodium structure analog is hard to remove, the purity can not achieve the requirements for medicinal use, the preparation process is complicated, and the like are solved. The process has the beneficial effects that by adopting a supplementary material warming and oxidization method, the preparation and the decolorization of the low-molecular heparin sodium are realized, and the heparin sodium structure analogue is effectively removed by using weakly-basic anion exchange resin; and the process has the advantages of shortened production period, saved cost, simple process, easiness in operation and is suitable for industrial production.
Owner:NANJING XINBAI PHARMA +1
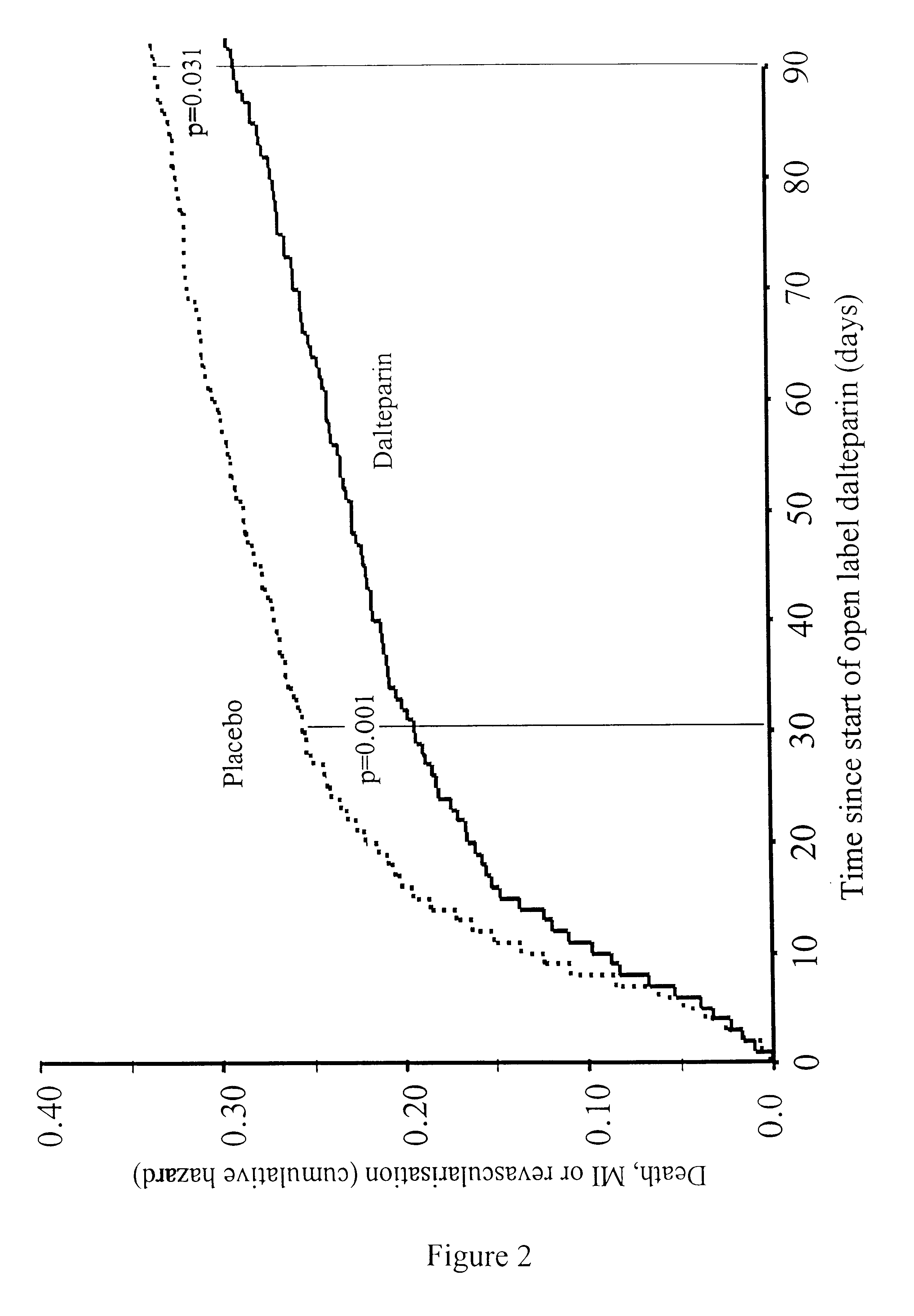
Method for treatment of unstable coronary artery disease by an early revascularisation together with administration of a low molecular weight heparin
InactiveUS6258798B1Reduce riskPromote resultsOrganic active ingredientsCardiovascular disorderDiseaseCoronary artery disease
The invention relates to a method for treatment of unstable coronary artery disease, which is characterised by an early revascularisation together with administration of a low molecular weight heparin. Preferably the low molecular weight heparin is administered up to revascularisation. The methods are also useful for treatment of unstable angina and not worsening chest pain and for treatment of non-Q-wave myocardial infarction (mild heart attack).
Owner:PHARMACIA AB
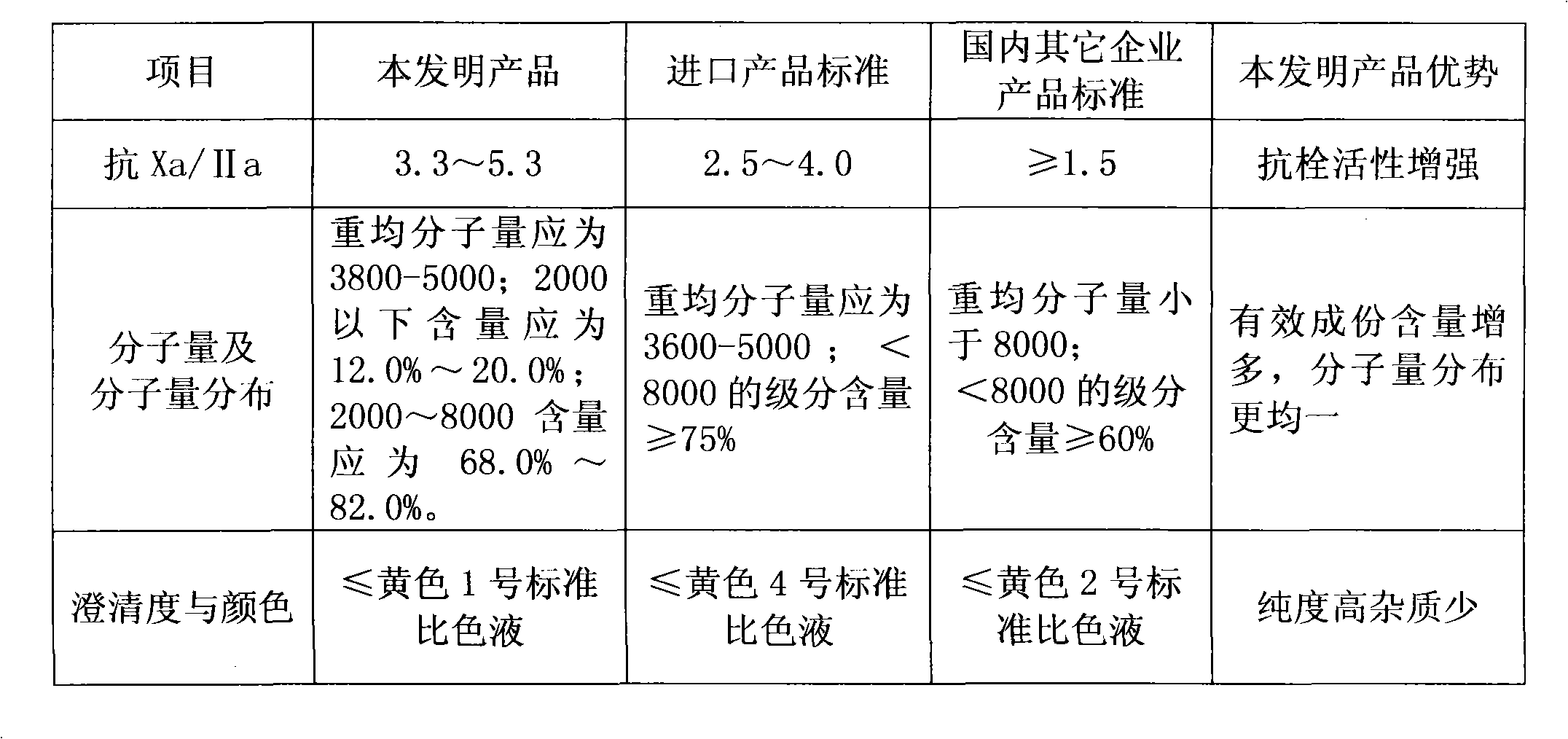
Process for preparing low moledule heparin calcium of low nitrite content
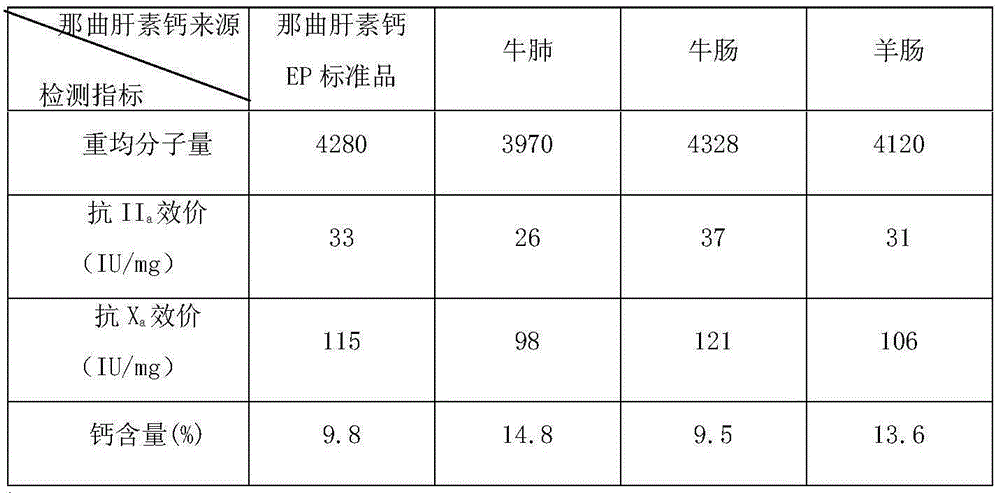
ActiveCN1554671ALow costSimple processOrganic active ingredientsBlood disorderLow molecular mass heparinChemistry
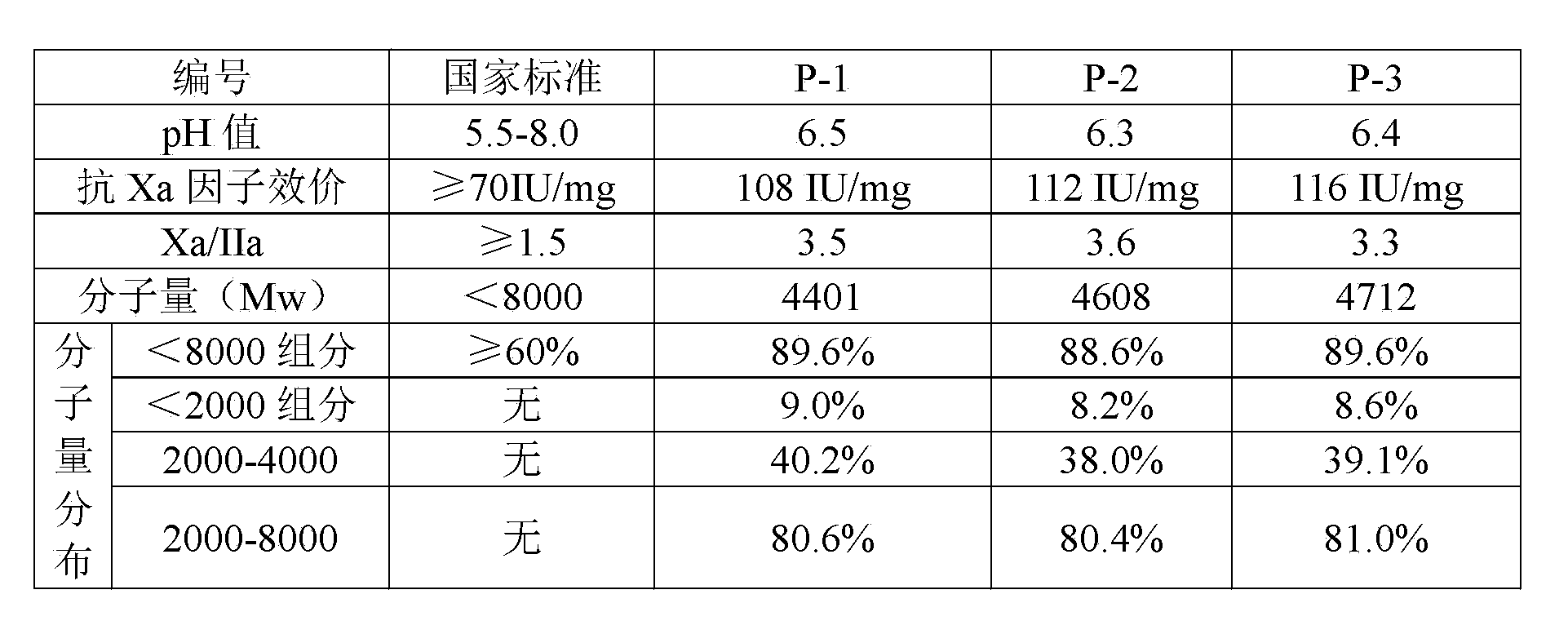
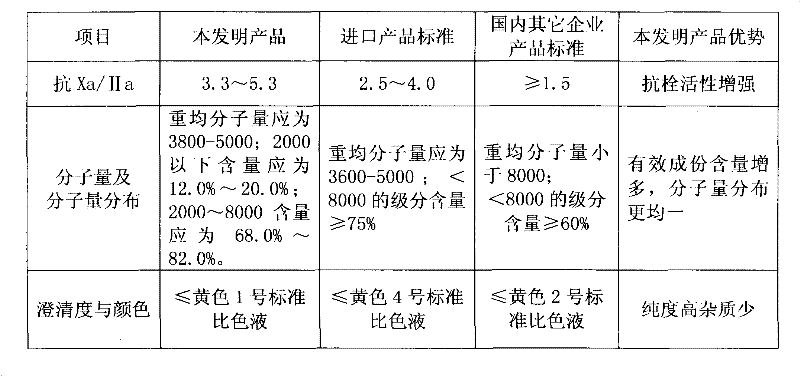
The preparation process of low molecular weight heparin calcium includes the following steps: dissolving refined heparin sodium in acetic acid aqua and adding sodium nitrite to the solution for cracking for 4-6 hr to obtain cracked solution; neutralizing the solution with calcium hydroxide to pH 5.5-6.0; filtering to eliminate insoluble matter, precipitating the solution with saturated ethanol solution of calcium chloride and collecting the precipitate; dissolving the precipitate in injection water and regulate pH with hydrochloric acid to 0.3-1.0; neutralizing the solution to pH 6.0-6.5; filtering to eliminate insoluble matter, precipitating the filtrate with saturated ethanol solution of calcium chloride and collecting the precipitate; and conventional decompression drying to obtain low molecular weight heparin calcium product. The product has average molecular weight of 3000-5000 D, Xa resisting activity not lower than 90 IU / mg, Xa resistance / IIa resistance ratio of 3-5 and nitrite content lower than 2 ppm.
Owner:ZHAOKE PHARMA HEFEI
Antimicrobial peptide freeze-dried preparation for treating colpitis and preparation method thereof
InactiveCN104043098AStrong anti-inflammatory, anti-itch and anti-bacterialProtect physical and mental healthOrganic active ingredientsPeptide/protein ingredientsProtozoaCancer cell
The invention provides a freeze-dried preparation for treating colpitis. The preparation includes an antimicrobial peptide freeze-dried powder and an antibacterial peptide basic liquid. The antibacterial peptide freeze-dried powder comprises the following components according to the total mass percentage: 0.1%-8% of an antibacterial peptide human beta defensin, 0.01%-0.2% of a low molecular weight heparin sodium and 1%-15% of a stabilizing agent; the antibacterial peptide basic liquid comprises the following components according to the total mass percentage: 0.01%-0.4% of hyaluronic acid, 0.5%-4% of menthoxypropanediol, 0.5%-5% of a honey extract, 0.5%-5% of PCA sodium, 0.5%-5% of dissolved protease, 0.3%-0.7% of 1,2 hexanediol, 1.5%-6% of oat beta dextran, 1%-5% soybean isoflavone, and the balance of deionized water or water for injection. The antimicrobial peptide freeze-dried preparation provided by the invention has broad-spectrum antibacterial activity, and strong killing effect on bacteria, fungi, viruses, protozoa and cancer cells, and even can enhance immunity, accelerate wound healing process; in addition, the preparation has obvious curative effect, and no rebound or side effect.
Owner:广州舒泰生物技术有限公司
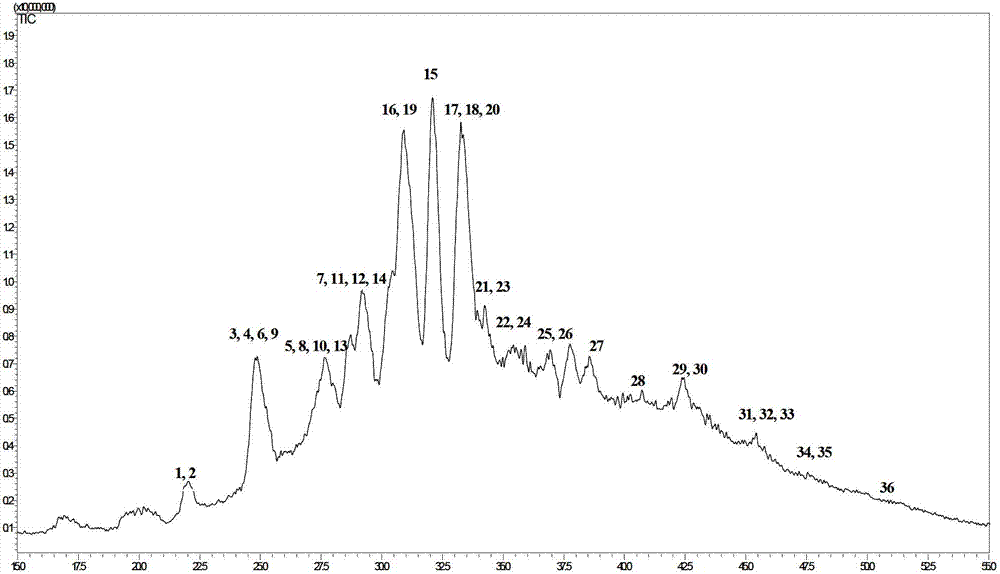
Reverse-phase chromatography and mass-spectrometry combined detection method for complete low-molecular-heparin degradation product through precolumn derivatization
ActiveCN103630647AEnsure safetySolve the problem that only 8 common heparin disaccharides can be detectedComponent separationReversed-Phase Liquid ChromatographyMass Spectrometry-Mass Spectrometry
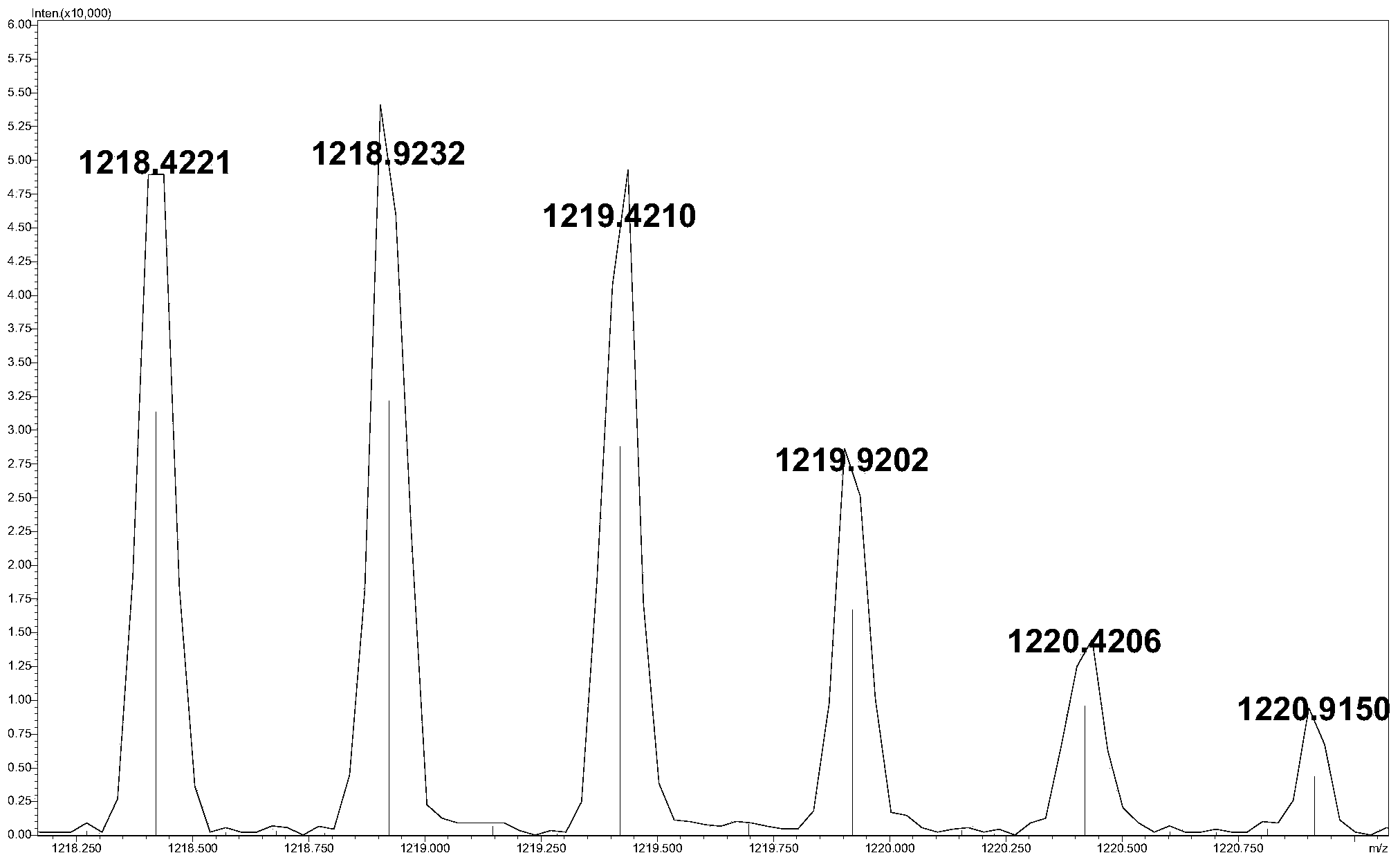
The invention relates to a reverse-phase chromatography and mass-spectrometry combined detection method for a complete low-molecular-heparin degradation product through precolumn derivatization. All components of the complete low-molecular-heparin degradation product are detected by combining reversed-phase chromatography and a high-resolution mass spectrum; all the components in a sample are separated through the reversed-phase chromatography, and a precision molecular weight can be obtained through the high-resolution mass spectrum; besides detection of eight common heparin disaccharides, tail end modification structures and special structures such as 3-O-tetrose-hydrosulfates relevant to anticoagulation and trisaccharides obtained through peeling reaction can be also detected; therefore, the structure of each complete low-molecular-heparin degradation product can be precisely represented. According to the reverse-phase chromatography and mass-spectrometry combined detection method, the problem that only the eight common heparin disaccharides can be detected in the prior art is solved; the reverse-phase chromatography and mass-spectrometry combined detection method has an extremely large practical value in research, development and production control of low-molecular-heparin imitating drugs and guarantee of the safety of the drugs.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Process for producing low molecular sodium heparin
The invention discloses a process for producing low molecular sodium heparin by using raw sodium heparin as a raw material, which comprises the steps of: producing a sodium heparin solution; degradingthe solution; neutralizing the solution; collecting a precipitation; producing low molecular raw sodium heparin; producing low molecular fine sodium heparin and the like. The low molecular sodium heparin has the characteristics of rich sources of the raw material, high yield, stable and reliable quality, high purity, low cost, long curative effect, no toxic or side effect, simple process, convenient operation and no 'three wastes' discharge, has the effects of coagulation resistance, thrombosis resistance, tumor resistance, inflammation resistance, allergy resistance and the like and the efficiency of regulating blood lipid, has significant curative effect and wide application range, can be produced into injections, capsules, ointments and the like, is convenient for massive production, and is widely used for producing coagulation resistant low molecular sodium heparin.
Owner:SICHUAN TIANCHENG BIOCHEM TECH
Preparation and purification process for nadroparin calcium
ActiveCN103382232AEfficient separationChange fractional precipitation methodBlood disorderExtracellular fluid disorderFractional PrecipitationDepolymerization
The invention relates to a preparation and purification process for nadroparin calcium. The process comprises the following steps: depolymerization; reduction; recrystallization; chromatography; and filtration. According to the invention, the process can effectively extract a fragment with a narrow molecular weight distribution range; nadroparin calcium is obtained through improvement of a preparation process for low-molecular-weight heparin calcium, an out-dated solvent fractional precipitation method is changed, conditions for anion exchange chromatography are optimized, and the fragment both meeting requirements for molecular weight of LMWH in China and according with molecular weight distribution of nadroparin calcium are obtained through gradient?elution.
Owner:CHANGSHAN BIOCHEM PHARM JIANGSU CO LTD
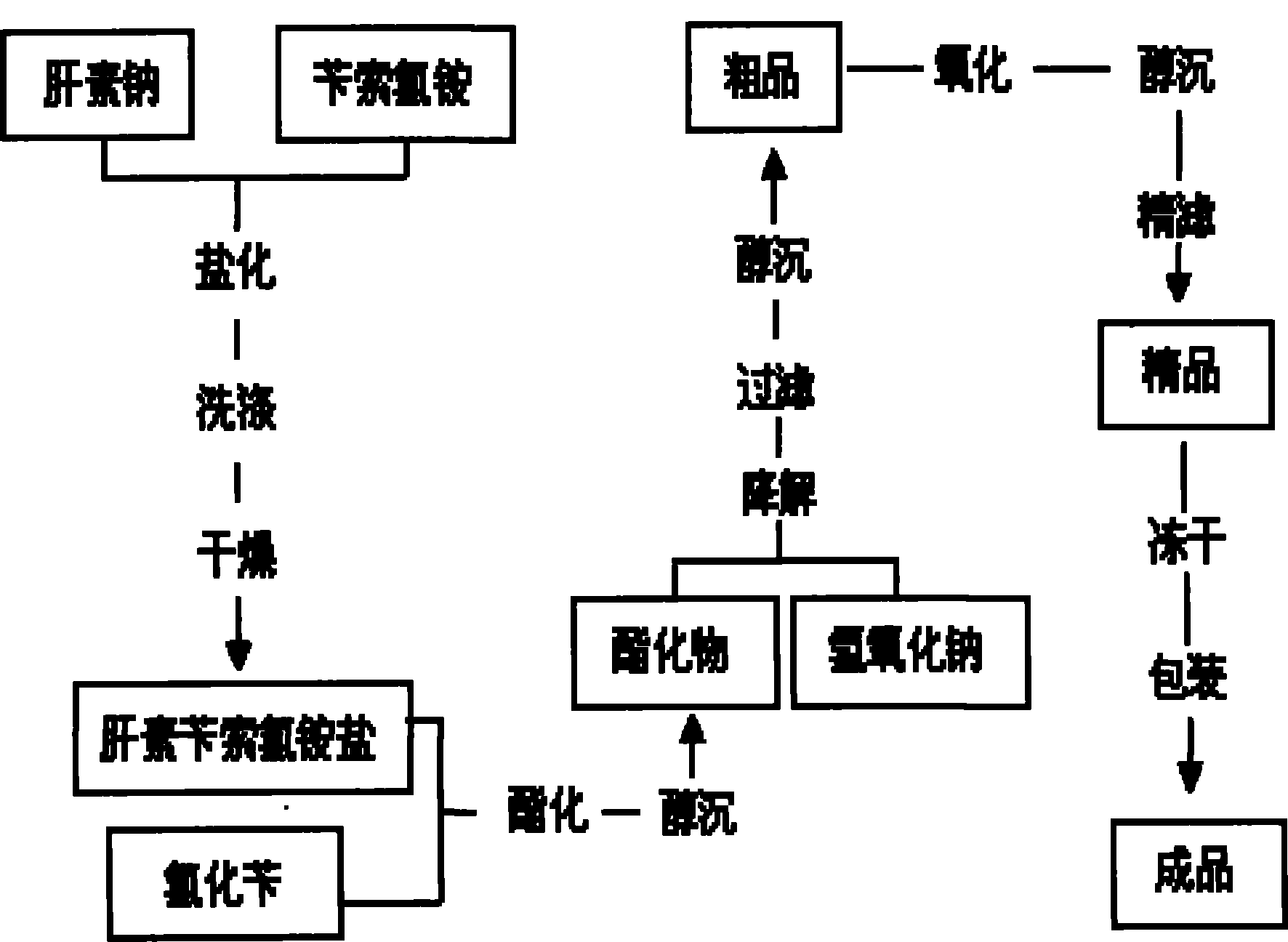
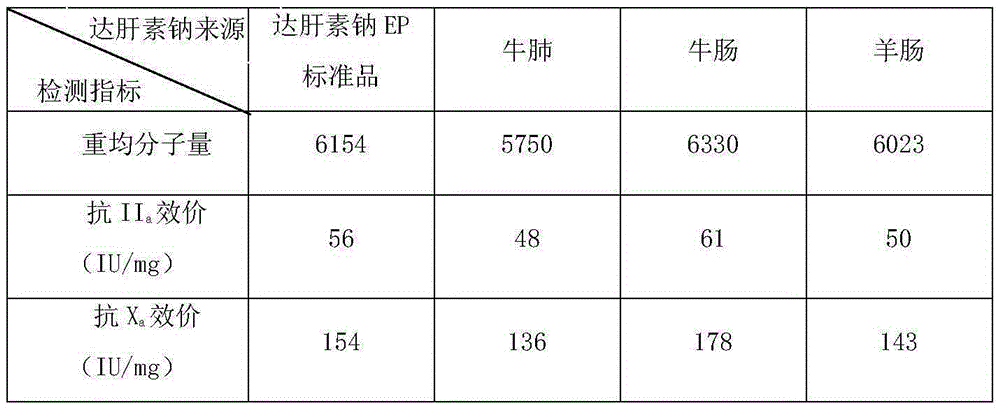
Production method of dalteparin sodium fine product
The invention discloses a production method of dalteparin sodium. According to the production method, a heparin sodium coarse product is taken as a raw material, a low-molecular-weight heparin sodium coarse product is obtained through degradation, reduction and ethanol precipitation; compared with a method taking a heparin sodium fine product as the raw material, the method is wider in application range and can better control the quality of a dalteparin sodium fine product from the source. Innovatively, molecular weight and distribution of the low-molecular-weight heparin sodium coarse product before chromatography are tested through HPLC (high performance liquid chromatography), corresponding required-to-be-removed areas of macromolecules and micromolecules and corresponding volumes are calculated according to the formula, filtrate in the chromatography process is collected, a low-molecular-weight heparin sodium fine product liquid which meets the requirements is accurately and quantitatively obtained, and use of an expensive molecular weight interception filtering membrane is avoided; a one-pot process is achieved through oxidation, sterilization filtration and sediment dehydration, the operation is simplified, and time and labor cost are saved; and a new method for preparing a raw dalteparin sodium material is developed on the basis of the prior art, and industrial production of dalteparin sodium is realized.
Owner:NANJING KING FRIEND BIOCHEM PHARMA CO LTD
Preparation process of nadroparin calcium
ActiveCN104086673AHigh activityHighly Active Nadroparin Calcium Product, Product QualityAntipyreticAnalgesicsNitro compoundFreeze-drying
The invention relates to a preparation process of nadroparin calcium. The preparation process comprises the following steps: S1, degrading heparin sodium; S2, reducing degradation liquid; S3, performing ultraviolet radiation, namely radiating reduction liquid by using an ultraviolet lamp to remove residual nitro compounds from the reduction liquid and reduce the content of N-NO groups; S4, performing calcium conversion and concentration, namely performing ultrafiltration on an ultrafiltration membrane by using a 5% calcium chloride solution, and concentrating the reduction liquid until the solid-liquid ratio is 1:10; S5, performing anion exchange chromatography, namely performing chromatography by adopting anion exchange resin, and collecting elution liquid; S6, freeze-drying to obtain nadroparin calcium. According to the process, the molecular weight of low-molecular-weight heparin calcium is controlled through the anion exchange chromatography, so that the molecular weight can be accurately controlled within 1% and the yield can be increased to more than 50%; moreover, the automation degree is high and the operation is convenient, so that a powerful guarantee is provided for increasing the production capacity.
Owner:CHANGZHOU QIANHONG BIOPHARMA
Purifying method for low molecule heparin
The present invention relates to the extraction and purification method of low molecular weight heparin for treating blood coagulation and thrombus. The coarse low molecular weight heparin material is dissolved in NaCl solution and chromatographically separated with molecular sieve gel layer to obtain the chromatographic separated liquid, and the chromatographic separated liquid is alcohol precipitated, dewatered and dried to obtain low molecular weight heparin segment. Before chromatography, the coarse product is irradiated with ultraviolet lamp or reacted with oxidant sodium hypochlorite or hydrobromic acid to eliminate harmful ¿CN-NO- radical, and treated with strong anionic exchange resin to eliminate methanol and other chemical impurity. The present invention has the low molecular weight heparin product reaching relevant international standard, and the method is simple and high in yield.
Owner:NANJING KING FRIEND BIOCHEM PHARMA CO LTD
Engineering strain construction method for improving activity of heparinase I
InactiveCN106497897AImprove the efficiency of enzymatic hydrolysis preparationLow costNucleic acid vectorFermentationProtein targetGenetic engineering
The invention relates to an engineering strain construction method for improving the activity of heparinase I. An amino acid sequence is optimized according to spatial structure analysis on the heparinase I to obtain Hep169 with the activity improved by 48% compared with the heparinase; a Hep169 gene is optimized according to codon preference, a gene DNA is obtained through artificial synthesis and is cloned into an expression vector to perform fusion expression with SUMO and other labels, host cell conversion and screening are performed to establish a soluble genetic engineering production system of the Hep169, analysis results show that a target protein obtains an efficient soluble expression, has very good biological activity and can efficiently crack heparin to generate low-molecular-weight heparin. By the adoption of the method, the highly active HepI is provided, a new method is provided for efficient soluble expression gene engineering production of the heparinase I, the production cost of the low-molecular-weight heparin and other drugs can be effectively reduced, and the engineering strain construction method has wide application prospect.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Mesenchymal stem cell preservation liquid, and preparation method and application thereof
ActiveCN102365933AProlong the duration of activityEasy to prepareDead animal preservationCord blood stem cellMedicine
The invention belongs to the field of biologic medicines and discloses mesenchymal stem cell preservation liquid, and a preparation method and application thereof. The mesenchymal stem cell preservation liquid contains AB cord blood plasma which is 5-15% of the volume of the preservation liquid and low molecular weight heparin calcium which is 70-80 AXaIU / ml. The AB cord blood plasma and mesenchymal stem cells come from the same provider. By means of the mesenchymal stem cell preservation liquid provided by the invention, the activity maintaining time of the mesenchymal stem cells can be prolonged (above 90% of activity is kept at 4-15 DEG C for 72 h); after the mesenchymal stem cells are preserved in the preservation liquid for 72h, the cells are normal in form, the proliferation and amplification capability of the cells cannot be influenced, and the phenotypic characteristics of the mesenchymal stem cells cannot be influenced; the mesenchymal stem cell preservation liquid disclosed by the invention is safe and reliable for clinical application; and, because the AB cord blood plasma and the cord mesenchymal stem cells come from the same placenta and belong to auto-plasma, the possibility of exogenous virus pollution is reduced.
Owner:广州市天河诺亚生物工程有限公司
Preparation method for low-molecular heparin originated from new species
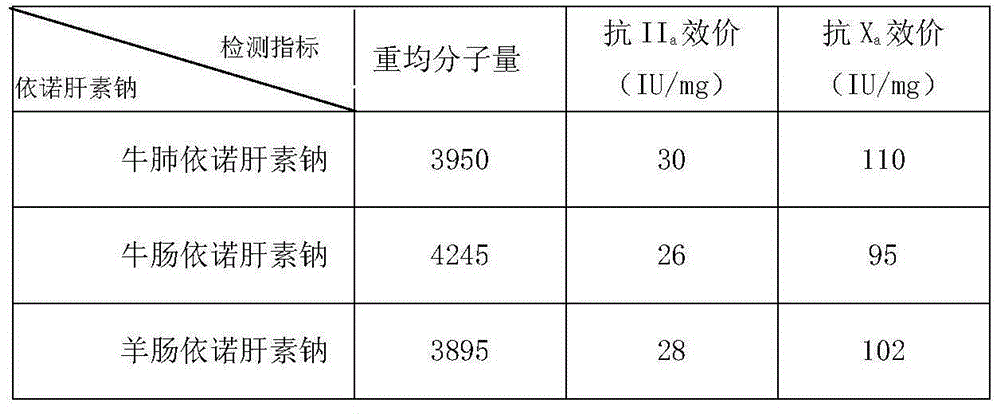
The invention relates to a preparation method for low-molecular heparin originated from a new species. The preparation method comprises the following steps: (1) fully dissolving heparin sodium prepared from raw materials bovine lungs, ox intestines and / or goat intestines in water, and adding a benzethonium chloride solution to prepare heparin benzethonium chloride salt; (2) dissolving the heparin benzethonium chloride salt in dichloromethane, adding benzyl chloride, stirring the mixture to react, reducing the temperature and adding a sodium acetate methanol solution to prepare heparin benzyl ester; (3) dissolving the heparin benzyl ester in water, adding sodium hydroxide, reducing the temperature after reaction, adjusting the pH to neutral, adding sodium chloride, and then adding alcohol to prepare an enoxaparin sodium crude product; and (4) dissolving the enoxaparin sodium coarse product in water, and purifying and drying the mixture to obtain enoxaparin sodium. According to the preparation method provided by the invention, the condition that the low-molecular heparin can be obtained by heparin sodium prepared from bovine lungs, ox intestines and goat intestines is found for the first time, and the prepared low-molecular heparin is lower in molecular weight compared with that of existing low-molecular heparin, so that the biological activity is higher and the low-molecular heparin has a wide market application prospect.
Owner:SHANDONG UNIV
Method for preparing enoxaparin sodium
The invention discloses a method for preparing enoxaparin sodium, comprising the steps of salinizing, drying, esterfying, alcohol precipitating, oxidizing, alcohol precipitating, fine filtering and freeze drying. In the method provided by the invention, a hydrophilic liquid phase reaction, a hydrophobic liquid phase reaction and a solid phase reaction are adopted, so that macromolecule sodium heparin is degraded into micromolecule sodium heparin with a specific structure, and the molecular weights of products and molecular weight distribution ranges are controlled, thus anti-FIIa activity resulting in bleeding risk is greatly reduced, the anti-FXa activity is relatively improved, and the product effectiveness and safety advantages are obvious. The enoxaparin sodium can be used for effectively preventing venous thromboembolism and pulmonary embolism, can be used for thrombosis before and after operations of orthopedic surgery and neurosurgery, and can be used for greatly reducing apoplexy risk, more effectively reducing death, cardiac failure and recurrent angina of patients suffering from unstable coronary artery syndromes, reducing hypertriglyceridemia and effectively eliminatingthe side effects of haemorrhage, osteoporosis and induced thrombocytopenia after long-term use of common unfractionated heparin sodium and derivates of common unfractionated heparin sodium.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
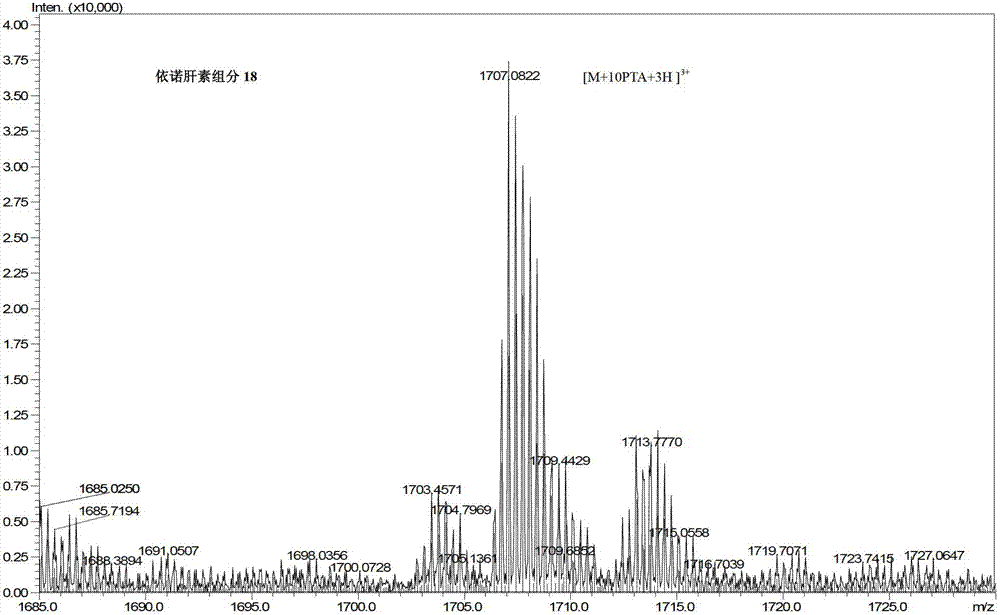
Ion pair antiphase chromatography and mass spectrometry combined detection method for low-molecule heparin part degradation products
ActiveCN103454372AFine characterizationImprove detection levelComponent separationChromatographic separationUltraviolet absorption
The invention relates to an ion pair antiphase chromatography and mass spectrometry combined detection method for low-molecule heparin part degradation products. All components of the low-molecule heparin part degradation products are detected in a manner of combining ion pair antiphase chromatography and high-resolution mass spectrometer; all the components in a sample are separated through the ion pair antiphase chromatography, and a precise molecule weight is obtained through the high-resolution mass spectrometer, so that sugar chain sequence information, including structures of two ends, lengths of sugar chains and substitution numbers of acetyl and sulfuric acid, of all the components is calculated, so that the structures of the low-molecule heparin part degradation products can be finely represented. The ion pair antiphase chromatography and mass spectrometry combined detection method can detect the structures of the two ends, the lengths of the sugar chains and the substitution numbers of the acetyl and the sulfuric acid of the low-molecule heparin part degradation products; the problem in the prior art that only an ultraviolet absorption atlas for degradation of low-molecule heparin parts can be obtained is solved; the ion pair antiphase chromatography and mass spectrometry combined detection method has a high practicability value in improvement of the detection level of heparin and guarantee of drug safety.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Method for preparing relaxing and face-cleansing cosmetics and application
ActiveCN102379838ACosmetic preparationsAntipyreticRecombinant Human Keratinocyte Growth FactorVitamin C
The invention relates to a method for preparing relaxing and face-cleansing cosmetics and application. The cosmetics comprises the following components in percentage by mass: 0.00001 to 0.001 percent of recombinant human keratinocyte growth factors, 0.00001 to 0.001 percent of recombinant human interleukin-1 receptor antagonist, 0.1 to 0.5 percent of low-molecular sodium heparin, 5 to 25 percent of stabilizer, 0.02 to 0.3 percent of hyaluronic acid, 0.5 to 8 percent of vitamin C sodium phosphate, 0.5 to 5 percent of methyl propanediol, 0.3 to 0.7 percent of 1,2 hexanediol, 0.5 to 3.5 percent of lactobacillus / mung bean fermentation liquor, 0.5 to 5 percent of dissolved protease and the balance of water. The relaxing and face-cleansing cosmetics are applied to sensitive skin, can be stored for a long time, and is suitable for long-term use.
Owner:广州泰润合投资有限公司
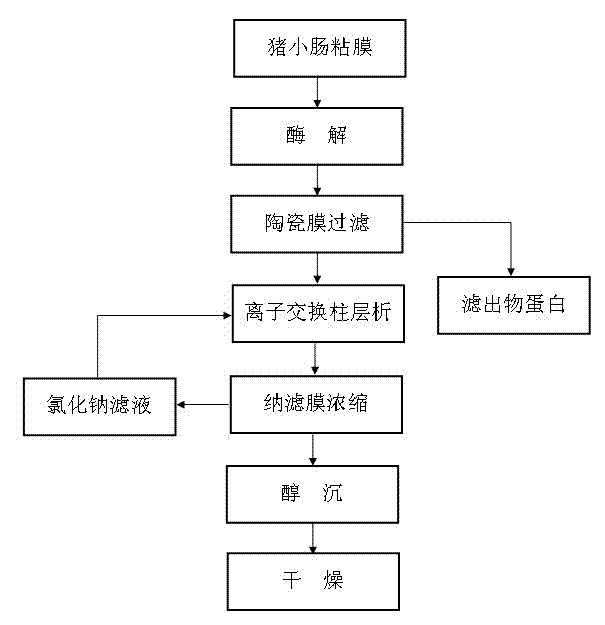
Process for preparing heparin sodium through membrane separation
The invention discloses a process for preparing heparin sodium through membrane separation. The process comprises the steps: intestinal mucosa enzymolysis, ceramic membrane filtration, ion exchange column chromatography, nanofiltraiton membrane concentration, alcohol precipitation and drying, wherein the optimal condition for the intestinal mucosa enzymolysis is formed by the proper proportion of intestinal mucosa to water, enzymolysis temperature and pH value of a system. A protease can take the optimal enzymolysis effect, the structure and activity of heparin are not damaged when the glucosidic bond linked between protein and the heparin is hydrolyzed, meanwhile, fewer low-molecular heparins are generated, the activity of the heparin can be improved by 20%, and the generated low-molecular heparins can be reduced by 10%. Due to the adoption of the ceramic membrane filtration, protein impurities and the heparin in an enzymatic hydrolysate can be effectively separated, so that the heparin loss is greatly reduced and can be reduced by 5-8% as comparison with that of the traditional process, the production cost is reduced, the discharged wastewater can be reduced by 50-60%, the resin utilization ratio can be increased by 40-50%, the consumption of auxiliary materials is low, the quality of the heparin sodium is stable, the process is convenient to operate, and the defects such as high impurity content in the enzymatic hydrolysate, serious waste liquid discharge pollution, high personnel utilization rate and the like are overcome.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Detection method for evaluating curative effect and residual condition of heparin drugs
The invention relates to the field of blood detection, in particular to a detection method for evaluating the curative effect and the residual condition of heparin drugs. The heparin drugs mainly comprise heparin, low-molecular-weight heparin and some heparinoid drugs. The detection method mainly comprises steps as follows: heparinase, a freeze-drying protective agent and an adhesive are mixed, then a mixed reagent in appropriate proportion is prepared from an obtained mixture by the aid of a buffer solution, the mixed reagent is enveloped in a test-cup and subjected to freezing and vacuum pumping treatment, and a heparinase cup is obtained. The detection method has a plurality of advantages that the operation is simple, the guarantee period is long, the stability is good and the like. Theheparinase cup and a common cup (a blank sample cup) are put in a thrombelastogram, an anticoagulant and a chelating agent are added to the cups respectively, endogenously activated whole blood samples are added to the test-cups, and thromboelastography is started for detection; values R of starting time of blood clot formation in the heparinase cup and the common cup are compared, and whether heparin exists in the blood can be judged; if heparin exists, the value R of the starting time of blood clot formation in the heparinase cup is smaller than that of the starting time of blood clot formation in the common cup. The method is simple to operate, a detection result is good in repeatability, and the accuracy is high.
Owner:北京乐普诊断科技股份有限公司
Compound low-molecular heparin sodium liposome gel and preparation method and application thereof
InactiveCN103356693AIncrease drug concentrationImprove permeabilityOrganic active ingredientsAerosol deliveryEthanol InjectionSkin Injury
The invention belongs to the technical field of medicines and relates to a compound low-molecular heparin sodium liposome gel and a preparation method and application thereof. The compound low-molecular heparin sodium liposome gel comprises low-molecular heparin sodium, asiaticoside, lipid components, a membrane softening agent and gel matrix components, wherein the low-molecular heparin sodium and asiaticoside are pharmacological active ingredients, and based on the total gel of 100g, the content of the low-molecular heparin sodium is 5000-35000IU, and the content of the asiaticoside is 0.1-2.5g. The preparation method comprises the following steps of: (1) preparing a compound low-molecular heparin sodium liposome by employing an ethanol injection-ultrasonic method or ethanol injection-high pressure homogenizer method; (2) preparing a blank gel; (3) adding the compound low-molecular heparin sodium liposome into the blank gel, uniformly stirring, and obtaining the compound low-molecular heparin sodium liposome gel. The gel is mainly used for treating skin injury and scar.
Owner:SHENZHEN HEPALINK PHARMA GRP CO LTD
Low molecule heparin and salt thereof, pharmaceutical composition comprising same and producing method thereof
InactiveCN1847268AGet efficientlyLow impurity contentOrganic active ingredientsDecompositionImpurity
To provide low-molecular-weight heparin or its salt that can be efficiently obtained by a simple method without requiring large-scale equipment and has extremely reduced impurity content, pharmaceutical compositions containing it, and production of low-molecular-weight heparin or its salt method. The low-molecular-weight heparin or its salt of the present invention can be treated with a reducing agent after reducing the molecular weight of the heparin and / or its salt, and after the reducing agent is removed and / or decomposed, the pH of the solution is adjusted to 4 Or get smaller.
Owner:Q P CORP
Mesenchymal stem cell injection as well as preparation method and application thereof
InactiveCN106177918AEasy to get materialsNo ethical issuesOrganic active ingredientsNervous disorderVitamin CMesenchymal stem cell
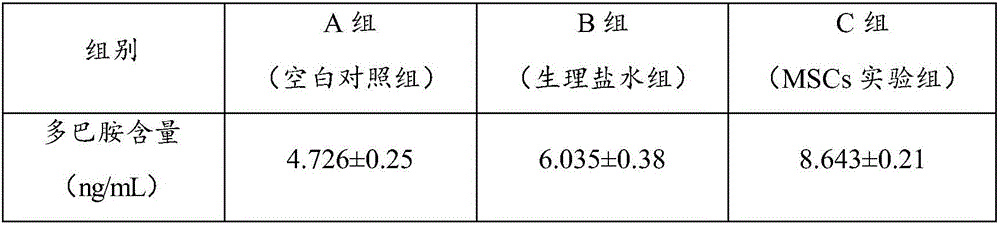
The invention relates to the technical field of stem cells, in particular to a mesenchymal stem cell injection as well as a preparation method and application thereof. The mesenchymal stem cell injection comprises mesenchymal stem cells, human serum albumins, low-molecular weight heparin calcium, vitamin C, lentinan, dimethyl sulfoxide and a menstruum. The mesenchymal stem cell injection provided by the invention can effectively maintain the activity of the stem cells, can increase the content of striatal dopamine and can effectively treat the Parkinson's disease.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Preparation method of novel source of low molecular weight heparin from nitrous acid degradation
The invention relates to a preparation method of a novel source of low molecular weight heparin from nitrous acid degradation. The steps are as follows: (I) completely dissolving raw materials in water, adding sodium nitrite to prepare a reaction solution A; (II), adding sodium borohydride, and reacting to prepare a reaction solution B; (III) adding an alcohol solution, conducting solid-liquid separation, drying to prepare a solid A, and then dissolving the solid A in water to prepare a reaction solution C; (IV) replacing sodium ions with calcium ions by cation resin exchange to prepare a reaction solution D; and (V) adding alcohol solution to the reaction solution D, fully mixing, conducting solid-liquid separation, purifying, and drying to prepare the low molecular weight heparin. The invention can fundamentally promote the diversification of species sources for low molecular heparin, expand the supply of raw materials for heparin drugs, and achieve a higher degree of stabilization of the market of heparin and low molecular weight heparin drugs.
Owner:SHANDONG UNIV
New purpose of pharmaceutical composition containing pioglitazone and heparin or low molecular heparin
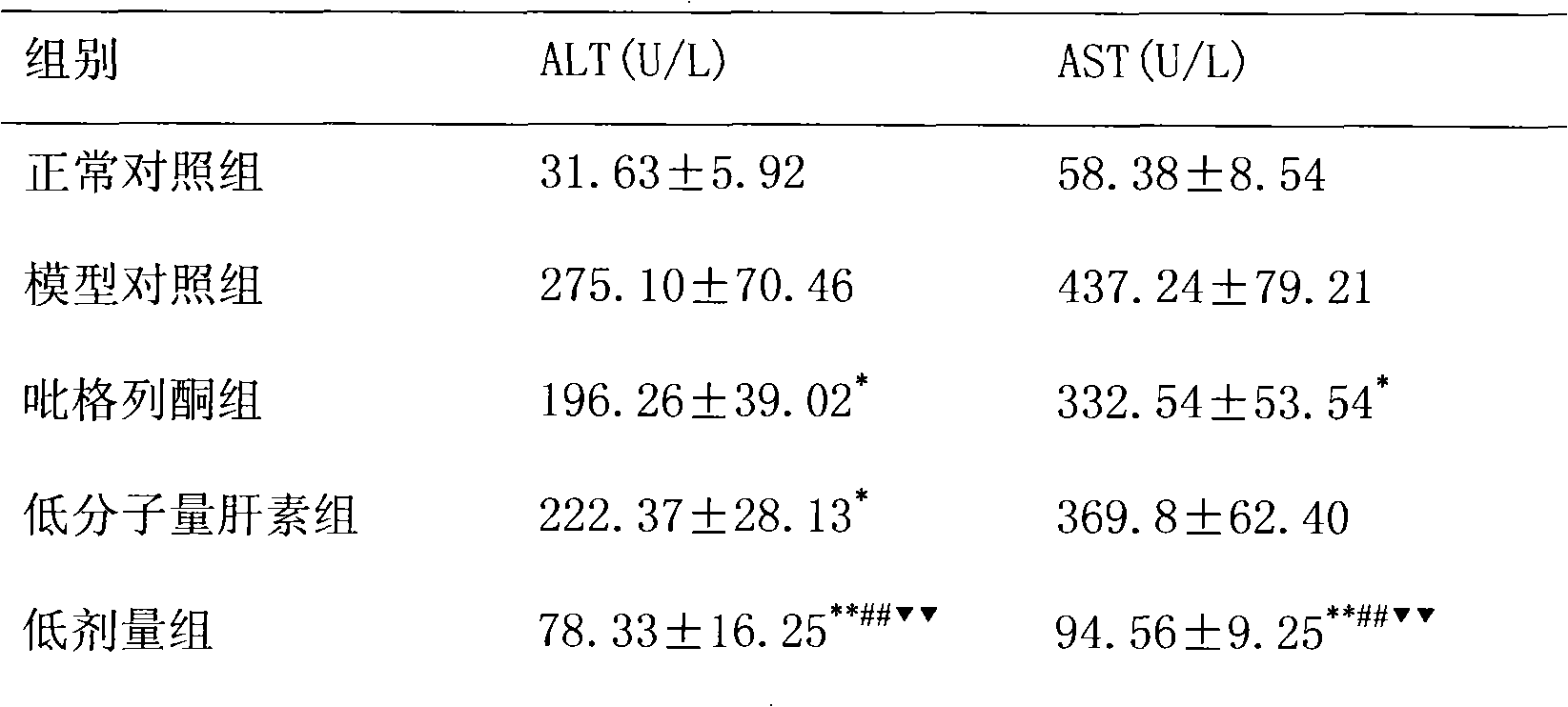
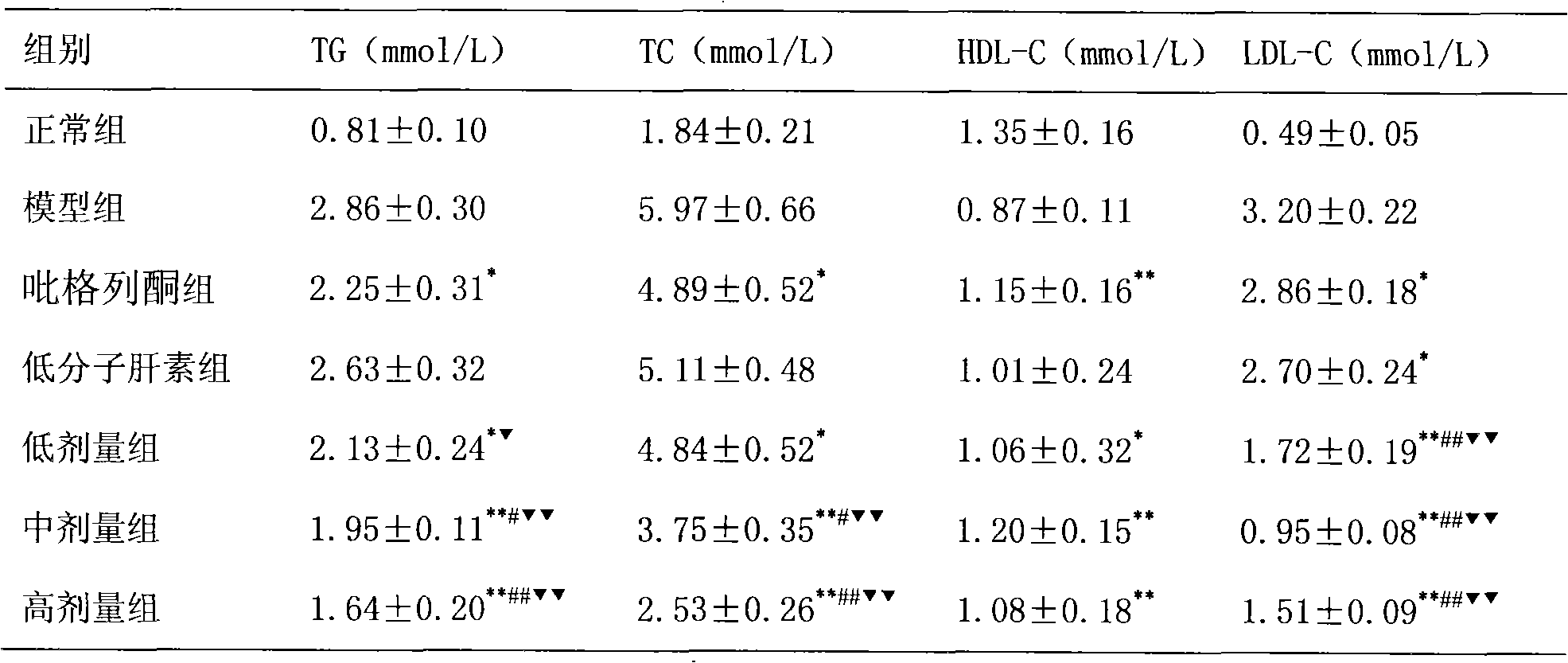
ActiveCN101884643AHighlight substantiveSignificant progressOrganic active ingredientsDigestive systemFatty liverPyrrole
The invention relates to the application of pioglitazone and heparin or low molecular heparin or pharmaceutically acceptable salts thereof or derivatives thereof to preparing a pharmaceutical composition, in particular to the application to preparing a medicament for preventing or treating fatty liver. the purpose of the pioglitazone and the heparin or the low molecular heparin or the pharmaceutically acceptable salts thereof in lessening fat deposition in rat hepatocytes and the synergy of a pioglitazone and heparin or low molecular heparin compound in preventing or treating the rat fatty liver are proved by animal tests. The invention provides a new candidate medicament for searching a medicament for lessening fat deposition in hepatocytes and treating diseases caused by clinic lipid metabolism, such as fatty liver, and the like and therefore enriches the prior art.
Owner:LUNAN PHARMA GROUP CORPORATION
Umbilical cord mesenchymal stem cell injection liquid, preparation method and application thereof
InactiveCN106237313AGreat differentiation potentialImprove proliferative abilityOrganic active ingredientsPeptide/protein ingredientsVitamin CHuman albumin
The invention relates to the technical field of stem cells and especially relates to an umbilical cord mesenchymal stem cell injection liquid, a preparation method and an application thereof. The injection liquid comprises umbilical cord mesenchymal stem cells, human albumin, low molecular heparin calcium, vitamin C, panax japonicus majoris saponin and a solvent. The injection liquid is prepared from the umbilical cord mesenchymal stem cells, so that the materials are convenient to obtain and the method does not violate ethical issue. The injection liquid can effectively prolong preservative time of the cells and the preserved stem cells have high activity, so that the injection liquid is convenient to use. After refusion, the injection can promote differentiation and maturation of the mesenchymal stem cell to liver cells, thus reducing incidence rate of GVHD.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com